Abstract
Background and Purpose
Patients with intracranial atherosclerotic disease (ICAD) have a 3.6% to 22% annual risk of stroke. In this study, we sought to evaluate the natural history and prognosis of patients with symptomatic ICAD who received medical therapy versus percutaneous transluminal angioplasty and stenting (PTAS) at our institution.
Methods
Charts of all patients with symptomatic ICAD from July 2004 to September 2007 were reviewed and assessed for history of transient ischemic attack (TIA) or stroke. Patients were either treated with “best medical therapy” (Medical Therapy Group) or PTAS plus antiplatelet agents (PTAS Group), and followed prospectively. A favorable outcome was defined as the absence of TIAs, strokes or vascular death, modified Rankin Scale of 3 or less and no endovascular re-intervention of symptomatic in-stent restenosis (ISR).
Results
One hundred eleven patients fulfilled entry criteria, with 58 (52.3%) and 53 patients (47.7%) enrolled in the Medical Therapy and PTAS Groups, respectively. Thirty-eight patients of the Medical Therapy group (65.5%) had a favorable outcome compared to 37 patients of the PTAS group (69.8%). Combined ischemic endpoint data for the occurrence of TIA, stroke and vascular death was similar with 14 (24%) events in the Medical Therapy group versus 15 (28.3%) events in the PTAS group.
Conclusion
Overall, the combined ischemic endpoint was the same in the Medical Therapy and PTAS groups.
Keywords: Angioplasty & Stenting, Atherosclerosis, Interventional Neuroradiology, Intracranial Stenosis
Introduction
Patients with intracranial atherosclerotic disease (ICAD) have a 3.6% to 22% annual risk of stroke. Approximately 10% of ischemic strokes have been related to ICAD. Based on the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial, ischemic stroke rates in the territory of a moderate to severe symptomatic intracranial stenosis were 11% and 12% at one year for warfarin and aspirin, respectively. 1 Among patients with symptomatic ICAD who “failed” antithrombotic therapy, the subsequent rates of stroke or vascular death were as high as 45% per year.2
There is no consistent evidence of “best medical therapy” to treat symptomatic ICAD. In most cases, therapeutic decisions are based on anecdotal evidence or personal experience. New treatments for ICAD such as intracranial percutaneous transluminal angioplasty and stenting (PTAS) have emerged as alternative approaches to medical therapy. However, there is limited data available regarding the success and durability of this modality. We report our retrospective, single center, non-randomized experience in the management of symptomatic ICAD to determine if patients receiving PTAS experienced better clinical outcomes than those receiving more traditional medical therapy.
Methods
Patient selection
We retrospectively reviewed the University of Wisconsin Comprehensive Stroke Center medical records of all patients with symptomatic ICAD who presented between July 2004 and September 2007. The study was approved by the University of Wisconsin Investigational Review Board.
Demographic characteristics, vascular risk factors, history of transient ischemic attacks (TIAs) or stroke were determined by medical records review. The extent and location of ICAD was documented by magnetic resonance angiography, computerized tomography angiography or intra-arterial digital subtraction angiography. Other possible stroke etiologies such as cardiogenic embolism, paroxysmal embolism with patent foramen ovale or cervical thromboembolism were ruled out by echocardiography and further imaging. Patients with coagulopathies and non-atherosclerotic causes of intracranial stenosis were excluded from this study.
Treatment modalities
Patients were treated with “best medical therapy” (Medical Therapy Group) or PTA and stenting plus antiplatelet agents (PTAS Group) after a multidisciplinary committee comprised by a vascular neurologist, a neuro-endovascular specialist and, frequently, a vascular neurosurgeon reviewed each case. Antithrombotic selection was based on various published Guidelines. 3–6 This study did not intend to look at antithrombotic agent-specific outcome data.
Lesions felt amenable for re-vascularization included those with angiographically verified 50% or greater stenosis of a major intracranial artery and TIA or stroke in the vascular territory of the target lesion. PTA/stenting was performed in thirty-one lesions by using the Wingspan system (Boston Scientific, Fremont, CA).7 Twelve lesions were treated using Neuroform stent systems (Boston Scientific, Fremont, CA) and fourteen using various balloon-expandable stent systems.
