Abstract
Objective
Defining an optimal staging strategy requires an evaluation of the effectiveness and costs of diagnostic tests and may include the burden of these tests for patients. This study evaluated the burden of cervical ultrasonography (US), endoscopic ultrasonography (EUS), computed tomography (CT) and positron emission tomography (PET) in patients with esophageal carcinoma (EC).
Methods
Consenting consecutive patients underwent a standard preoperative work-up. Burden of testing was evaluated with a self-report questionnaire addressing anxiety, embarrassment, and discomfort, each measured on a 1(none) to 5 (extreme) point-scale. An overall burden score was calculated by summing the three item scores. In addition, patients were asked to rank the four tests from least to most inconvenient. Statistical analysis was performed with nonparametric tests.
Results
82 patients (67 , 15 ; mean age 64.3 yrs) participated. For most tests and most dimensions of burden, the large majority of subjects was in categories 1 and 2.With respect to anxiety, the rank order (from highest burden to lowest burden) was EUS, US, PET, and CT (average scores 1.7, 1.5, 1.4, and 1.2, respectively). For embarrassment, the rank order was EUS, PET, US, and CT (1.9, 1.5, 1.4, and 1.3 respectively). For discomfort, the rank order was EUS, PET, US and CT (2.0, 1.6, 1.4, and 1.2, respectively). And for total burden, the rank order was EUS, PET, US and CT (5.6, 4.6, 4.2, and 3.7). PET was ranked as least inconvenient by 35% of patients and as most inconvenient by 16% compared with the other tests.
Conclusion
Significant but small differences were observed in patient burden for imaging tests to evaluate EC. The perceived burden of PET was lower than that of EUS, but higher than the burden of CT. However absolute values were low for all tests and therefore patient burden will not be a key feature for the construction of an optimal staging algorithm for EC.
Keywords: esophageal carcinoma, perceived burden, cervical ultrasonography, endoscopic ultrasonograhy, computed tomography, positron emission tomography
Introduction
The incidence of adenocarcinomas of the esophagus or gastroesophageal junction (GEJ) is rising (Falk 2002; Jenkins et al 2002; Wijnhoven et al 2001). Surgical resection is currently the best curative treatment in patients without distant metastases and/or locally irresectable tumor growth (Daly et al 2000). Conventional imaging techniques are employed to select only patients with potentially curable disease for esophagectomy. Currently, the most common conventional modalities for staging of esophageal cancer (EC) are cervical ultrasonography (US) with fine needle aspiration (FNA), endoscopic ultrasonography (EUS) with FNA, and computed tomography (CT).
Despite these efforts preoperatively, distant metastatic spread is encountered during operation in 10%–20% of patients (Sariego et al 1993; Clements et al 2004; van Westreenen et al 2005). During the last decade, positron emission tomography (PET) using 18F- Fluorodeoxyglucose has been introduced as a noninvasive method for staging esophageal cancer, especially for the detection of distant lymphatic and hematogenous metastases (Block et al 1997; Flanagan et al 1997; Luketich et al 1997; Flamen et al 2000; van Westreenen 2004). Selection of patients for esophageal cancer resection with curative intent could be improved by the implementation of PET, which has been suggested by former studies (Flamen et al 2000).
The exact role of PET in the staging pathway of esophageal cancer is not clear yet. The pathway will be predominantly based on maximizing diagnostic accuracy at acceptable costs. If differences in the accuracy of the various tests are small, or, when added, the additional value of a procedure is limited, patient burden of the various tests may become an important feature for the construction of an optimal staging algorithm.
We designed a study to evaluate and compare the patient burden of cervical ultrasonography (US), endoscopic ultrasonograhy (EUS), computed tomography (CT) and positron emission tomograhy (PET) in the work-up of patients with esophageal cancer.
Patients and methods
Between November 2003 and July 2004, a consecutive group of 82 consenting patients with esophageal carcinoma visited one of two participating medical centers (Academic Medical Center (AMC), Amsterdam and University Medical Center Groningen (UMCG), the Netherlands) and were asked about the impact of the diagnostic procedures (AMC: 51, UMCG: 31 patients). The medical ethics committees of both hospitals approved this study.
