Abstract
The CYP3A5*1 allele has been associated with differences in the metabolism of some CYP3A substrates. CYP3A5 polymorphism may also influence susceptibility for certain drug interactions. We have previously noted a correlation between basal CYP3A activity and the inductive effects of dexamethasone using the erythromycin breath test (ERBT). To determine if CYP3A5 polymorphism influences induction of CYP3A activity, we examined the effect of an anti-emetic regimen of dexamethasone, and the prototypical inducer rifampin, on the ERBT in a African American volunteers prospectively stratified by CYP3A5*1 allele carrier-status. Mean basal ERBTs were significantly higher in CYP3A5*1 carriers (2.71% ± 0.53%) versus non-carriers (2.12% ± 0.37%, P = 0.006). Rifampin increased ERBTs in CYP3A5*1 carriers (4.68% vs 2.60%, P = 0.0008) and non-carriers (3.55% vs 2.11%, P = 0.0017), while dexamethasone increased ERBTs only in CYP3A5*1 non-carriers (3.03% vs 2.14%, P = 0.031). CYP3A5 polymorphism appears to influence susceptibility to induction-type drug interactions for some inducers, and CYP3A5*1 non-carriers may be more susceptible to the inductive effects of dexamethasone due to lower basal CYP3A activity.
INTRODUCTION
Members of the cytochrome P450 3A (CYP3A) subfamily of drug metabolizing enzymes are the most abundantly expressed cytochrome P450 enzymes in human liver and are involved in the metabolism of nearly 50% of clinically used drugs (Wilkinson, 2005).
In adults, hepatic CYP3A activity reflects primarily the net contributions of CYP3A4 and CYP3A5 which share overlapping substrate specificities but differ with regard to tissue expression and transcriptional regulation (Gibson et al., 2002; Goodwin et al., 2002; Burk and Wojnowski, 2004). Recent efforts to understand inter-individual variability in CYP3A activity have focused primarily on CYP3A5 polymorphisms since variability in the contribution of functional CYP3A5 activity could influence an individual’s susceptibility to inducer- or inhibitor-mediated drug interactions. The major CYP3A5 polymorphisms include the CYP3A5*3, *6 and *7 alleles which are functionally inactive due to single nucleotide polymorphisms that result in either splice defects or the introduction of an early stop codon that result in reduced production of the full length active protein (Lamba et al., 2002; Wojnowski, 2004; Xie et al., 2004). CYP3A5*1 is the only functional CYP3A5 allele known to contribute to total CYP3A activity and the frequency of the CYP3A5*1 allele has been shown to differ among ethnic groups (Kuehl et al., 2001). The CYP3A5*1 allele has been associated with higher midazolam systemic clearance and tacrolimus dose requirements (Kuehl et al., 2001; Lin et al., 2002; Wong et al., 2004), and greater metabolism of quinidine and saquinavir (Mouly et al., 2005; Mirghani et al., 2006).
Less is known about the influence of the CYP3A5*1 allele on susceptibility for drug interactions caused by different CYP3A inducers and inhibitors. Recently, the CYP3A5*1 allele was shown to influence susceptibility to the inhibitory effects of fluconazole but only in a substrate-dependent manner (Isoherranen et al., 2007). We have previously noted a correlation between basal hepatic CYP3A activity and the inductive effects of dexamethasone in healthy volunteers using the erythromycin breath test (McCune et al., 2000). While the effect of CYP3A5 polymorphism on the induction of CYP3A activity by glucocorticoids has not been explored, the presence of the CYP3A5*1 allele does not appear to influence induction of hepatic CYP3A activity by the potent CYP3A4 inducer, rifampin when assessed by the systemic clearance of midazolam or by the ERBT (Floyd et al., 2003; Yu et al., 2004). We hypothesized that the influence of CYP3A5 polymorphism on induction-type drug interactions could vary with different inducers. Therefore, the effect of an anti-emetic dose of dexamethasone on CYP3A activity, as determined by the erythromycin breath test, was compared to that of the prototypical inducer rifampin in a cohort of young, healthy African American volunteers prospectively genotyped for the presence of the CYP3A5*1 allele.
