Introduction
In Part 1 we saw that cancer is a multistep process involving complex genetic abnormalities that deregulate signalling pathways, and it involves the cooperation of multiple deregulating genetic pathways.1 Given the widespread involvement of HERVs and related products in human genetic chemistry it is likely that they will be involved in carcinogenesis. We might anticipate that such viral involvement will arise from the known oncogenic potential of viruses, particularly retroviruses. We also need to consider that HERVs, unlike exogenous retroviruses, have been subject to selection working at holobiontic level within the human genome over long time periods. From this we might anticipate additional potential for carcinogenesis deriving from the cooption, or dysregulation, of established symbiotic roles of whole viruses, viral genes and viral regulatory sequences involved in normal genetic pathways.
Viruses and cancer
Approximately 20% of human cancers have been attributed to virus infection, and, in the opinion of virologist, Robin A Weiss, other cancers may also have a viral component.2 In 2008 the German pathologist, Harald zur Hausen, was awarded the Nobel Prize for Medicine for his discovery that the human papilloma virus (HPV) is the cause of 99% of cancers of the cervix, and a majority of vulval, vaginal and penile cancers – leading to the current vaccination programme.3–5 Other examples include the hepatitis B and C viruses, which cause hepatocellular carcinoma,6 and the Epstein-Barr virus, which causes Burkitt's lymphoma, and may also be linked to nasopharyngeal carcinoma and some of the lymphomas that complicate AIDS.7 Many such viruses are DNA-based, and contain genes that are directly oncogenic through insertion into the host DNA. For example, HPV causes cervical cancer through two viral proteins, E6 and E7, which interfere with the regulation of normal cell division by two key human proteins, Rb and p53.8
Exogenous retroviruses are carcinogenic throughout the animal kingdom, including marine invertebrates, birds, marsupials and a wide variety of placental mammals.9,10 These RNA-based retroviruses are usually oncogenic through common indirect pathways, such as integration of the virus adjacent to a cellular oncogene, or incorporation of a host oncogene within the retroviral genome, or through more complex interactions involving viral LTRs and host regulatory pathways, such as tumour suppression genes.11,12 HIV-1 also involves virus-specific oncogenic pathways. For example, the various non-Hodgkin's lymphomas associated with AIDS may involve activation of the oncogene c-MYC, inactivation of p53, and co-infection with the Epstein-Barr virus.13 With effective modern treatment, the once-frequent Kaposi's sarcoma is now a rarity, confirming the importance of AIDS-related immunosuppression in the genesis of tumour progression.14 HTLV-1 also induces oncogenesis through a virus-specific regulatory protein, called Tax, although the precise oncological pathway is still under evaluation.15 Genetic screening for exogenous retroviral oncogenesis in experimental mice has revealed more than a hundred loci with carcinogenic potential, including the involvement of established human oncogenes.16–18 This suggested that HERVs might also possess significant oncogenic potential.
HERVs in carcinogenesis
Endogenous retroviral particles were first reported in platelets from patients with myeloproliferative disorders in 1975.19 Subsequently, reverse transcriptase activity coupled with EM pictures of HERVs have been detected in platelets from patients with primary proliferative polycythaemia and essential thrombocythaemia,20 HERV-K protein synthesis (gag gene) has been detected in megakaryocytes from stem cells cultured from the peripheral blood of patients with essential thrombocythaemia,21 with packaging of the gag protein into HERV-K viruses budding from the cell membrane of the megakaryocytes. While this suggested that HERV-Ks might be implicated in the myeloproliferative disorders, no firm conclusions could be drawn as to whether the HERVs were causative or acting in a responsive role.
HERV-H has been found in leukaemia and various cancer cell lines as well as cancers of the lung, stomach, intestine, bone marrow, bladder, prostate and cervix,22–25 HERV-K with melanoma, seminomas, the blood of leukaemia patients, teratocarcinomas and breast cancer lines,26–34 and HERV-E in prostate carcinoma.35 Other researchers have linked HERV-related sequences, such as LINE-1s, SINES and Alus to a variety of cancers,36 including oesophageal adenocarcinoma.37 It is too early to confirm any putative role of HERVs and products in such associations, but one study has reported a possible mechanism of HERV-induced malignancy. A relatively rare pattern of stem-cell myeloproliferative disorder has been linked to translocations on chromosome 8 in a region involving the FGFR1 gene, which encodes one of the tyrosine kinase receptors for fibroblast growth factors. The resulting syndrome is characterized by myeloid hyperplasia, frequent peripheral blood eosinophilia and B- or T-cell lymphoblastic leukaemia or lymphoma.38,39 Guasch and colleagues have reported the fusion of a HERV-K element sequence with FGFR1 sequences at the break point on chromosome 8 in one out of eight ‘partner gene’ examples of this disorder, with subsequent translocation to chromosome 19 in a patient suffering from an atypical myeloproliferative syndrome.40 This might imply non-allelic recombination between HERV elements on the two chromosomes.