Patients who were in sufficiently good condition for major surgery, with histologically confirmed potentially curable esophageal or GEJ carcinoma as estimated by history and physical examination underwent a cervical US with or without FNA, EUS with or without FNA, and multidetector CT of neck, chest, and abdomen as part of routine work-up. These patients were also asked to undergo PET.
Staging procedures
All patients underwent the four test procedures: US ± FNA, EUS ± FNA, CT, and PET (see Table 1). All tests were performed within two weeks; the order was determined based upon waiting times and availability. All diagnostic tests were performed according to a standard procedure that had been established during joint meetings of the research group members of the two participating hospitals. Patients received a written information sheet prior to all tests.
Table 1.
Characteristics of the four test procedures
| Procedure | Fasting | IV puncture | Sedation | Pharmaceutical | FNA | Duration(min.) |
|---|---|---|---|---|---|---|
| US | No | No | No | None | Yes | 15 |
| EUS | Yes | Yes | Yes | None | Yes | 150 |
| CT | No | Yes | No | i.v. contrast | No | 20 |
| PET | Yes | Yes | No | 18F-FDG | No | 150 |
Abbreviations: US, cervical ultrasonography; CT, computed tomography; EUS, endoscopic ultrasonography; PET, positron emission tomography; IV, intravenous; 18F-FDG, fluoro-deoxyglucose; FNA, fine needle aspiration (prevalence see results section in text).
Cervical ultrasonography
Cervical US with cytological biopsy of suspicious lesions was performed using either a 15.2 MHz or 7.5 MHz linear array transducer. Round echogenic lymph nodes with a diameter of more than 5 mm were considered suspected and were investigated by cytology.
Ultrasound-guided FNA was performed for cytological analysis using a standard 21-gauge intravenous needle in 14 of the 82 (17%) patients. Estimated room-time was 15 minutes.
Endoscopic ultrasonography
A radial scanner (GF-UM130 or GF-UM160; 5–20 MHz, Olympus Medical Systems, Tokyo, Japan) was used for the performance of EUS. EUS-guided FNA was obtained by a separate linear array echo-endoscope (FF-UC140P, Olympus Medical Systems, Tokyo, Japan). FNA was performed with a 22-gauge needle (Echotip, Wilson-Cook Medical Inc., Winston Salem, USA) in 8 of the 82 (10%) of the patients. In 7 patients (8%) a stenotic tumor did not allow the standard echo-endoscope to pass, and a small-caliber probe (MH-908, 7.5 MHz, Olympus Medical Systems, Tokyo, Japan) was used in an attempt to traverse the tumor.
EUS was performed with conscious sedation using 2.5–10 mg midazolam intravenously with the patient in a left decubitus position. The duration of the procedure was about 30 min. After the procedure, the patient was observed for at least 120 min.
Computed tomography
Multidetector CT was performed with a 4-ring or 16-ring CT scanner (Philips MX 8000, Best, The Netherlands, -AMC or Somatom Sensation, Siemens, Erlangen, Germany, -UMCG). Scans were obtained of the lower neck, chest, and upper abdomen including the liver. The patient had to drink 500 ml of oral contrast directly before the CT-examination. The CT-examination comprised two consecutively performed scans during breath hold after an intravenous injection of contrast medium (120 ml low osmolar contrast medium at 3.5 ml/s). First scan after a delay of 20–25 seconds after start of contrast injection included the chest and supraclavicular region. After a delay of 90 seconds after start of contrast injection a second spiral scan was performed of the upper abdomen, at least including the liver and celiac region. The room-time of the CT-examination was approximately 20 minutes.
Positron emission tomography
The studies were performed using an ECAT EXACT HR+ (Siemens/CTI Inc., Knoxville, TN, USA). After an overnight or >6 hr fast period, 370–555 MBq was injected intravenously for 2D acquisitions (lower limit for patients >85 kg, upper limits for patients >85 kg). Patients were prehydrated with 500–1500 ml water. Interval between FDG injection and image acquisition was 60–90 min, during this time period bedrest was prescribed for the first half-hour. Scans were obtained from the midfemoral region up to the skull. Emission scan-duration was 5 min per bed position, transmission imaging (always-performed) 3 min. Duration of the scan was approximately 60 minutes. Duration of the entire procedure was 2 ½ hours.