MATERIALS AND METHODS
Study participants
Healthy, unrelated volunteers self-identified as African American (n = 84) were prospectively genotyped to identify potential study participants. The volunteers, ranging in age from 18–45 years, were genotyped for CYP3A4*1B and CYP3A5*3, *6, and *7 alleles from a mouthwash sample using polymerase chain reaction-restriction fragment length polymorphism (Lin et al., 2002). Subjects were prospectively stratified according to CYP3A5 genotype without regard to smoking status or to the presence of the CYP3A4*1B allele. To determine the effect of CYP3A5 genotype on rifampin induction, 14 subjects were grouped according to the presence (CYP3A5*1/*1 and *1/*3, *1/*6, or *1/*7) or absence (CYP3A5*3/*3 or CYP3A5*3, *6, or *7/*6, *7) of the CYP3A5*1 allele. Likewise, 12 subjects were similarly stratified to determine the effect of CYP3A5 genotype on CYP3A induction by dexamethasone. Prior to enrollment, all subjects received a physical examination consisting of vital signs (pulse, blood pressure, respiration and body temperature), auscultation of heart and lungs and routine clinical laboratory tests. Individuals with a diagnosis or history of cancer, significant organ dysfunction or disease, HIV or taking medications known to induce or inhibit CYP3A were excluded. Individuals with risk factors for glucocorticoid-associated osteonecrosis, such as diagnosis or history of systemic lupus erythematous, recent radiation exposure, recent history of major trauma, history of steroid use in the previous 12 months, histological disease, gout/hyperuricemia or hyperlipidemia were excluded from participation in the dexamethasone treatment group. A negative tuberculin skin test was required for all subjects. In addition, a negative pregnancy test within seven days of ERBT administration was required for female subjects. No alcohol, herbal medications, or foods or beverages containing grapefruit juice were permitted for the duration of the study.
Study protocol
The study protocol was approved by the University of North Carolina Biomedical Institutional Review Board, and all subjects provided written informed consent before enrollment. This was a parallel design study that allowed crossover into the other treatment arm. After the screening visit, basal hepatic CYP3A activity was determined by the ERBT as previously described (Watkins et al., 1989), and the percent of the erythromycin dose exhaled per hour was calculated from the measured amount of [14C]-CO2 (Isoherranen et al., 2007). Subjects then received one of two CYP3A inducers for 5 days: rifampin 600mg orally once daily or dexamethasone 8mg orally twice daily and then on day 6 the ERBT was repeated. The extent of induction in the ERBT was calculated by expressing the difference between ERBT values, obtained before and after each inducer, as a percent of each subject’s baseline ERBT.
Dexamethasone and rifampin were self-administered on an outpatient basis. Compliance was assessed through study calendars in which subjects documented the time of drug self-administration. Subjects qualifying and consenting to both inducer treatments underwent a minimum 14-day washout period between receipt of rifampin and dexamethasone. During the dexamethasone phase of the study, subjects were asked to monitor, daily, their blood pressure with a home monitoring cuff, as well as glucose levels with a urine dipstick kit. Subjects were contacted daily by phone during the induction phase to assess compliance, monitoring parameters and adverse events.
Statistical Analysis
All statistical analyses were performed using SAS-JMP (SAS Institute Inc, Cary, NC). The choice of sample size was guided by considerations of the expected statistical precision, expected power of the test procedure for the primary outcome which was selected based on findings from our previous study (McCune et al., 2000), study feasibility, cost, and the aims of the study. A sample size of 6 per group provided sufficient power to detect an effect size of 50%, however, a sample size of 8 per group was selected because of the uncertainty in the expected variance in an African American population identified by CYP3A5 genotype status. The primary outcome, the effect of dexamethasone on the ERBT in a cohort who were non-carriers of the CYP3A5*1 allele, was analyzed by use of a paired Student’s t-test. We also determined the effect of dexamethasone on the ERBT in a cohort who carried the CYP3A5*1 allele. The difference in the inductive effects of rifampin and dexamethasone between CYP3A5*1 allele carriers and non-carriers were secondary endpoints for this study. In all analyses, P < 0.05 was considered statistically significant and data are presented as mean + standard deviation. In the planning stage, estimated power curves were plotted as functions of plausible magnitudes of treatment effects and variance based on previous inductive effects observed with rifampin and dexamethasone (Gharaibeh et al., 1998; McCune et al., 2000).