In a series of papers, Schulte et al. have described how the insertion of a HERV into the intron sequence immediately upstream of the first coding exon of the human growth factor gene, pleiotrophin (PTN), generated an additional promoter with trophoblast-specific activity.41 Further studies of the HERV suggested that it derived from the recombination of a HERV-E and RTVL-1, generating a novel viral element containing one gag, two pols and two env domains, flanked by LTRs. Since it retained the defining primer binding of a HERV-E, the authors classed it as a novel HERV-E.42 PTN stimulates growth and transformation in fibroblasts and epithelial cells, and it plays an important role in the developments of human melanoma and human trophoblast-derived choriocarcinoma. The authors also demonstrated transcription of messenger RNA for the fused HERV-E.PTN domain in normal human trophoblast cell cultures as early as 9 weeks after gestation as well as in full-term placentae. The fused domain was not present in mice, or rhesus monkeys, but was common to humans, chimpanzees and gorillas, confirming a holobiontic evolutionary event dating to about 25 million years ago that had resulted in a new PTN promoter. Transcription of the fused domain was also a feature of chorioncarcinoma cell lines but not tumour cell lines derived from the embryoblast (teratocarcinomas) or other lineages. This suggests that the chorioncarcinoma might be co-opting a symbiotic HERV function involved in the proliferative and invasive behaviour of normal trophoblasts during placentation. Deletion of the retroviral-derived section of the promoter sequence prevented the growth, invasion and angiogenesis that would normally accompany the tumour development.43
In an elegant body of work over the last decade, Roemer, Armbruester and colleagues have presented a growing litany of evidence for the fact that HERV-K viruses may play significant roles in carcinogenesis. They first noticed the association between high titres of antibodies to HERV gag and env and germ cell tumours, such as seminomas and teratocarcinomas.44,45 HERV-K viruses possess a virus-specific gene known as rec, or cORF, which is the functional homologue of the HIV-1 gene, REV. This codes for proteins that enable daughter virus production after infection. The same authors have shown that HERV-K rec expression interferes with germ cell development in transgenic mice, where it may also cause carcinoma in situ.46 In particular they have identified a novel gene, Np9, within the HERV-K env genetic domain that gives rise to a protein localized predominantly in the cell nucleus. The expression of Np9 in various tumours suggest that it may be playing a role in carcinogenesis,47,48 and this in turn may be mediated through interactions between HERV proteins Np9 and Rec with the promyelocytic leukaemia zinc finger protein, a transcriptional repressor and chromatin remodeller that has been implicated in cancer and the self-renewal of spermatogonial stem cells.49,50 However, even this well-researched line of evidence, while increasingly suggestive of a carcinogenic role for certain HERV-K genes, does not yet amount to conclusive evidence.
Members of the HTDV/HERV-K family can express themselves as viral particles in testicular cells. This is also dependent on the HERV Rec protein – a role that may have as yet unknown physiological implications. This uncertainty makes it difficult to interpret high levels of expression of the same viruses and their genes in testicular tumours, though some authors question if dysregulation of cORF expression might contribute to the onset and progression of germ cell tumours.51 Despite the uncertainty, Nelson and colleagues conclude that the weight of evidence amounts to convincing evidence of the involvement of HERVs and their products in carcinogenesis.52,53 As we saw with mutation, the difficulty in proving direct causation is due to the involvement of multiple genetic pathways together with an ill-defined interplay between environmental and epigenetic factors in a multistep, complex aetiology.
Possible ways in which HERVs might be involved in carcinogenesis are outlined in a helpful review article by Ruprecht and colleagues, with a simplified scheme depicting a theoretical series of steps.54 An interesting possibility is the potential of HERV proteins to act as tumour recognition antigens, thus provoking an anti-tumour immune response that might be beneficial in immunological surveillance and defence against cancers. Conversely, Manganey and colleagues have confirmed, in a series of studies, that the expression of the env protein of both exogenous retroviruses and HERV-H on the surface of tumour cells allows them to evade immune rejection.55,56 For Ruprecht this adds the potential for ‘tumour immune escape’ mediated by HERV env proteins expressed on tumour cells, which in turn might provoke dysregulation of the normal immunosuppressive role of HERV proteins, such as syncytin-2. In a related study, Ruggieri and colleagues have discovered a novel signal peptide coded by the env gene of HERV-K(HML-2), a family of viruses known to be associated with testicular germ cell tumours, raising the possibility that this might also contribute to immune evasion of the tumour cells.57 Recruitment of the expression of HERV-W syncytin-1 protein in certain breast and endometrial cancers may result in fusions involving cancer-to-endothelial cells, or cancer cell-to-cell fusions, which may promote tumour growth.54,58
HERV-related retroposons in carcinogenesis
Where the position of HERVs is believed to be fixed in the chromosomes following endogenization, other HERV-related products, such as LINEs, SINEs and Alus, are capable of multiplying themselves and reinserting within the genome. These insertions can result in mutation-like disruption of coding regions, interference with regulation within introns and regulatory sequences, or translocations and deletions through virus-style recombination between homologous and non-homologous chromosomes. Morse and colleagues have reported a tumour-specific rearrangement of a MYC oncogene locus brought about by L1 insertion into an intron, which was associated with ductal adenocarcinoma of the breast.59 Miki and colleagues have reported a disruptive L1 insertion into the APC tumour suppressor gene in a case of colon cancer.60 Given that there are many other tumour suppressor genes, such as RB (retinoblastoma), WT (Wilm's tumour), DCC and MCC (colorectal carcinomas), and p53, and given that L1 insertions are thought to be widespread and random, these must now be considered as one of the potential mechanisms for carcinogenesis through suppressor gene disruption.