Patients were followed prospectively on monthly basis. In some cases, structured telephone interviews were conducted in patients who did not have recent assessments. Favorable outcome was defined as symptom resolution, no new events and a modified Rankin scale score (mRS) of 3 or less. Events were defined as TIA, stroke, vascular death, performance of an extracranial-intracranial (EC-IC) bypass in the originally treated vascular territory due to TIA or stroke symptoms and performance of another PTA or additional placement of a stent in a symptomatic in-stent restenosis (ISR). ISR was defined as a lesion demonstrating stenosis greater than 50% adjacent to the stent and absolute luminal loss greater than 20% on follow-up imaging, as described previously.8 ISR was considered symptomatic if it was accompanied by TIA symptoms or stroke. In order to facilitate data analysis, stent occlusion and EC-IC bypass surgery were recorded by their accompanying ischemic event, TIA or stroke. Events and favorable outcomes were registered at each clinic visit or interview session. As part of a post-hoc analysis, a combined ischemic endpoint of TIA, stroke and vascular death was complied.
Statistical analysis
Demographic comparisons were made between the Medical Therapy and PTAS groups using either a Pearson's Chi-Square test, Fisher's exact test, Student’s t-test or Wilcoxon rank sum test. The time from start-of-observation to time of either loss-to-follow-up or first ischemic event was used to generate Kaplan-Meier curves. Second and third ischemic events (TIA or stroke), were also recorded under the Kaplan-Meier curves. Lost -to-follow-up was considered censored data. A log rank test was used to compare the two Kaplan-Meier curves. The start-of-observation defined in this paper was either the initial clinical assessment by our Stroke Team or, in the PTAS group, the day the stent was placed.
Subgroup analysis of favorable outcome was performed in the PTAS group. Comparisons by stent type, deployment of single or multiple stents and stent location were made using Pearson Chi-Square tests. Number of favorable outcomes using these variables were examined and statistically compared with Pearson's Chi-Square test.
Results
One hundred eleven patients fulfilled entry criteria, with 58 patients (52.3%) and 53 patients (47.7%) enrolled in the Medical Therapy and PTAS groups, respectively (Table 1). The mean age among both groups was 65-years. Patients were predominantly white, with 48 (82.7%) in the Medical Therapy group and 52 (98.1%) in the PTAS group. Hypertension, hyperlipidemia, coronary artery disease and previous stroke were the most common vascular risk factors among both groups. History of prior TIA was documented in 18.9 and 54.7% of the Medical Therapy and PTAS groups, respectively (p < 0.001). Location of atherosclerotic stenoses also varied significantly between groups (p < 0.001). Medical Therapy patients had more diffuse stenoses and less isolated anterior or posterior circulation lesions, 67.2%, 22.4% and 10.3%, respectively. PTAS patients had less diffuse stenoses and more isolated anterior or posterior circulation atherosclerotic lesions, 28.3%, 28.3% and 43.4%, respectively.
Table 1.
General data of the 111 patients
| Medical Therapy (n=58) |
PTAS (n=53) |
||
|---|---|---|---|
| Age, mean (range) | 65.17 (39 – 87) | 65.42 (40 – 88) | NS |
| Male sex, n (%) | 25 (43.1) | 16 (30.1) | NS |
| White, n (%) | 48 (82.7) | 52 (98.1) | NS |
| Hypertension, n (%) | 46 (79.3) | 47 (88.6) | NS |
| Diabetes, n (%) | 29 (50.0) | 25 (47.1) | NS |
| Hyperlipidemia, n (%) | 37 (63.7) | 33 (62.2) | NS |
| Smoker, n (%) | 19 (32.7) | 10 (18.8) | NS |
| Coronary artery disease, n (%) | 22 (37.9) | 20 (37.3) | NS |
| Previous TIA, n (%) | 11 (18.9) | 29 (54.7) | < 0.001 |
| Previous stroke, n (%) | 22 (37.9) | 20 (37.3) | NS |
| Location of stenosis, n (%) | < 0.001 | ||
| Anterior circulation | 13 (22.4) | 15 (28.3) | |
| Posterior circulation | 6 (10.3) | 23 (43.4) | |
| Diffuse | 39 (67.2) | 15 (28.3) | |
| Patient referral, n (%) | < 0.001 | ||
| Clinic | 11 (18.9) | 25 (47.1) | |
| Inpatient consult | 7 (12.0) | --- | |
| Emergency department | 31 (53.4) | 15 (28.3) | |
| Transferred from OSH | 9 (15.5) | 13 (24.5) | |
| Presenting condition, n (%) | NS | ||
| Stroke | 43 (74.1) | 30 (56.6) | |
| TIA | 15 (25.6) | 23 (43.4) | |
| Stoke at presentation, mean (range) | |||
| NIHSS | 5.44 (0 – 23) | 3.47 (0 – 16) | 0.016 |
| mRS | 2.98 (1 – 5) | 1.83 (1 – 5) | < 0.001 |
| TIA at presentation, mean (range) | |||
| NIHSS | 1.53 (0 – 13) | 0.7 (0 – 7) | NS |
| mRS | 1.33 (0 – 5) | 1.57 (0 – 5) | NS |
| Favorable outcome, n (%) | 38 (65.5) | 37 (69.8) | NS |
PTAS indicates percutaneous transluminal angioplasty and stenting; TIA, transient ischemic attack; OSH, outside hospital.