Test questionnaire
Two weeks after having finished all tests, patients were requested to complete a self-report questionnaire. The questionnaire consisted of three modules. First, a standard formatted Likert scoring module (Likert 1932). was used with two items addressing embarrassment and discomfort for each of the four staging modalities. The two items have previously been used in a study of the acceptance of computer tomographic colonoscopy by patients (van Gelder et al 2004). We added anxiety as a third item (Katz et al 1994; Melendez and McCrank 1993; MacKenzie et al 1995). Responses were scored on a five-point anchored scale with 1 indicating ‘none’, 2 indicating ‘ little’, 3 indicating ‘quite’, 4 indicating ‘very’, and 5 indicating ‘very much’. An overall burden score was calculated as the sum of the three items scores.
In addition, a comparative assessment item was used, inviting patients to rank the different tests from least to most inconvenient.
Statistical analyses
The nonparametric Friedman test for related samples was used to compare the burden and ranking of the different tests. The Mann-Whitney U test was used to test for differences between groups of patients with respect to sum of burden scores. Subgroup analyses were performed based on US and/or EUS with or without FNA.
Results
During the study period 82 patients were enrolled in this study (67 male; 15 female). Their mean age was 64.3 (SD ± 8.3) years. After conventional work-up 5 patients had a T1 tumor, 10 patients a T2 tumor, 63 patients a T3 tumor, and 4 patients had a T4 tumor.
All test data were available and could be analyzed. For most tests and most dimensions of burden, the large majority of subjects was in categories 1 and 2 (Table 2). Patients scored more often 4 (very) or 5 (very much) for EUS regarding embarrassment and discomfort (both 7 patients) in comparison with US (both 3 patients), CT (1 and no patient respectively) and PET (both 3 patients). Regarding anxiety, respectively 5 and 6 patients scored 4 or 5 on EUS and PET, while only 2 patients scored 4 or 5 on US and CT.
Table 2.
Reported burden scores (1 ‘none’, 2 ‘little’, 3 ‘quite’, 4 ‘very’, and 5 ‘very much’) for anxiety, embarrassment, discomfort, and their total for all staging procedures
| Score | Average | SD | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||
| US | anxiety | 56 | 18 | 6 | 1 | 1 | 1.5 | 0.8 | EUS p = 0.01 | CT p = 0.00 | PET p = 0.76 |
| embarrassment | 60 | 14 | 5 | 1 | 2 | 1.4 | 0.9 | EUS p = 0.00 | CT p = 0.06 | PET p = 0.24 | |
| discomfort | 62 | 14 | 3 | 3 | 0 | 1.4 | 0.7 | EUS p = 0.00 | CT p = 0.06 | PET p = 0.01 | |
| total | 4.2 | 2.0 | EUS p = 0.00 | CT p = 0.00 | PET p = 0.11 | ||||||
| EUS | anxiety | 47 | 20 | 9 | 1 | 4 | 1.7 | 1.0 | US p = 0.01 | CT p = 0.00 | PET p = 0.03 |
| embarrassment | 36 | 25 | 13 | 6 | 1 | 1.9 | 1.0 | US p = 0.00 | CT p = 0.00 | PET p = 0.00 | |
| discomfort | 28 | 32 | 14 | 6 | 1 | 2.0 | 1.0 | US p = 0.00 | CT p = 0.00 | PET p = 0.00 | |
| total | 5.6 | 2.6 | US p = 0.00 | CT p = 0.00 | PET p = 0.00 | ||||||
| CT | anxiety | 71 | 8 | 1 | 1 | 1 | 1.2 | 0.6 | US p = 0.00 | EUS p = 0.00 | PET p = 0.01 |
| embarrassment | 62 | 16 | 3 | 1 | 0 | 1.3 | 0.6 | US p = 0.06 | EUS p = 0.00 | PET p = 0.00 | |
| discomfort | 68 | 13 | 1 | 0 | 0 | 1.2 | 0.4 | US p = 0.06 | EUS p = 0.00 | PET p = 0.00 | |
| total | 3.7 | 1.3 | US p = 0.00 | EUS p = 0.00 | PET p = 0.00 | ||||||
| PET | anxiety | 63 | 11 | 2 | 3 | 3 | 1.4 | 1.0 | US p = 0.76 | EUS p = 0.03 | CT p = 0.01 |
| embarrassment | 53 | 19 | 7 | 1 | 2 | 1.5 | 0.9 | US p = 0.24 | EUS p = 0.00 | CT p = 0.00 | |
| discomfort | 44 | 30 | 5 | 3 | 0 | 1.6 | 0.8 | US p = 0.01 | EUS p = 0.00 | CT p = 0.00 | |
| total | 4.6 | 2.2 | US p = 0.11 | EUS p = 0.00 | CT p = 0.00 | ||||||
Abbreviations: US, ultrasonography; EUS, endoscopic ultrasonography; CT, computed tomography; PET, positron emission tomography; SD, standard deviation.