SINEs are of different origins to Alus, with which they are often grouped, but both depend on HERVs or LINEs for transposition and they show behavioural similarities. Misra and colleagues have reported the loss of a band of 443 nucleotide bases in tumour DNA taken from a grade IV glioblastoma multiforme, which, on sequencing, was found to comprise a group of SINE-R sequences that were derived from HERV-K.61 Related sequences were also found in the tumour suppressor gene BRCA2 and the DNA repair gene XRCC1. While this does not demonstrate a cause–effect relationship, it suggests a possible role for the HERV sequences in gene inactivation through viral recombination during carcinogenesis.
The enormously high frequency of Alus in the human genome and the fact that they can interrupt normal genetic function through inserting into coding exons, or into introns, where they cause alternative splicing, or by unequal homologous recombination, means that Alus are a major consideration in the genetics of cancer.62–64 For example O'Neil and her colleagues have shown that the MYB oncogene, which is frequently duplicated during the pathogenesis of human T cell acute lymphoblastic leukaemia (T-ALL), is flanked by Alu repeats, making it susceptible to tandem duplication as a result of Alu-to-Alu recombination on homologous chromatids. These duplications occur at low frequency during normal thymocyte development in healthy people, but they are clonally selected during the molecular pathogenesis of human T-ALL, a thymocyte malignancy.65 Roughly one in four hereditary breast cancers are attributed to mutations affecting BRCA1 or BRCA2 genes. Montagna and colleagues describe a 3kb deletion in BRCA1 in two high-risk families, which encompassed exon 17 and gave rise to a frameshift mutation.66 The rearrangement was the result of recombination between two very similar Alu repeats. This type of mutation would not have been identified by the common methods of detection, based on single exon amplification using PCR. Homologous recombination between Alu elements has also been associated with acute myeloid leukaemia, sometimes with a normal karyotype.67–69 Multiple endocrine neoplasia type 1 is an autosomal dominant cancer syndrome, which is caused by germline mutations of the tumour suppressor gene MEN1. Fukuuchi and colleagues have reported an Alu-linked germline deletion of the MEN1 flanked by Alu sequences in a family where, again, the deletion would have been undetectable by conventional sequencing analysis.70 Alu repeats have also been associated with familial colorectal cancer,71,72 breast and ovarian cancer,73,74 Ewing's sarcoma75 and glioma,76 meanwhile it seems likely that this list of associations will increase as the database grows.
Two illustrative approaches to remedial therapy, based on HERVs
Spadafora and colleagues have investigated the effects of blocking reverse transcriptase in a variety of cancers in murine and human cell lines, including teratocarcinomas, fibrosarcoma, osteosarcoma, gliomas, melanoma, and carcinomas of the colon, breast, prostate and thyroid, to show that, regardless of histological origin, RT inhibition caused a dramatic reduction in tumour cell proliferation, meanwhile inducing a more normal cell differentiation, which included a reprogramming of gene expression to a pattern that was more typical of normality.77,78 They also demonstrated qualitatively different responses in vitro and in vivo after blocking the effects of the reverse transcriptases of HERV-K and LINE-1 origin, suggesting important differences in function between the two retroviral components. Unlike conventional therapy, these studies showed a novel pattern of reversal of malignant transformation, behaviour and genetic expression in the affected cell lines as well as a marked reduction in the proliferation of human cell tumours in laboratory strains of mice ( Figure 1).79,80 The reduction in cell proliferation and reprogramming of differentiation reverted to malignant behaviour when they removed the anti-RT drug from the cellular or animal models. In a recent study of the process of malignant transformation of melanoma cells, the same authors have reported HERV-K activation during two of the key stages of carcinogenesis: in the initial transformation to malignancy; and in the ability of malignant cells to escape immune detection by the body's surveillance.81 In cell cultures, these changes were accompanied by vigorous HERV-K expression, including massive production of virus-like particles. Reduction of the expression of HERV-K, using RNA interference, prevented some of the changes involved in malignant transformation.