The referral for the first encounter with the Stroke Team was significantly different between groups (p < 0.001). Forty of the 58 (69%) patients in the Medical Therapy group were either admitted through our Emergency Department or were transferred from another facility. Nearly 50% of the PTAS group was initially seen in our stroke clinics.
At the time of presentation, 43 patients (74.4%) of the Medical Therapy group suffered an ischemic stroke. These patients had more stroke-related disability with a significantly higher NIHSS (average of 5.44, p = 0.016), a wider NIHSS range (from 0 to 23), and higher mRS values (average of 2.98, p < 0.001).
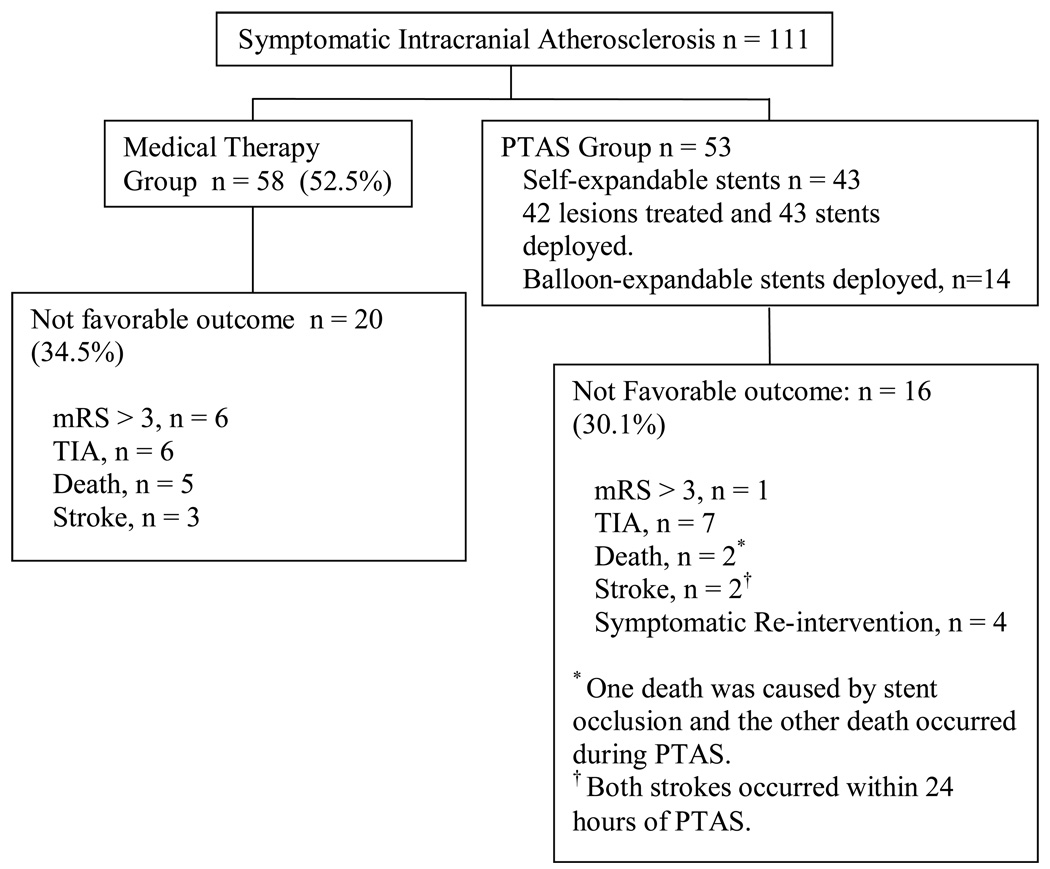
In follow up, thirty eight (65.5%) of the Medical Therapy group did not have an event, compared to thirty seven patients (69.8%) in the PTAS group (Figure 1). The Medical Therapy group experienced 5 TIAs (8.6%), 5 deaths (8.6%), 3 strokes (5.1%) and 1 EC-IC bypass surgery (1.7%) due to recurrent TIAs. The PTAS group experienced 8 neuroendovascular re-interventions (15%), 4 for asymptomatic and 4 for symptomatic ISR; 5 TIAs (9.4%), 2 treatment-related vascular deaths (3.7%), 2 periprocedural strokes (3.7%) and 2 EC-IC bypass surgeries (3.7%) due to recurrent TIAs. One of the vascular deaths in the PTAS group was related to an acute middle cerebral artery in-stent occlusion with the development of a fatal ischemic stroke. This occurred during discontinuation of all antithrombotic medications because of a gastro-intestinal hemorrhage. The other death in the PTAS group occurred during an attempted basilar artery angioplasty and stenting, which was complicated with dissection and vessel rupture. Combined ischemic endpoint data for the occurrence of TIA, stroke and vascular death showed no difference in incidence of ischemic events. Table 2 summarizes ischemic endpoints, 14 of 58 (24%) patients in the Medical Therapy group suffered an ischemic event (6 TIAs, 3 strokes and 5 vascular deaths), while 15 of 53 (28.3%) patients in the PTAS group had an ischemic event (11 TIAs, 2 strokes and 2 stroke-related vascular deaths).