RESULTS
CYP3A5 Genotyping
For the 84 subjects who were originally consented and genotyped for this study, the frequency of the CYP3A5*1 allele was 66%. Genotype distribution showed 24 individuals carrying the CYP3A5*1/*1 genotype (29%), 20 individuals carrying the CYP3A5*1/*3 genotype (24%), 7 individuals carrying the CYP3A5*1/*6 genotype (8.3%), and 4 carrying the CYP3A5*1/*7 genotype (4.8%). One individual each was genotyped as CYP3A5*6/*7 and CYP3A5*6/*6. Eight individuals carried the CYP3A5*3/*6 genotype (9.5%), and 6 carried the CYP3A5*3/*7 genotype (7.1%). The homozygous variant genotype, CYP3A5*3/*3, was carried by 13 individuals (15.5%). The frequency distributions of all CYP3A5 alleles were in Hardy-Weinberg equilibrium (p ≥ 0.35). These allelic frequencies were in agreement with previous reports involving African American subjects (Kuehl et al., 2001).
Linkage disequilibrium (LD) between CYP3A4*1B and CYP3A5*1 alleles was not observed (D’ = 0.025, Haploview ver3.11) when an additional 51 subjects genotyped for major CYP3A4 and CYP3A5 polymorphisms were pooled for a total of 135 African Americans for this analysis. This result was consistent with our observation from the HapMap data (http://www.hapmap.org) that CYP3A4*1B and CYP3A5*1 genotypes were not in LD in African Americans when CYP3A5 genotypes were visually sorted by CYP3A4*1B (data not shown).
Effect of CYP3A5 Genotype on Extent of Induction
Of the 27 volunteers who consented to participate, 21 completed the study. Of the six dropouts, two in the rifampin arm did not return for treatment. In the dexamethasone arm, two did not return for treatment, and two withdrew after initiating dexamethasone treatment. Of these latter two subjects, one experienced irretractable hiccups beginning on day 3 of dexamethasone treatment, which lasted greater than 24 hrs but resolved 24 hours after discontinuing the dexamethasone; the other subject completed the 5-day course of dexamethasone but IV access could not be obtained to draw labs or administer the second ERBT. Fourteen subjects completed the rifampin arm of the study (7 CYP3A5*1 carriers, 7 non-carriers), and 12 subjects completed the dexamethasone arm (6 carriers, 6 non-carriers). Three CYP3A5*1 carriers and two non-carriers participated in both arms of the study.