Figure 1.
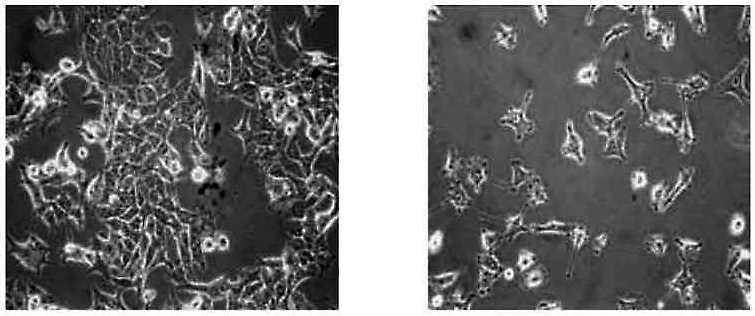
Effects of L1 silencing in cancer. The left view shows melanoma cells of the A-375 culture line. The right view shows the same cells after L1 expression was blocked by RNA interference. The clumping of rounded tumour cells has reverted to the more normal stellate forms with dendritic processes. This was accompanied by some normalisation of the genetic processes associated with cell proliferation. Kindly provided by Corrado Spadafora
Another pioneering approach has been adopted by Palmarini and colleagues studying Jaagsiekte sheep retrovirus (JSRV), which causes a major infectious epidemic in sheep leading to fatal ovine pulmonary adenocarcinoma.82 This is of specific interest to the study of carcinogenesis since, uniquely among the known retroviruses, the JSRV env gene is directly oncogenic, inducing cancerous transformation of the alveolar type II cells and non-ciliated bronchiolar cells (Clara cells) in the lungs of infected sheep as well as inducing cancer in a number of cell lines in vitro.83 The exogenous JSRV is closely related to the endogenous form of the same virus, enJSRV, which is expressed at high level in the genital tract of the ewe. The original portal of entry was surely genital, but Palmarini's group have shown that expression of a specific genetic locus derived from the gag protein of the endogenous virus (the enJS56A1 locus) now blocks viral entry and replication in the cells lining the genital tract, thus preventing genital transmission.84 Meanwhile the exogenous virus has evolved a new transmission strategy, via pulmonary infection. Palmarini and his colleagues have been studying the molecular basis of the enJS56A1 block, with the aim of inducing expression of the enJS56A1 locus within the cells lining the pulmonary tract in germ line experiments ( Figure 2). In essence they are looking at the possibility of inducing ERV-induced genetic change in sheep that would promote species resistance to the exogenous virus, a novel approach to a carcinogenic retroviral epidemic in a mammal.
Figure 2.
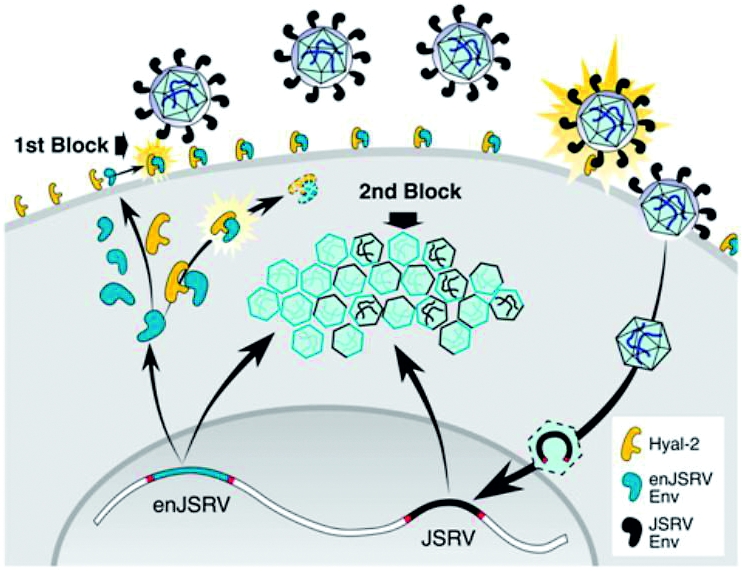
The two sites of enJSRV block. The endogenous JSRV blocks genital invasion by the exogenous virus at two sites, the attachment of the env gene at the epithelial cell surface and at the stage of exogenous viral replication in the cytoplasm. Kindly provided by Massimo Palmarini
In summary
Although our current understanding of the contribution of HERVs to carcinogenesis is limited by our lack of understanding of their normal physiological roles, it would appear that they play significant, and very likely, a variety of different roles in both anti-cancer protection and pathogenesis. It is important that future mass genetic screening systems should include the possibility of HERVs and related genetic sequences in their programmes. A fuller understanding of this HERV role, coupled with a deeper epigenetic understanding, is likely to contribute to new lines of therapy, including immunotherapy.25,85
Part 5 of this series will examine the role of epigenetics and genomic duplications in health and disease.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding None
Ethical approval Not applicable
Guarantor FPR
Contributorship FPR is the sole contributor
Acknowledgements
The author would like to thank Massimo Palmarini, Klaus Roemer, Corrado Spadafora and Oliver Quarrell for their assistance with this paper
References
- 1.Ryan FP. An alternative approach to medical genetics based on modern evolutionary biology. Part 1: mutation and symbiogenesis. J R Soc Med 2009;102:272–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss RA. Retroviruses and cancer. Curr Sci 2001;81:528–34 [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907 [DOI] [PubMed] [Google Scholar]
- 4.See http://en.wikipedia.org/wiki/Harald_zur_Hausen
- 5.Madkan VK, Cook-Norris RH, Steadman MC, et al. The oncogenic potential of human papillomaviruses: a review on the role of host genetics and environmental cofactors. Br J Dermatol 2007;157:228–41 [DOI] [PubMed] [Google Scholar]