Figure 1.
Patient outcome by study group
Table 2.
Event distribution
| Event | TIA | Stroke | Death | |
|---|---|---|---|---|
| Medical Therapy, n (%) | 14 (24.1) | 6 (10.3) | 3 (5.1) | 5 (8.6) |
| PTAS, n (%) | 15 (28.3) | 11 (20.7)* | 2 (3.7)† | 2 (3.7) ‡ |
Four TIAs occurred with symptomatic ISR and one as a periprocedural complication.
Strokes occurred within 24 hours of PTAS.
One death occurred after stent occlusion and the other one as a procedural complication.
Periprocedural event rate
Two ischemic strokes and one death occurred within 24 hours of PTAS for a periprocedural complication rate of 5.6%. One patient had a cerebral infarct on MRI with neurologic signs lasting less than 24 hours and another patient had TIA (Table 3).
Table 3.
PTAS complications
| Event | No. | System | Outcome |
|---|---|---|---|
| Stent occlusion | 2 | Balloon-expandable stents | |
| 2 days after PTAS | EC-IC bypass surgery | ||
| 9 days after PTAS | MCA stroke and death | ||
| Ischemic strokes within 24 hours |
2 | Balloon-expandable stents | Symptoms persisted in both cases |
| Deaths within 24 hours |
1 | Self-expandable stent |
MCA indicates middle cerebral artery.
The two stent occlusions reported in this study occurred with balloon-expandable systems; the first stent occlusion happened two days after PTAS and the patient underwent EC-IC bypass surgery because of recurrent TIAs. The second stent occlusion occurred 9 days after PTAS, while the patient was off antiplatelet medications because of a gastro-intestinal hemorrhage, and as described earlier, had a large middle cerebral artery territory stroke and died.