To evaluate the influence of CYP3A5 genotype on basal CYP3A activity, the basal ERBT values from both rifampin and dexamethasone arms of the study were pooled. For the five subjects who completed both arms of the study, the mean of both basal ERBT values (one from the rifampin arm and one from the dexamethasone arm) was included in a weighted least-squares analysis depicted in Figure 1. In addition, neither the level of significance nor conclusion was changed if either basal value for these five subjects was used in separate analyses. The mean basal ERBT value for all subjects was found to be significantly higher for individuals who carried at least one CYP3A5*1 allele compared to individuals who lacked a CYP3A5*1 allele (2.71% ± 0.53% versus 2.12% ± 0.37%, P = 0.006).
Figure 1. Effect of CYP3A5 genotype on basal hepatic CYP3A activity as determined by the erythromycin breath test (ERBT).
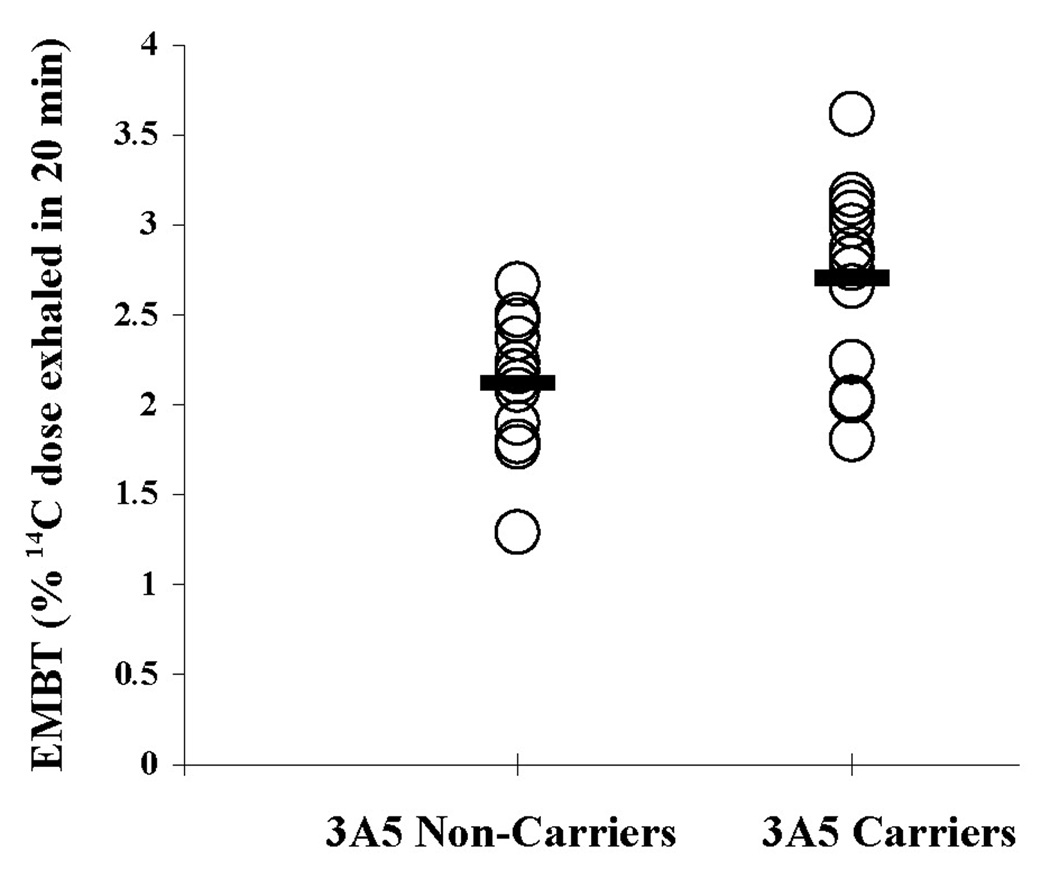
The mean ERBT was significantly higher for CYP3A5*1 carriers compared to non-carriers (2.71% ± 0.53% versus 2.12% ± 0.37%, P = 0.006) using a weighted least squares analysis to correct for 5 subjects who participated in both study arms. Open circles denote individual values. Closed bars denote the group mean.
Rifampin significantly increased CYP3A activity in carriers of the CYP3A5*1 allele from a baseline mean ERBT of 2.60 ± 0.66% to 4.68% ± 1.03% (P = 0.0008), and in non-carriers of the CYP3A5*1 allele from a baseline mean ERBT of 2.11 ± 0.43% to 3.55% ± 0.9% (P = 0.0017) (Figure 2A). Similarly, and anti-emetic regimen of dexamethasone increased CYP3A activity in non-carriers of the CYP3A5*1 allele from a baseline mean ERBT of 2.14 ± 0.31% to 3.03% ± 0.94% (P = 0.031) (Figure 2B). In contrast to the effect of rifampin, an increase in CYP3A activity following dexamethasone administration was not detected in African Americans who carried the CYP3A5*1 allele (mean baseline ERBT of 2.83 ± 0.35% to 3.30% ± 0.85%, P = 0.160) (Figure 2B). Because baseline values differed between the two CYP3A5 genotypes, the change from baseline to induced level of the ERBT was also compared between CYP3A5*1 carriers and non-carriers for both inducers. The change in the ERBT following rifampin treatment was similar for both carriers (2.09 ± 1.02%) and non-carriers (1.44 ± 0.81%) of the CYP3A5*1 allele. While an approximately 2-fold difference in the change in the ERBT was observed between CYP3A5*1 carriers (0.47 ± 1.05%) and non-carriers (0.89 ± 0.91%) following administration of dexamethasone, this difference did not reach statistical significance due to greater variance observed with this inducer compared to rifampin.
Figure 2. Effect of rifampin or dexamethasone on CYP3A activity in African Americans who were stratified according to CYP3A5*1 allele carrier status.