- 6.Koike K. Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways. J Gastroenterol Hepatol 2007;22 (Suppl. 1):S108–S11 [DOI] [PubMed] [Google Scholar]
- 7.Young LS, Murray PG. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene 2003;22:5108–21 [DOI] [PubMed] [Google Scholar]
- 8.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci 2006;110:525–41 [DOI] [PubMed] [Google Scholar]
- 9.Romalde JL, Vilariño ML, Beaz R, et al. Evidence of retroviral etiology for disseminated neoplasia in cockles. J Invertebr Pathol 2006;94:95–101 [DOI] [PubMed] [Google Scholar]
- 10.Martin J, Herniou W, Cook J, et al. Human endogenous retrovirus type I-related viruses have an apparently widespread distribution within vertebrates. J Virology 1997;71:437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Löwer R. The pathogenic potential of endogenous retroviruses: facts and fantasies. Trends Microbiol 1999;7:350–6 [DOI] [PubMed] [Google Scholar]
- 12.Hayward WS, Neel BG, Astrin SM. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leucosis. Nature 1981;290:475–80 [DOI] [PubMed] [Google Scholar]
- 13.Carbone A. AIDS-related non-Hodgkin's lymphomas: from pathology and molecular pathogenesis to treatment. Human Path 2002;33:392–404 [DOI] [PubMed] [Google Scholar]
- 14.Scadden DT. Epstein-Barr virus, the CNS, and AIDS-related lymphomas: as close as flame to smoke. J Clin Oncol 2000;18:3323–4 [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Ren T, Guan H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IkB kinases to the microdomains for persistent activation of NF-kB. J Biol Chem 2009;284:6208–17 [DOI] [PubMed] [Google Scholar]
- 16.Neil JC, Cameron ER. Retroviral insertion sites and cancer: fountain of all knowledge? Cancer Cell 2002;2:253–5 [DOI] [PubMed] [Google Scholar]
- 17.Mikkers H, Allen J, Knipscheer P, et al. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat Genet 2002;32:153–9 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Shen H, Keiko A, et al. New genes involved in cancer identified by retroviral tagging. Nat Genet 2002;32:166–74 [DOI] [PubMed] [Google Scholar]
- 19.Brodsky I, Fuscaldo A, Erlick BJ, et al. Analysis of platelets from patients with thrombocythemia for reverse transcriptase and virus-like particles. J Natl Cancer Inst 1975;55:1069–74 [DOI] [PubMed] [Google Scholar]
- 20.Boyd MT, Oscier DG, Maclean N. Detection of retrovirus in patients with myeloproliferative disease. Lancet 1989;333:814–17 [DOI] [PubMed] [Google Scholar]
- 21.Morgan D, Brodsky I. Human endogenous retrovirus (HERV-K) particles in megakaryocytes cultured from essential thrombocythemia peripheral blood stem cells. Expt Haemat 2004;32:520–5 [DOI] [PubMed] [Google Scholar]
- 22.Lindeskog M, Blomberg J. Spliced human endogenous retroviral HERV-H env transcripts in T-cell leukaemia cell lines and normal leukocytes: alternative splicing pattern of HERV-H transcripts. J Gen Virol 1997;78:275–85 [DOI] [PubMed] [Google Scholar]
- 23.Hirose Y, Takamatsu M, Harada F. Presence of env genes in members of the RTVL-H family of human endogenous retrovirus-like elements. Virology 1993;192:52–61 [DOI] [PubMed] [Google Scholar]
- 24.Yi J-M, Kim H-M, Kim HS. Human endogenous retrovirus HERV-H family in human tissues and cancer cells: expression, identification, and phylogeny. Canc Lett 2006;231:228–39 [DOI] [PubMed] [Google Scholar]
- 25.Stauffer Y, Theiler G, Sperisen P, et al. Digital expression profiles of human endogenous retroviral families in normal and cancerous tissue. Canc Immun 2004;4:2. [PubMed] [Google Scholar]
- 26.Schiavetti F, Thonnard J, Colau D, et al. A human retroviral sequence encoding an antigen recognized on melanoma by cytolytic L lymphocytes. Canc Res 2002;62:5510–16 [PubMed] [Google Scholar]
- 27.Buscher K, Trefzer U, Hofmann M, et al. Expression of human endogenous retrovirus K in melanomas and melanoma cell lines. Canc Res 2005;65:4172–80 [DOI] [PubMed] [Google Scholar]