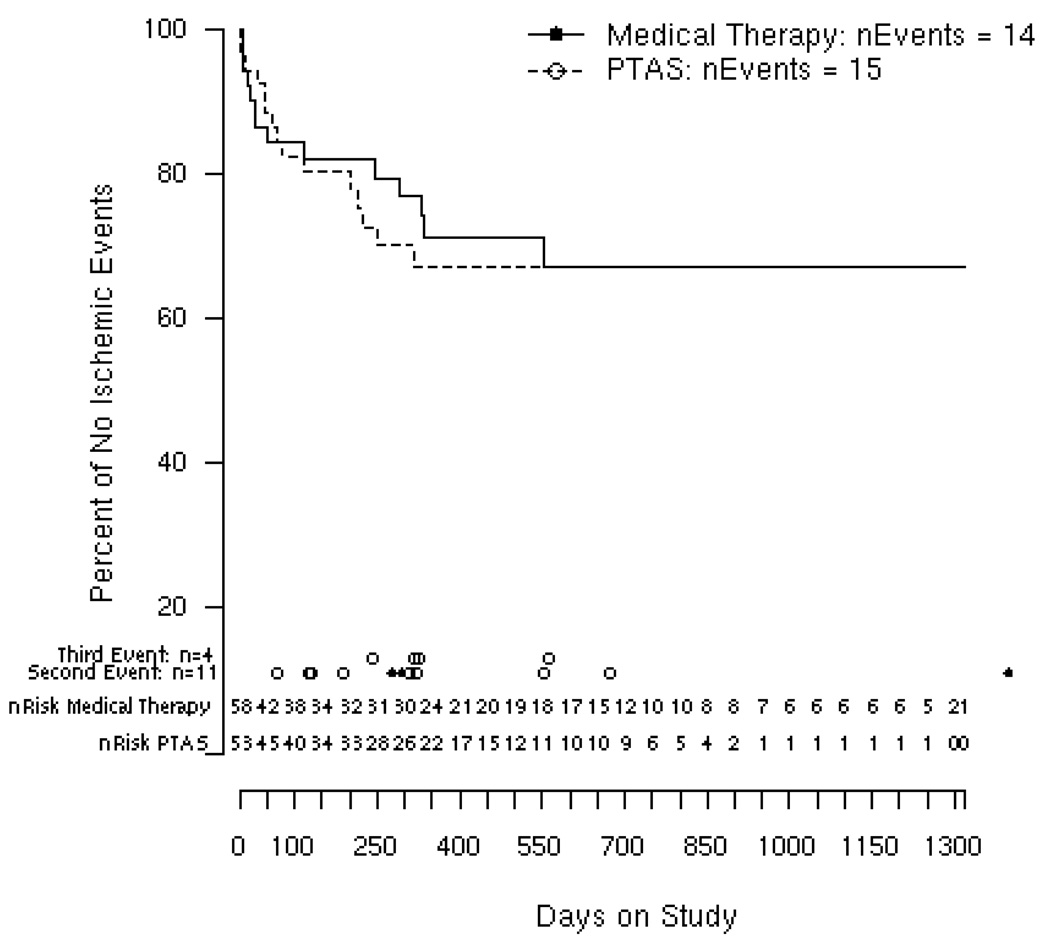
Kaplan-Meier analysis showed that Medical Therapy and PTAS patients tend to achieve event-free survival by 12 months of enrollment. Kaplan-Meier curves are practically identical over the first 3 months. PTAS patients tended to have a higher incidence of first ischemic events between 3 and 12 months, achieving the exact Kaplan-Meier estimate by 18 months (Figure 2). Average follow-up was 14 months for both groups.
Figure 2.
Kaplan-Meier estimates of event rates among Medical Therapy and PTAS groups
Kaplan Meier curves for time to first ischemic event for Medical Therapy and PTAS. Numbers of patients at risk are given for every 50 days on study. nEvents is the total number of first ischemic events. Dots indicate time of second and third events. Open circle for PTAS and full circle for Medical Therapy patients
PTAS Group Sub-analysis
Subgroup analysis of the PTAS Group did not show a significant difference in outcome based on the type of stent used. Favorable outcomes were seen at a similar rate for patients who were treated with a single stent (n=30, 68.2%) and for those with multiple stents (n=7, 77.8%) (p=0.706). There tended to be more favorable outcomes in patients who had a stent placed only in a posterior circulation lesion (n=22, 73.3%) compared to anterior circulation (n=14, 63.6%), but this difference was not statistically different (p = 0.827).