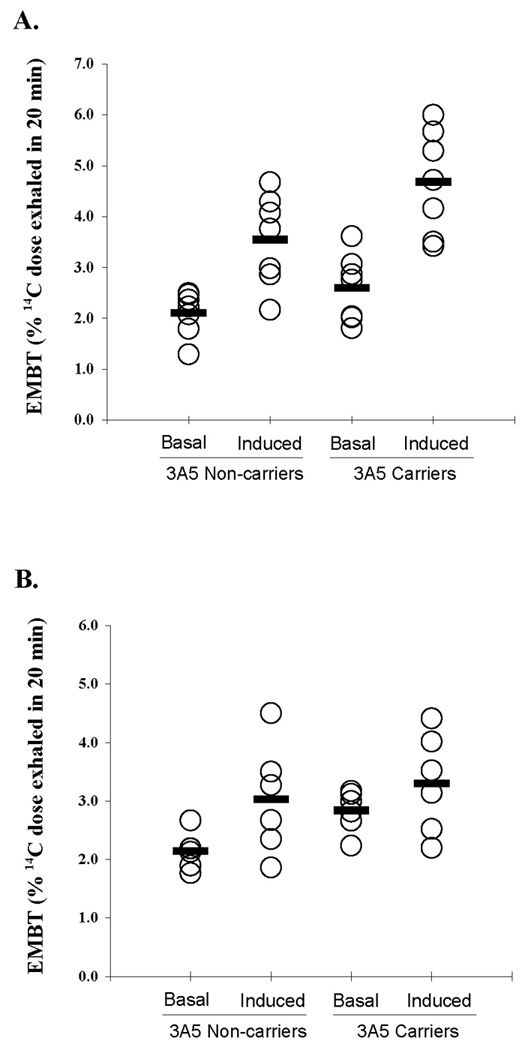
(A) Rifampin significantly induced CYP3A activity in non-carriers (P = 0.0017) and carriers (P = 0.0008) of the CYP3A5*1 allele. (B) A significant increase in CYP3A activity was observed in CYP3A5*1 non-carriers after dexamethasone administration (P = 0.031). In contrast, an effect of dexamethasone on CYP3A activity was not detected in CYP3A5*1 carriers (P = 0.160). Open circles denote individual values. Closed bars denote the group mean.
Several studies have suggested that women have higher hepatic CYP3A activity (as measured by the ERBT) than men (Watkins et al., 1989; Watkins et al., 1992; Kinirons et al., 1999). In the dexamethasone arm of this study, all CYP3A5*1 carriers were women, whereas 4 out of 6 of the CYP3A5*1 non-carriers were women. In the rifampin arm, there were 6 women who were CYP3A5*1 carriers and 5 who were non-carriers. To verify that sex effects on basal CYP3A activity did not account for differences observed with either inducer, we compared basal ERBT values between women who were either carriers or non-carriers of the CYP3A5*1 allele. Women who carried the CYP3A5*1 allele had a significantly higher mean basal ERBT value compared to women non-carriers (2.84% versus 2.01%; P = 0.003). From this post-hoc analysis, we conclude that effects of the two inducers on CYP3A activity were not confounded by sex.
DISCUSSION
The current study was conducted in African Americans because of the higher frequency of the CYP3A5*1 allele in this population, and to control for the effects of genetic admixture when subjects were stratified according to CYP3A5*1 allele carrier status (Kuehl et al., 2001; Lamba et al., 2002; Zeigler-Johnson et al., 2004; Roy et al., 2005). The presence of CYP3A5 protein has been used to explain disease associations linked to the CYP3A4*1B allele since CYP3A4*1B and CYP3A5*1 alleles appeared to be strongly linked in some populations. The CYP3A5*1 allele did not appear to be in LD with the CYP3A4*1B allele in our study although strong LD has been observed in non-African populations (Thompson et al., 2006). Recently, the CYP3A7*2 allele was shown to be in high LD with the CYP3A5*1 allele in African Americans (Rodriguez-Antona et al., 2005; Thompson et al., 2006).
Our results suggest that African Americans carrying at least one CYP3A5*1 allele had higher basal ERBT values compared to those lacking the CYP3A5*1 allele, and differences in basal activity may be associated with difference in susceptibility for induction-type drug interactions with glucocorticoids. It has been estimated that CYP3A5 can account for 6% to 85% of total hepatic CYP3A content and may contribute to increases in basal CYP3A activity in individuals who carry a functional CYP3A5*1 allele (Wrighton et al., 1990; Kuehl et al., 2001; Lin et al., 2002). Individuals carrying at least one CYP3A5*1 allele exhibit a higher oral clearance, lower steady-state blood concentrations, and require higher tacrolimus doses to achieve therapeutic blood concentrations than patients lacking the CYP3A5*1 allele (Bader et al., 2000; Hesselink et al., 2003; Thervet et al., 2003; Haufroid et al., 2004; Hesselink et al., 2004). Recent studies evaluating saquinavir, indinavir, and quinidine have found a positive correlation between the presence of a CYP3A5*1 allele and higher oral clearance (Frohlich et al., 2004; Mouly et al., 2005; Anderson et al., 2006; Mirghani et al., 2006).