- 28.Rakoff-Nahoum S, Kuebler PJ, Heymann JJ, et al. Detection of T lymphocytes specific for human endogenous retrovirus K (HERV-K) in patients with seminomas. AIDS Res Hum Retroviruses 2006;22:52–6 [DOI] [PubMed] [Google Scholar]
- 29.Herbst H, Kuhler-Obbarius C, Lauke H, et al. Human endogenous retrovirus (HERV)-K transcripts in gonadoblastomas and gonadoblastoma-derived germ cell tumours. Virchows Arch 1999;434:11–15 [DOI] [PubMed] [Google Scholar]
- 30.Depil S, Roche C, Dussart P, et al. Expression of a human endogenous retrovirus, HERV-K, in the blood cells of leukaemia patients. Leukaemia 2002;16:254–9 [DOI] [PubMed] [Google Scholar]
- 31.Boller K, König H, Sauter M, et al. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinomas-derived retrovirus HTDV. Virology 1993;196:349–53 [DOI] [PubMed] [Google Scholar]
- 32.Löwer R, Löwer J, Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. PNAS 1996;93:5177–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bieda K, Hoffmann A, Boller K. Phenotypic heterogeneity of human endogenous retrovirus particles produced by teratocarcinomas cell lines. J Gen Virol 2001;82:591–6 [DOI] [PubMed] [Google Scholar]
- 34.Wang-Johanning F, Frost AR, Johanning GL, et al. Expression of human endogenous retrovirus K envelope transcripts in human breast cancer. Clin Cancer Res 2001;7:1553–60 [PubMed] [Google Scholar]
- 35.Wang-Johanning F, Frost AR, Jian B, et al. Detecting the expression of human endogenous retrovirus E envelope transcripts in human prostate adenocarcinoma. Cancer 2003;98:187–97 [DOI] [PubMed] [Google Scholar]
- 36.Kim D-S, Huh JW, Kim HS. Transposable elements in human cancers by genome-wide EST alignment. Genes Genet Syst 2007;82:145–56 [DOI] [PubMed] [Google Scholar]
- 37.Lin L, Zhuwen W, Prescott MS, et al. Multiple forms of genetic instability within a 2-Mb chromosomal segment of 3q26.3-q27 are associated with development of esophageal adenocarcinoma. Gene Chromosome Canc 2006;45:319–31 [DOI] [PubMed] [Google Scholar]
- 38.Chaffanet M, Popovici C, Leroux D, et al. t(6;8), t(8;9) and t(8;13) translocations associated with stem cell myeloproliferative disorders have close or identical breakpoints in chromosome region 8p11–12. Oncogene 1998;19:945–9 [DOI] [PubMed] [Google Scholar]
- 39.MacDonald D, Aguiar RC, Mason PJ, et al. A new proliferative disorder associated with chromosomal translocations involving 8p12: a review. Leukemia 1995;9:1628–30 [PubMed] [Google Scholar]
- 40.Guasch G, Popovici C, Mugneret F, et al. Endogenous retroviral sequence is fused to FGFR1 kinase in the 8p12 stem-cell myeloproliferative disorder with t(8;19)(p12;q13.3). Blood 2003;101:286–8 [DOI] [PubMed] [Google Scholar]
- 41.Schulte AM, Lai S, Kurtz A, Czubayko F, Riegel AT, Wellstein A. Human trophoblast and chorioncarcinoma expression of the growth factor pleiotrophin attributable to germ-line insertion of an endogenous retrovirus. PNAS 1996;93:14759–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulte AM, Wellstein A. Structure and phylogenetic analysis of an endogenous retrovirus inserted into the human growth factor gene pleiotrophin. J Virol 1998;72:6065–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulte AM, Malerczyk C, Cabal-Manzano R, et al. Influence of the human endogenous retrovirus-like element HERV-E.PTN on the expression of growth factor pleiotrophin: a critical role of a retroviral Sp1-binding site. Oncogene 2000;19:3988–98 [DOI] [PubMed] [Google Scholar]
- 44.Sauter M, Schommer S, Kremmer E, et al. Human endogenous retrovirus K10: expression of gag protein and detection of antibodies in patients with seminomas. J Virol 1995;69:414–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauter M, Roemer K, Best B, et al. Specificity of antibodies directed against env proteins of human endogenous retroviruses in patients with germ cell tumors. Canc Res 1996;56:4362–5 [PubMed] [Google Scholar]
- 46.Galli UM, Sauter M, Lecher B, et al. Human endogenous retrovirus rec interferes with germ cell development in mice and may cause carcinoma in situ, the predecessor of germ cell tumours. Oncogene 2005;24:3223–8 [DOI] [PubMed] [Google Scholar]