Discussion
In our series, patients who did not undergo PTA/stenting typically presented to either the emergency department or were transferred from another facility. More often they presented with ischemic stroke, higher first-encounter NIHSS and mRS, and diffuse ICAD. PTAS patients typically were first seen at the Stroke Clinic and more often presented with at least one TIA, lower first encounter NIHSS and mRS when their clinical presentation was stroke, and had isolated anterior or posterior ICAD.
The American Stroke Association advocates the use of medical therapies such as antithrombotic agents, statins and risk factor control for patients with symptomatic ICAD. Endovascular therapies such as PTA/stenting, while appear to be promising, continue to be considered investigational 6 and are used with a humanitarian device exemption. However, endovascular treatment also incorporates medical strategies used in secondary stroke prevention. “Best medical therapy” has been widely used for the treatment ICAD but combined ischemic endpoints of TIA, stroke and vascular death remain high. 1, 2, 9, 10 Furthermore, WASID showed that neither high-dose aspirin nor warfarin were effective in preventing ipsilateral strokes referable to diseased intracranial vascular territories. 1, 9 For all these reasons, the outcome of randomized trials such as the ongoing SAMMPRIS trial will hopefully clarify this complex issue.
Although the long term outcome of these interventions is unknown, multiple centers have reported technically successful endovascular therapies for the treatment of symptomatic intracranial atherosclerotic lesions. 11–13 The Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral and Intracranial Arteries (SSYLVIA) trial reported moderate to severe in-stent re-stenosis within 6 months of intervention in 32.4% of treated intracranial lesions. 14 Even with newer self-expandable stents, the frequency of moderate to severe ISR appears to be as high as 35 %. 7, 8 Re-interventions are required in approximately 19% of cases, exposing patients to periprocedural risks. 12, 13
The periprocedural 24 hour complication rate (stroke or death) in this study (5.6%) was similar to the reported in the literature. 15 Although low, the 2 strokes and 1 procedural related-death represent 20% of the combined ischemic endpoint in the PTAS group. The two strokes occurred with balloon-expandable systems and the vascular death with a Neuroform stent system. Better technology and technical approaches may lower even further periprocedural complications and improve outcome.
Patient selection for endovascular therapy is often based on imaging findings, failure of “best medical therapy”, timing of the ischemic event and clinical significance of the stenosis 16. In this study, the qualifying event for endovascular treatment in the PTAS group was more likely a TIA, despite medical therapy, suggesting that the decision to intervene was based on symptom recurrence in healthier patients often electively referred from an outpatient setting. As has been described in the literature, even in symptomatic ICAD patients referred emergently for endovascular treatment, the decision to intervene was typically made at least after twenty-four hours of admission. 17 Urgent endovascular treatments have shown a periprocedural complication rate as high as 50%. 18 Therefore, most procedures are performed preventively in patients with repeated TIAs or small strokes. Additionally, several smaller series of acute intervention in ischemic strokes with intracranial self-expanding stents have reported periprocedural hemorrhage and mortality rates of 11 and 33%, respectively. 19, 20 On the other hand, ICAD natural history data reveals a median time of recurrent TIA, stroke or death of 36 days, with 53% of patients experiencing a recurrent stroke or vascular death after being treated with “best medical therapy”. 2
A Kaplan-Meier analysis used to adjust for variable follow-up between the Medical Therapy and PTAS Groups, showed that after 18 months, stabilized clinical trends were maintained (Figure 2). Zaidat el at, did not show a trend favorable to PTA/stenting beyond 90 days 21. Most of the PTAS group ischemic events occurred within the first three months of follow up, and although the majority of procedures were technically successful, periprocedural complications accounted for 20% of first ischemic events. Another major drawback of angioplasty and stenting is the development of ISR. In an attempt to maintain clinical equipoise and stent patency, patients are often subjected to additional imaging and endovascular procedures during follow-up. This approach unfortunately increases health care costs as well as the risk of periprocedural complications. Nevertheless, ISR is only worrisome if it becomes symptomatic, in our series, 50% of ISR were symptomatic.