Notes: Significance of differences between the scores of the staging procedures are mentioned in the last column.
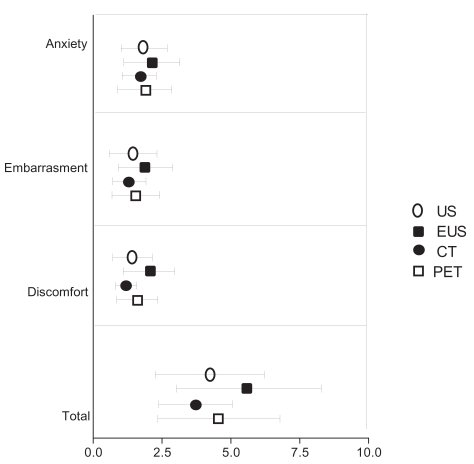
The averages scores were between 1.0 and 2.0 for all items (see Table 2 and Figure 1).
Figure 1.
Burden scores of the four tests in the diagnostic work-up of patients with esophageal cancer with respect to anxiety, embarrassment, discomfort, and sum burden.
Notes: Values indicate mean and standard deviation (SD); n = 82.
Abbreviations: US, cervical ultrasonography; EUS, endoscopic ultrasonograhy; CT, computed tomography; PET, positron emission tomography.
With respect to anxiety, the rank order (from highest to lowest scores) was: EUS (1.7, SD 1.0), US (1.5, SD 0.8, P = 0.01), PET (1.4, SD 1.0, P = 0.03), CT (1.2, SD 0.8 P = 0.00). For embarrassment, the rank order was: EUS (1.9, SD 1.0), PET (1.5, SD 0.9), US (1.4, SD 0.8), CT (1.3, SD 0.6). For discomfort, the rank order was: EUS (2.0, SD 1.0), PET (1.6, SD 0.8), US 1.4 (SD 0.7), and CT (1.2, SD 0.4). For total burden, CT had significantly the lowest average score: 3.7 (SD 1.3, range 3–10) (all P = 0.00) and EUS significantly the highest: 5.6 (2.6, range 3–14) (all P = 0.00). For PET, the average score for total burden was 4.6 (SD 2.2, range 3–14) and was comparable with US 4.2 (SD 2.0, range 3–12) (see Table 2, Figure 1). EUS causes the most burden for all dimensions and CT the least. US is more associated with anxiety than with embarrassment or discomfort.
In a subgroup analysis of patients with or without a FNA in US and EUS, there was a significant higher perceived total burden for US with FNA (n = 14, mean 5.1, SD 2.2) versus US without FNA (n = 68, mean 4.0 SD 1.9) (P = 0.02), which was due to difference in perceived discomfort (1.7 versus 1.3, respectively; P = 0.004). There was no significant difference in total perceived burden for EUS with (n = 8, mean 6.6 SD 1.8) or without FNA (n = 74, mean 5.5 SD 2.7).