- 47.Armbruester V, Sauter, M, Krautkraemer E, et al. A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clin Canc Res 2002;8:1800–7 [PubMed] [Google Scholar]
- 48.Armbruester, Sauter M, Roemer K, et al. Np9 protein of human endogenous retrovirus K interacts with ligand of Numb Protein X. J Virol 2004;78:10310–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denne M, Sauter M, Armbruester V, et al. Physical and functional interactions of human endogenous retrovirus proteins Np9 and Rec with the promyelocytic leukaemia zinc finger protein. J Virol 2007;81:5607–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boese A, Sauter M, Galli U, et al. Human endogenous retrovirus protein cORF supports cell transformation and associates with promyelocytic leukaemia zinc finger protein. Oncogene 2000;19:4328–36 [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Bogerd HP, Peng S, et al. An ancient family of human endogenous retroviruses encodes a functional homolog of the HIV-1 Rev protein. PNAS 1999;96:13404–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson PN, Carnegie PR, Martin J, et al. Demystified … Human endogenous retroviruses. J Clin Path: Mol Pathol 2003;56:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson PN, Hooley P, Roden D, et al. Human endogenous retroviruses: transposable elements with potential? Clin Exp Immunol 2004;138:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruprecht K, Mayer J, Sauter M, et al. Endogenous retroviruses and cancer. Cell Mol Life Sci 2008;65:3366–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manganey M, Heidmann T. Tumour cells expressing a retroviral envelope escape immune rejection in vivo. PNAS 1998;95:14920–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manganey M, de Parseval N, Thomas G, Heidmann T. The full-length envelope of an HERV-H endogenous retrovirus has immunosuppressive properties. J Gen Virol 2001;82:2515–18 [DOI] [PubMed] [Google Scholar]
- 57.Ruggieri A, Maldener E, Sauter M, et al. Human endogenous retrovirus HERV-K(HML-2) encodes a stable signal peptide with biological properties distinct from Rec. Retrovirology 2009;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duelli D, Lazebnik Y. Cell fusion: a hidden enemy? Canc Cell 2003;3:445–8 [DOI] [PubMed] [Google Scholar]
- 59.Morse B, Rotherg PG, South VJ, et al. Insertional mutagenesis of the myc locus by a LINE-1 sequence in human breast carcinoma. Nature 1988;333:87–90 [DOI] [PubMed] [Google Scholar]
- 60.Miki Y, Nishisho I, Horii A, et al. Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Canc Res 1992;52:643–5 [PubMed] [Google Scholar]
- 61.Misra A, Chosdol K, Sarkar C, et al. Alteration of a sequence with homology to human endogenous retrovirus (HERV-K) in primary human glioma: implications for viral repeat mediated arrangement. Mutat Res Fund Mol Mech Mutagen 2001;484:53–9 [DOI] [PubMed] [Google Scholar]
- 62.Deininger PL, Batzer MA. Alu repeats and human disease. Mol Genet Metab 1999;67:183–93 [DOI] [PubMed] [Google Scholar]
- 63.Gibbons R, Dugaiczyk Phylogenetic roots of Alu-mediated rearrangements leading to cancer. Genome 2005;48:160–7 [DOI] [PubMed] [Google Scholar]
- 64.Kolomietz E, Meyn MS, Pandita A, et al. The role of Alu repeat clusters as mediators of recurrent chromosomal aberration in tumours. Gene Chromosome Canc 2002;35;97–112 [DOI] [PubMed] [Google Scholar]
- 65.O'Neil J, Tchinda J, Gutierrez A, et al. Alu elements mediate MYB gene tandem duplication in human T-ALL. J Expt Med 2007;204:3059–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montagna M, Santacatterina M, Torri A, et al. Identification of a 3kb Alu-mediated BRCA1 gene rearrangement in two breast/ovarian cancer families. Oncogene 1999;18:4160–5 [DOI] [PubMed] [Google Scholar]
- 67.Schichman SA, Caliguiri MA, Strout MP, et al. ALL-1 tandem duplication in acute myeloid leukaemia with a normal karyotype involves homologous recombination between Alu elements. Canc Res 1994;54:4277–80 [PubMed] [Google Scholar]