We acknowledge the limitations of this single center retrospective study. Because treatment allocation between the Medical Therapy and PTAS Groups was not randomized, it is conceivable that the different clinical and angiographic characteristics among the two groups accounts for different outcomes. The treatment choices made between “best medical therapy” and PTAS were based primarily on clinical presentation, lesion location, lesion accessibility and the overall suitability for an endovascular procedure. These inherent differences might have affected the rates of clinical end points, mainly because of referral bias. The heterogeneity of endovascular therapies used in this study reflects an evolving technology that has achieved a high technical success. Nevertheless, the variety of endovascular systems used undermines the homogeneity required for a suitable comparison with other treatment modalities.
Further subgroup analysis of the PTAS group showed that stent location was possibly related to a better outcome. When all outcome measures were considered, posterior circulation endovascular interventions trended toward better outcomes than anterior circulation lesions, 73.3% and 63.6 % respectively. Levy et al reported a higher incidence of ISR within the anterior circulation as well 8.
Most neurointerventional studies compare their results to the WASID trial. 17, 21 This a valid alternative given the lack of control groups in the medical arm; nevertheless, this comparison might be deceiving since in WASID there was a time-selection bias and some patients deemed to be at high risk of stroke were not enrolled early to allow for a period of observation 9. This single center study describes the outcome of two treatment modalities in a relatively homogeneous population with similar demographics and risk factors. The trend observed of a similar pattern of events for both groups should be verified with a larger trial comparing medical therapy with intracranial stenting 22; this is a crucial issue since the validity of self-expandable stents has been recently questioned. 23, 24
Acknowledgements
This project was supported in part by the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), funded through an NIH Clinical and Translational Science Award (CTSA), grant number 1 UL1 RR025011.
Contributor Information
Edgar A. Samaniego, Neurology Resident, University of Wisconsin - Madison 701 Welch Road, Suite B 325, Palo Alto, CA 94304, Fax: (650) 723 - 4448, phone: (650) 723 - 4451 esamanie@stanford.edu
Scott Hetzel, Biostatistics and Medical Informatics, University of Wisconsin, K6/434, Clinical Sciences Center, University of Wisconsin Hospitals, 600 Highland Avenue, Madison, WI 53792, Phone: (608) 265 - 4311 hetzel@biostat.wisc.edu.
Supriya Thirunarayanan, Neurology Resident, University of Wisconsin, H6/574, Clinical Sciences Center, University of Wisconsin Hospitals, 600 Highland Avenue, Madison, WI 53792, Fax: (608) 263 - 5443, Phone: (608) 262 - 2646 supriya@neurology.wisc.edu
Beverly Aagaard-Kienitz, Associate Professor of Neuroradiology, E3/366, Clinical Sciences Center, University of Wisconsin Hospitals, 600 Highland Avenue, Madison, WI 53792-3252, Phone (608) 263-8328 baagaard-kienitz@uwhealth.org
Ross Levine, Associate Professor of Neurology and Radiology, University of Wisconsin Department of Neurology and Radiology, H6/532, Clinical Sciences Center, University of Wisconsin Hospitals, 600 Highland Avenue, Madison, WI 53792, Phone: (608) 263 - 5415 levine@neurology.wisc.edu
References
- 1.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 2.Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: Outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490–497. doi: 10.1212/wnl.55.4.490. [DOI] [PubMed] [Google Scholar]
- 3.Adams H, Adams R, Del Zoppo G, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the stroke council of the american heart association/american stroke association. Stroke. 2005;36:916–923. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, Grubb RL, Higashida R, Kidwell C, Kwiatkowski TG, Marler JR, Hademenos GJ. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the stroke council of the american stroke association. Stroke. 2003;34:1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Hart RG, Lutsep HL, Newell DW, Sacco RL. Aha scientific statement. Supplement to the guidelines for the management of transient ischemic attacks: A statement from the ad hoc committee on guidelines for the management of transient ischemic attacks, stroke council, american heart association. Stroke. 1999;30:2502–2511. doi: 10.1161/01.str.30.11.2502. [DOI] [PubMed] [Google Scholar]