Similar to previous observations (Floyd et al., 2003; Yu et al., 2004), the CYP3A5*1 allele was not associated with differences in the ability of rifampin to induce ERBT values in our African American cohort. In contrast, induction of ERBT values by dexamethasone was only detected in non-carriers of the CYP3A5*1 allele. Differences in potencies between these two inducers could explain this observation. Compared to the potent inductive effects of rifampin on CYP3A4 protein, the inductive effects of a less potent PXR activator, such as dexamethasone, could be obscured if a large proportion of the total CYP3A activity was due to functional CYP3A5 since CYP3A5 expression is constitutive and not altered by PXR activation (Bertilsson et al., 1998; Lehmann et al., 1998; Burk et al., 2004). The proximal promoter of CYP3A5 contains a consensus ER-6 response element which may be important for its constitutive expression, although it lacks the distal PXR-response element shown to enhance transcription of CYP3A4 by xenobiotics (Goodwin et al., 2002; Burk and Wojnowski, 2004). Therefore, compared to CYP3A4, CYP3A5 appears to be less sensitive to induction by PXR activators and this has been confirmed in studies with human hepatocytes (Burk et al., 2004).
Several recent reports suggest the CYP3A5*1 allele may impart important clinical consequences due to the higher clearance of some CYP3A drugs (Mouly et al., 2005; Isoherranen et al., 2007; Jin et al., 2007; Josephson et al., 2007). More recently, low hepatic CYP3A activity was found to be associated with the risk for corticosteroid-induced osteonecrosis (Kaneshiro et al., 2006). Our finding that CYP3A5 polymorphism may influence basal levels of hepatic CYP3A activity and the inductive effects of corticosteroids suggests that absence of CYP3A5*1 alleles may be associated with risk for corticosteroid-induced osteonecrosis.
In summary, CYP3A5 polymorphism appears to influence susceptibility to induction-type drug interactions for certain inducers, and CYP3A5*1 non-carriers may be more susceptible to the inductive effects of dexamethasone due to lower CYP3A4-dependent basal activity. These results may be particularly relevant for patients undergoing chemotherapy and receiving dexamethasone for delayed nausea. Our data also suggests that CYP3A5 genotype may have played a role in our previous finding of an inverse correlation between baseline hepatic CYP3A activity and the extent of CYP3A induction following a 5-day course of dexamethasone.
ACKNOWLEDGEMENTS
We would like to acknowledge Paul B. Watkins and Raymond C. Givens, UNC School Medicine, for providing 51 additional African American genotypes for the linkage disequilibrium analysis; Alison C. Lyke, and Michael L. Lim, UNC School of Pharmacy, and Anthony Gachie for coordinating subject recruitment; June Park, UNC School of Pharmacy, for help with the ERBT for CYP3A phenotyping; and Nina Isoherraneri for help with genotyping.
This work was supported through grants from the General Clinical Research Centers program of the Division of Research Resources (RR00046), National Institutes of Health; a Center on Minority Aging Award from the University of North Carolina Institute on Aging (R.L.H.); National Institute of Child Health and Human Development (U10 HD045962–03); and from National Institutes of Health/National Institute of General Medical Sciences Pharmacogenetics Research Network and Database (U01 GM61374, http://pharmgkb.org) under grant U01 GM61393 (E.G.S.).