- 68.So CW, Ma ZG, Price CM, et al. MLL self fusion mediated by Alu repeat homologous recombination and prognosis of AML-M4/M5 subtypes. Canc Res 1997;57:117–22 [PubMed] [Google Scholar]
- 69.Strout MP, Marcucci G, Bloomfield CD, et al. The partial tandem duplication of ALL1 (MLL) is consistently generated by Alu-mediated homologous recombination in acute myeloid leukemia. PNAS 1998;95:2390–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukuuchi A, Nagamura Y, Yaguchi H, et al. A whole MEN1 gene deletion flanked by Alu repeats in a family with multiple endocrine neoplasia type 1. Jpn J Clin Oncol 2006;36:739–44 [DOI] [PubMed] [Google Scholar]
- 71.Mauillon JL, Michel P, Limacher JM, et al. Identification of novel germline hMLH1 mutations including a 22kb alu-mediated deletion in patients with familiar colorectal cancer. Canc Res 1996;56:5728–33 [PubMed] [Google Scholar]
- 72.Nyström-Lahti M, Kristo P, Nicolaides NC, et al. Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nat Med 1995;1:1203–6 [DOI] [PubMed] [Google Scholar]
- 73.Swensen J, Hoffman M, Skolnick MH, et al. Identification of a 14 kb deletion involving the promoter region of BRCA1 in a breast cancer family. Hum Mol Genet 1997;6:1513–17 [DOI] [PubMed] [Google Scholar]
- 74.Puget N, Sinilnikova OM, Stoppa-Lyonnet D, et al. An Alu-mediated 6-kb duplication in the BRCA1 gene: a new founder mutation. Ann Hum Genet 1999;64:300–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Onno M, Nakamura T, Hillova J, et al. Rearrangement of the human tre oncogene by homologous recombination between Alu repeats of nucleotide sequences from two different chromosomes. Oncogene 1992;7:2519–23 [PubMed] [Google Scholar]
- 76.Rothberg PG, Ponnuru S, Baker D, et al. A deletion polymorphism due to Alu-Alu recombination in intron 2 of the retinoblastoma gene: Association with human gliomas. Mol Carcinogen 1997;19:69–73 [DOI] [PubMed] [Google Scholar]
- 77.Mangiacasale R, Pittoggi C, Sciamanna I, et al. Exposure of normal and transformed cells to nevirapine, a reverse transcriptase inhibitor, reduces cell growth and promotes differentiation. Oncogene 2003;22:2750–61 [DOI] [PubMed] [Google Scholar]
- 78.Landriscina M, Fabiano A, Altamura S, et al. Reverse transcriptase inhibitors down-regulate cell proliferation in vitro and in vivo and restore thyrotrophin signaling and iodine uptake in human thyroid anaplastic carcinoma. J Clin Endocrinol Metab 2005;90:5663–71 [DOI] [PubMed] [Google Scholar]
- 79.Sciamanna I, Landriscina M, Pittoggi C, et al. Inhibition of endogenous reverse transcriptase antagonises human tumour growth. Oncogene 2005;24:3923–31 [DOI] [PubMed] [Google Scholar]
- 80.Oricchio E, Sciamanna I, Beraldi R, et al. Distinct roles for LINE-1 and HERV-K retroelements in cell proliferation, differentiation and tumor progression. Oncogene 2007;26:4226–33 [DOI] [PubMed] [Google Scholar]
- 81.Serafino A, Balestrieri E, Pierimarchi P, et al. The activation of human endogenous retrovirus K (HERV-K) is implicated in melanoma cell malignant transformation. Exper Cell Res 2009;315:849–62 [DOI] [PubMed] [Google Scholar]
- 82.DeMartini JC, York DF. Retrovirus-associated neoplasms of the respiratory system of sheep and goats. Ovine pulmonary carcinoma and enzootic nasal tumor. Vet Clin N Am Food Anim Pract 1997;13:55–70 [DOI] [PubMed] [Google Scholar]
- 83.Palmarini M, Datta S, Omid R, et al. The long terminal repeat of Jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. J Virol 2000;74:5776–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cousens C, Bishop JV, Philbey AW, et al. Analysis of integration sites of Jaagsiekte sheep retrovirus in ovine pulmonary adenocarcinoma. J Virol 2004;78:8506–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spadafora C. A reverse transcriptase-dependent mechanism plays central roles in fundamental biological processes. Syst Biol Reprod Med 2008;54:11–21 [DOI] [PubMed] [Google Scholar]