The 4’s and 5’s for the various tests and dimensions were distributed in one limited group of 3 patients (these 3 patients reported much burden from all tests on all dimensions) and a larger group of 12 patients who reported much burden from one test (EUS and CT).
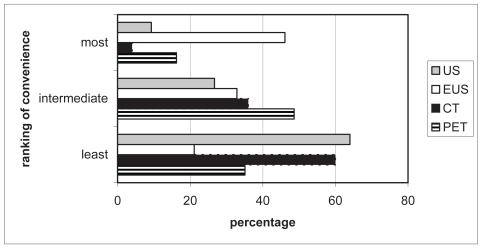
Seventy-five patients (91%) completed the ranking question. Half of the patients double-ranked tests (eg, 2 tests were perceived as least inconvenient), which was corrected for. US and CT were most often ranked as least inconvenient (64% and 60%, respectively, Figure 2). Patients perceived EUS as the most inconvenient test (46% of rankings). PET was mostly ranked in the middle of the spectrum of inconvenience. Yet PET was reported 35% of rankings as least inconvenient and 16% as most inconvenient.
Figure 2.
Inconvenience ranking of four tests in the diagnostic work-up of patients with esophageal carcinoma.
Notes: Grey bar: cervical ultrasonography; white bar: endosonography; black bar: computed tomography; striped bar: positron emission tomography.
Discussion
To our knowledge this is the first study to systematically investigate the patient burden of US, EUS, CT, and PET. This study shows that US, EUS, CT and PET are associated with low average levels of patient burden when used for the evaluation of esophageal carcinoma. Significant but small differences in the absolute amount of perceived burden between the tests could be observed. The average burden scores between 1.0 and 2.0 indicate that all patients experienced a low burden for all tests, but the individual scores per test reveals that a substantial number of patients experienced a high or even very high burden for a particular test. Especially anxiety in case of PET and all 3 items in case of EUS should be noticed.
The invasiveness of EUS is often mentioned as a drawback of this procedure. This is reflected in its relatively high burden scores for all items in this study. These values were still lower than anticipated, which might be explained by the availability of a thin scope and the application of sedation. Due to sedation the patient does not experience excessive burden of the investigation, and even forgets the experience, because of retrograde amnesia.
For PET a low average overall burden was observed (although some individual patients reported a high burden), which may be due to the context of diagnostic staging of a life threatening disease. Nevertheless, in our opinion, this issue will not hamper implementation of PET in the standard preoperative work-up of patient with esophageal cancer. In fact, PET is in general well accepted by the patients and adverse effects are rarely found. However, in this study CT is perceived even less uncomfortable than PET, probably due to the long room-time (especially scan-time) of PET compared with CT. Although, this item was not listed in the questionnaire, the long room-time of PET was often spontaneously reported by the patients as a drawback of the test. In the future, scanning time will be significantly reduced by the fusion of the established technologies of PET and CT into a single PET/CT system. In addition, some patients reported to be anxious for radioactivity, despite proper information provided in advance. In case of CT, no serious side effects of administered contrast medium were reported in this study population, which may have contributed to the favorable scores for CT in this study. Claustrophobia was not reported or noticed in the present study population; nevertheless it is well-known that claustrophobia may seriously hamper diagnostic tests like CT or PET.
In the present study, a 2-week interval between test and questionnaire was chosen, because we assumed that experience and preference are preferably measured after a certain time because this may better reflect future behavior than if experience and preference are measured under stressful circumstances. The study of van Gelder and colleagues (2004) showed that the experienced burden is the highest immediately after the tests. From then on, burden decreases. If a very long time period after testing should be chosen, it can be assumed that patients will forget the tests and burden could be underestimated. However it remains unknown when these opinions change and which interval should be used to optimally measure patient preference. Future studies will have to address the optimal time interval between the experience and evaluation.
A number of potential limitations of this study should be taken into account. Our study group consisted of patients with a life-threatening disease, and this may have influenced the relatively low degree to which they experience diagnostic procedures as a burden. In addition, the observed imbalance between men and women in the present study was not due to a form of selection bias but is inherent to the disease of esophageal carcinoma (Wijnhoven 2002). Further, an additional FNA was needed during US or EUS in some but not all patients. In a subanalysis there were higher perceived burden scores in the US-FNA subgroup, but not in de EUS-FNA subgroup. Finally, we have tried to standardize the information given to patients by handing out, prior to testing, a written information sheet. However we cannot claim that all patients received exactly the same oral information by their specialists.