Non-Standard Abbreviations
- CYP3A
cytochrome P450 3A subfamily
- ERBT
erythromycin breath test
- LD
linkage disequilibrium
REFERENCES
- Anderson PL, Lamba J, Aquilante CL, Schuetz E, Fletcher CV. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr. 2006;42:441–449. doi: 10.1097/01.qai.0000225013.53568.69. [DOI] [PubMed] [Google Scholar]
- Bader A, Hansen T, Kirchner G, Allmeling C, Haverich A, Borlak JT. Primary porcine enterocyte and hepatocyte cultures to study drug oxidation reactions. Br J Pharmacol. 2000;129:331–342. doi: 10.1038/sj.bjp.0703062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk O, Koch I, Raucy J, Hustert E, Eichelbaum M, Brockmoller J, Zanger UM, Wojnowski L. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR) J Biol Chem. 2004;279:38379–38385. doi: 10.1074/jbc.M404949200. [DOI] [PubMed] [Google Scholar]
- Burk O, Wojnowski L. Cytochrome P450 3A and their regulation. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:105–124. doi: 10.1007/s00210-003-0815-3. [DOI] [PubMed] [Google Scholar]
- Floyd MD, Gervasini G, Masica AL, Mayo G, George AL, Jr, Bhat K, Kim RB, Wilkinson GR. Genotype-phenotype associations for common CYP3A4 and CYP3A5 variants in the basal and induced metabolism of midazolam in European- and African-American men and women. Pharmacogenetics. 2003;13:595–606. doi: 10.1097/00008571-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Frohlich M, Hoffmann MM, Burhenne J, Mikus G, Weiss J, Haefeli WE. Association of the CYP3A5 A6986G (CYP3A5*3) polymorphism with saquinavir pharmacokinetics. Br J Clin Pharmacol. 2004;58:443–444. doi: 10.1111/j.1365-2125.2004.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaibeh MN, Gillen LP, Osborne B, Schwartz JI, Waldman SA. Effect of multiple doses of rifampin on the [14C N-methyl] erythromycin breath test in healthy male volunteers. J Clin Pharmacol. 1998;38:492–495. doi: 10.1002/j.1552-4604.1998.tb05785.x. [DOI] [PubMed] [Google Scholar]
- Gibson GG, Plant NJ, Swales KE, Ayrton A, El-Sankary W. Receptor-dependent transcriptional activation of cytochrome P4503A genes: induction mechanisms, species differences and interindividual variation in man. Xenobiotica. 2002;32:165–206. doi: 10.1080/00498250110102674. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, Malaise J, Lison D, Squifflet JP, Wallemacq P. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–154. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- Hesselink DA, van Gelder T, van Schaik RH, Balk AH, van der Heiden IP, van Dam T, van der Werf M, Weimar W, Mathot RA. Population pharmacokinetics of cyclosporine in kidney and heart transplant recipients and the influence of ethnicity and genetic polymorphisms in the MDR-1, CYP3A4, and CYP3A5 genes. Clin Pharmacol Ther. 2004;76:545–556. doi: 10.1016/j.clpt.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Isoherranen N, Ludington SR, Givens RC, Lamba JK, Pusek SN, Dees EC, Blough DK, Iwanaga K, Hawke RL, Schuetz EG, Watkins PB, Thummel KE, Paine MF. The Influence Of Cyp3a5 Expression On The Extent Of Hepatic Cyp3a Inhibition Is Substrate-Dependent: An In Vitro-In Vivo Evaluation. Drug Metab Dispos. 2007 doi: 10.1124/dmd.107.018382. [DOI] [PubMed] [Google Scholar]
- Jin Y, Wang YH, Miao J, Li L, Kovacs RJ, Marunde R, Hamman MA, Phillips S, Hilligoss J, Hall SD. Cytochrome P450 3A5 Genotype is Associated with Verapamil Response in Healthy Subjects. Clin Pharmacol Ther. 2007;82:579–585. doi: 10.1038/sj.clpt.6100208. [DOI] [PubMed] [Google Scholar]
- Josephson F, Allqvist A, Janabi M, Sayi J, Aklillu E, Jande M, Mahindi M, Burhenne J, Bottiger Y, Gustafsson LL, Haefeli WE, Bertilsson L. CYP3A5 genotype has an impact on the metabolism of the HIV protease inhibitor saquinavir. Clin Pharmacol Ther. 2007;81:708–712. doi: 10.1038/sj.clpt.6100117. [DOI] [PubMed] [Google Scholar]
- Kaneshiro Y, Oda Y, Iwakiri K, Masada T, Iwaki H, Hirota Y, Kondo K, Takaoka K. Low hepatic cytochrome P450 3A activity is a risk for corticosteroid-induced osteonecrosis. Clin Pharmacol Ther. 2006;80:396–402. doi: 10.1016/j.clpt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Kinirons MT, O'Shea D, Kim RB, Groopman JD, Thummel KE, Wood AJ, Wilkinson GR. Failure of erythromycin breath test to correlate with midazolam clearance as a probe of cytochrome P4503A. Clin Pharmacol Ther. 1999;66:224–231. doi: 10.1016/S0009-9236(99)70029-9. [DOI] [PubMed] [Google Scholar]