- 6.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the american heart association/american stroke association council on stroke: Co-sponsored by the council on cardiovascular radiology and intervention: The american academy of neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 7.Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Hanel RA, Woo H, Rasmussen PA, Hopkins LN, Masaryk TJ, McDougall CG. Us multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: Periprocedural results. Stroke. 2007;38:881–887. doi: 10.1161/01.STR.0000257963.65728.e8. [DOI] [PubMed] [Google Scholar]
- 8.Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Pride L, Purdy P, Welch B, Woo H, Rasmussen PA, Hopkins LN, Masaryk TJ, McDougall CG, Fiorella DJ. Wingspan in-stent restenosis and thrombosis: Incidence, clinical presentation, and management. Neurosurgery. 2007;61:644–650. doi: 10.1227/01.NEU.0000290914.24976.83. discussion 650-641. [DOI] [PubMed] [Google Scholar]
- 9.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, Sila CA, Jovin TG, Romano JG, Cloft HJ. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P, Zaidat OO, Suarez JI, Wityk RJ. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: A multicenter study. Neurosurgery. 2003;52:1033–1039. discussion 1039–1040. [PubMed] [Google Scholar]
- 11.de Rochemont Rdu M, Turowski B, Buchkremer M, Sitzer M, Zanella FE, Berkefeld J. Recurrent symptomatic high-grade intracranial stenoses: Safety and efficacy of undersized stents--initial experience. Radiology. 2004;231:45–49. doi: 10.1148/radiol.2311030183. [DOI] [PubMed] [Google Scholar]
- 12.Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, Do HM. Angioplasty for symptomatic intracranial stenosis: Clinical outcome. Stroke. 2006;37:1016–1020. doi: 10.1161/01.STR.0000206142.03677.c2. [DOI] [PubMed] [Google Scholar]
- 13.Mazighi M, Yadav JS, Abou-Chebl A. Durability of endovascular therapy for symptomatic intracranial atherosclerosis. Stroke. 2008;39:1766–1769. doi: 10.1161/STROKEAHA.107.500587. [DOI] [PubMed] [Google Scholar]
- 14.Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (ssylvia): Study results. Stroke. 2004;35:1388–1392. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]
- 15.Groschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009 doi: 10.1161/STROKEAHA.108.532713. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi AI, Hussein HM, El-Gengaihy A, Abdelmoula M, MF KS. Concurrent comparison of outcomes of primary angioplasty and of stent placement in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 2008;62:1053–1060. doi: 10.1227/01.neu.0000325867.06764.3a. discussion 1060-1052. [DOI] [PubMed] [Google Scholar]
- 17.Jiang WJ, Xu XT, Du B, Dong KH, Jin M, Wang QH, Ma N. Comparison of elective stenting of severe vs moderate intracranial atherosclerotic stenosis. Neurology. 2007;68:420–426. doi: 10.1212/01.wnl.0000252939.60764.8e. [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Schumacher HC, Mangla S, Meyers PM, Duong H, Khandji AG, Marshall RS, Mohr JP, Pile-Spellman J. Urgent endovascular revascularization for symptomatic intracranial atherosclerotic stenosis. Neurology. 2003;61:1729–1735. doi: 10.1212/01.wnl.0000103900.65021.5b. [DOI] [PubMed] [Google Scholar]
- 19.Levy EI, Mehta R, Gupta R, Hanel RA, Chamczuk AJ, Fiorella D, Woo HH, Albuquerque FC, Jovin TG, Horowitz MB, Hopkins LN. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28:816–822. [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidat OO, Wolfe T, Hussain SI, Lynch JR, Gupta R, Delap J, Torbey MT, Fitzsimmons BF. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke. 2008;39:2392–2395. doi: 10.1161/STROKEAHA.107.510966. [DOI] [PubMed] [Google Scholar]
- 21.Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, Mawad M, Lane B, Lynn MJ, Chimowitz M. The nih registry on use of the wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology. 2008;70:1518–1524. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derdeyn CP, Chimowitz MI. Angioplasty and stenting for atherosclerotic intracranial stenosis: Rationale for a randomized clinical trial. Neuroimaging Clin N Am. 2007;17:355–363. viii–ix. doi: 10.1016/j.nic.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallmes DF, Cloft HJ. How do we spin wingspan? AJNR Am J Neuroradiol. 2008;29:28–29. doi: 10.3174/ajnr.A0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallmes DF, Do HM. Wherefore wingspan? AJNR Am J Neuroradiol. 2007;28:997–998. doi: 10.3174/ajnr.A0614. [DOI] [PMC free article] [PubMed] [Google Scholar]