In conclusion, significant but small differences were observed in patient burden for imaging tests to evaluate esophageal carcinoma. The perceived burden of PET was lower than the perceived burden of EUS, but higher than the perceived burden of CT. As the average burden values were low for all tests, we find it safe to conclude that the role of patient perception in the search for an optimal staging algorithm for esophageal cancer patients will be limited. The preferred diagnostic pathway will most likely be based on maximizing diagnostic accuracy.
Footnotes
Disclosure
The authors report no conflicts of interest.
Reference
- Block MI, Patterson GA, Sundaresan RS, et al. Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg. 1997;64:770–6. doi: 10.1016/s0003-4975(97)00619-x. [DOI] [PubMed] [Google Scholar]
- Clements DM, Bowrey DJ, Havard TJ. The role of staging investigations for oesophago-gastric carcinoma. Eur J Surg Oncol. 2004;30:309–12. doi: 10.1016/j.ejso.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562–72. doi: 10.1016/s1072-7515(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122:1569–91. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–10. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- Flanagan FL, Dehdashti F, Siegel BA, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. Am J Roentgenol. 1997;168:417–24. doi: 10.2214/ajr.168.2.9016218. [DOI] [PubMed] [Google Scholar]
- Jenkins GJ, Doak SH, Parry JM, et al. Genetic pathways involved in the progression of Barrett’s metaplasia to adenocarcinoma. Br J Surg. 2002;89:824–37. doi: 10.1046/j.1365-2168.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- Katz RC, Wilson L, Frazer N. Anxiety and its determinants in patients undergoing magnetic resonance imaging. J Behav Ther Exp Psychiatry. 1994;25:131–4. doi: 10.1016/0005-7916(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Likert R. A technique for the measurement of attitude scales. Arch Psychol. 1932;140:44–53. [Google Scholar]
- Luketich JD, Schauer PR, Meltzer CC, et al. Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg. 1997;64:765–9. doi: 10.1016/s0003-4975(97)00624-3. [DOI] [PubMed] [Google Scholar]
- MacKenzie R, Sims C, Owens RG, et al. Patients’ perceptions of magnetic resonance imaging. Clin Radiol. 1995;50:137–43. doi: 10.1016/s0009-9260(05)83042-9. [DOI] [PubMed] [Google Scholar]
- Melendez JC, McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examinations. JAMA. 1993;270:745–7. doi: 10.1001/jama.1993.03510060091039. [DOI] [PubMed] [Google Scholar]
- Sariego J, Mosher S, Byrd M, et al. Prediction of outcome in “resectable” esophageal carcinoma. J Surg Oncol. 1993;54:223–5. doi: 10.1002/jso.2930540407. [DOI] [PubMed] [Google Scholar]
- Van Gelder RE, Birnie E, Florie J, et al. CT colonography and colonoscopy: assessment of patient preference in a 5-week follow-up study. Radiology. 2004;233:328–37. doi: 10.1148/radiol.2331031208. [DOI] [PubMed] [Google Scholar]
- van Westreenen HL, Heeren PA, van Dullemen HM, et al. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54–61. doi: 10.1016/j.gassur.2004.09.055. [DOI] [PubMed] [Google Scholar]
- van Westreenen HL, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805–12. doi: 10.1200/JCO.2004.01.083. [DOI] [PubMed] [Google Scholar]
- Wijnhoven BP, Louwman MW, Tilanus HW, et al. Increased incidence of adenocarcinomas at the gastro-oesophageal junction in Dutch males since the 1990s. Eur J Gastroenterol Hepatol. 2002;14:115–22. doi: 10.1097/00042737-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Wijnhoven BP, Tilanus HW, Dinjens WN. Molecular biology of Barrett’s adenocarcinoma. Ann Surg. 2001;233:322–37. doi: 10.1097/00000658-200103000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]