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Dowling AL, Quigley SD, Farin FM, Zhang J, Lamba J, Schuetz EG, Thummel KE. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol. 2002;62:162–172. doi: 10.1124/mol.62.1.162. [DOI] [PubMed] [Google Scholar]
- McCune JS, Hawke RL, LeCluyse EL, Gillenwater HH, Hamilton G, Ritchie J, Lindley C. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther. 2000;68:356–366. doi: 10.1067/mcp.2000.110215. [DOI] [PubMed] [Google Scholar]
- Mirghani RA, Sayi J, Aklillu E, Allqvist A, Jande M, Wennerholm A, Eriksen J, Herben VM, Jones BC, Gustafsson LL, Bertilsson L. CYP3A5 genotype has significant effect on quinine 3-hydroxylation in Tanzanians, who have lower total CYP3A activity than a Swedish population. Pharmacogenet Genomics. 2006;16:637–645. doi: 10.1097/01.fpc.0000230411.89973.1b. [DOI] [PubMed] [Google Scholar]
- Mouly SJ, Matheny C, Paine MF, Smith G, Lamba J, Lamba V, Pusek SN, Schuetz EG, Stewart PW, Watkins PB. Variation in oral clearance of saquinavir is predicted by CYP3A5*1 genotype but not by enterocyte content of cytochrome P450 3A5. Clin Pharmacol Ther. 2005;78:605–618. doi: 10.1016/j.clpt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Antona C, Axelson M, Otter C, Rane A, Ingelman-Sundberg M. A novel polymorphic cytochrome P450 formed by splicing of CYP3A7 and the pseudogene CYP3AP1. J Biol Chem. 2005;280:28324–28331. doi: 10.1074/jbc.M502309200. [DOI] [PubMed] [Google Scholar]
- Roy JN, Lajoie J, Zijenah LS, Barama A, Poirier C, Ward BJ, Roger M. CYP3A5 genetic polymorphisms in different ethnic populations. Drug Metab Dispos. 2005;33:884–887. doi: 10.1124/dmd.105.003822. [DOI] [PubMed] [Google Scholar]
- Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P, Legendre C, Daly AK. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233–1235. doi: 10.1097/01.TP.0000090753.99170.89. [DOI] [PubMed] [Google Scholar]
- Thompson EE, Kuttab-Boulos H, Yang L, Roe BA, Di Rienzo A. Sequence diversity and haplotype structure at the human CYP3A cluster. Pharmacogenomics J. 2006;6:105–114. doi: 10.1038/sj.tpj.6500347. [DOI] [PubMed] [Google Scholar]
- Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J Clin Invest. 1989;83:688–697. doi: 10.1172/JCI113933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PB, Turgeon DK, Saenger P, Lown KS, Kolars JC, Hamilton T, Fishman K, Guzelian PS, Voorhees JJ. Comparison of urinary 6-beta-cortisol and the erythromycin breath test as measures of hepatic P450IIIA (CYP3A) activity. Clin Pharmacol Ther. 1992;52:265–273. doi: 10.1038/clpt.1992.140. [DOI] [PubMed] [Google Scholar]
- Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- Wojnowski L. Genetics of the variable expression of CYP3A in humans. Ther Drug Monit. 2004;26:192–199. doi: 10.1097/00007691-200404000-00019. [DOI] [PubMed] [Google Scholar]
- Wong M, Balleine RL, Collins M, Liddle C, Clarke CL, Gurney H. CYP3A5 genotype and midazolam clearance in Australian patients receiving chemotherapy. Clin Pharmacol Ther. 2004;75:529–538. doi: 10.1016/j.clpt.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Wrighton SA, Brian WR, Sari MA, Iwasaki M, Guengerich FP, Raucy JL, Molowa DT, Vandenbranden M. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3) Mol Pharmacol. 1990;38:207–213. [PubMed] [Google Scholar]
- Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR. Genetic variability in CYP3A5 and its possible consequences. Pharmacogenomics. 2004;5:243–272. doi: 10.1517/phgs.5.3.243.29833. [DOI] [PubMed] [Google Scholar]
- Yu KS, Cho JY, Jang IJ, Hong KS, Chung JY, Kim JR, Lim HS, Oh DS, Yi SY, Liu KH, Shin JG, Shin SG. Effect of the CYP3A5 genotype on the pharmacokinetics of intravenous midazolam during inhibited and induced metabolic states. Clin Pharmacol Ther. 2004;76:104–112. doi: 10.1016/j.clpt.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Zeigler-Johnson C, Friebel T, Walker AH, Wang Y, Spangler E, Panossian S, Patacsil M, Aplenc R, Wein AJ, Malkowicz SB, Rebbeck TR. CYP3A4, CYP3A5, and CYP3A43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer Res. 2004;64:8461–8467. doi: 10.1158/0008-5472.CAN-04-1651. [DOI] [PubMed] [Google Scholar]


