Abstract
The objective of this study was to assess current treatment patterns, blood glucose test strip usage, and treatment compliance in patients with type 2 diabetes mellitus (T2DM) in primary care centers in Spain, and to assess factors related to glycemic control. We conducted a retrospective chart review of patients with T2DM and measured treatment compliance using the Morisky-Green questionnaire. 294 patients were included in the study from a population of patients attending 30 primary care centers throughout Spain. Results showed that the majority of patients were treated with oral monotherapy (36%) and oral combination therapy (35%). Less than half of the patients had good glycemic control (HbA1c ≤ 6.5%). Half of the patients treated pharmacologically reported good compliance with treatment. Logistic regression analyses performed to identify factors associated with glycemic control showed that high body mass index (BMI) and poor compliance were the strongest predictors of poor HbA1c control (OR: 2.198 and 1.789, respectively, p < 0.05). In conclusion, in the course of managing diabetes, physicians and patients should attempt to improve compliance and lower BMI, which could lead to better glycemic control.
Keywords: treatment adherence, treatment pattern, glucose control, diabetes, primary care
Introduction
Diabetes mellitus is a highly prevalent, chronic disease which can significantly impact morbidity and mortality. Type 2 diabetes mellitus (T2DM) is the most common form of diabetes and represents approximately 90% of all diagnosed cases of diabetes (Benito et al 2004). Although it is difficult to calculate the prevalence of T2DM accurately because of variations in the rate of nondiagnosed cases ranging from 15% to 50% (Bayo et al 1996; Laing and Williams 1996; King et al 1998; Zimmet et al 2001), estimates of the prevalence of diabetes in Europe and North America range from 5% to 10% (Laing et al 1996). Prevalence in Spain is estimated to be about 6% (Bayo et al 1996; Tamayo-Marco et al 1997). The number of cases of diabetes in Spain is therefore high and is expected to continue rising. According to the World Health Organization (WHO), in the year 2000, there were approximately 2.7 million cases of diabetes in Spain and the number was expected to increase to over 3.7 million by 2030 (WHO 2007). Diabetes is associated with chronic microvascular and macrovascular complications, such as a blindness and cardiovascular disease, and is one of the leading causes of death worldwide and in Spain (Benach et al 2001; Roglic et al 2005). In 2004, the standardized mortality rate was estimated to be approximately 14 per 100,000 in Spain (WHO Regional Office for Europe 2007).
In addition to physician-prescribed treatment, patient behavior is also critical in determining the success of the treatment. For patients with type 2 diabetes, attention to diet, exercise, blood glucose monitoring, and medication are necessary to achieve good glycemic control (Cramer 2004). Poor compliance may also lead to poor outcomes with pharmacological treatment, though compliance with diabetes treatment has not been widely studied. Self-monitoring of blood glucose could also play a role in improving treatment outcomes. This is especially true with insulin treatment because patients can adjust their dose depending on the outcome of their blood glucose test results. The American Diabetes Association (ADA) recommends self-monitoring of blood glucose (SMBG) as an important component of the care of all patients treated with insulin. However, the role and optimum frequency of such monitoring in patients with T2DM is still under debate (Farmer et al 2007), particularly in the case of patients receiving noninsulin treatment. Excessive use of SMBG can also lead to significant economic expense and psychological impact (Gallichan 1997). To inform this debate, it would be useful to know the current level of test strip usage for patients with diabetes in Spain.
Because little information was available on how patients with T2DM are treated in the primary care setting in Spain, this study was designed to identify current treatment patterns, to assess patients’ compliance with their treatment plans, and to estimate the use of blood glucose test strips. A further objective of the study was to determine which sociodemographic and clinical variables were most closely associated with the likelihood of achieving target levels of glycemic control and thus with reducing the risk of morbidity and mortality.
Methods
This was a retrospective medical chart review of patients with T2DM. Data collected included patient background information and a patient self-assessment of treatment compliance. A total of 30 primary care physicians took part in the study from July 2005 to September 2006 and were selected from 16 autonomous regions based on the geographical distribution of the population of Spain (Table 1).
Table 1.
Distribution of sample by geographical zones and autonomous regions
| Geographical areas | Autonomous regions | n (%) |
|---|---|---|
| East (34.6%)* | Aragon | 10 (3.4%) |
| Balearic Islands | 8 (2.7%) | |
| Catalonia | 51 (17.3%) | |
| Murcia | 10 (3.4%) | |
| Valencia | 28 (9.5%) | |
| Total | 107 (36.4%) | |
| North (16.8%)* | Asturias | 13 (4.4%) |
| Cantabria | 10 (3.4%) | |
| Galicia | 10 (3.4%) | |
| La Rioja | 10 (3.4%) | |
| Navarre | 6 (2%) | |
| Total | 49 (16.7%) | |
| South (25.1%)* | Andalusia | 47 (16%) |
| Canary Islands | 12 (4.1%) | |
| Extremadura | 13 (4.4%) | |
| Total | 72 (24.5%) | |
| Center (23.5%)* | Castilla-La Mancha | 13 (4.4%) |
| Castilla y León | 14 (4.8%) | |
| Madrid | 39 (13.3%) | |
| Total | 66 (22.4%) |
Notes:Percentage of the total Spanish population (INE 2006).
To be included in the study, patients had to be over 20 years of age and to have had T2DM (diagnosed using ADA criteria) (ADA 2005) for at least one year. Inclusion was also dependent on the availability in the healthcare center of a clinical record containing minimum basic information (ie, age, gender, educational level, duration of diabetes/age at diagnosis, ≥ 1 HbA1c record within the last 12 months, a list of any prescribed glucose-lowering medications that were taken during the three months prior to the HbA1c test, a body mass index (BMI) value within the six months prior to the HbA1c test, and documentation concerning the current glucose-lowering treatment regimen). Each physician included up to 10 patients in the study. Potential candidates for inclusion were asked to participate during one of their scheduled visits. Patients agreeing to participate were requested to sign an informed consent form and were then given the study materials including the Morisky-Green questionnaire on treatment compliance. Sociodemographic and clinical variables relating to diabetes history, treatment patterns (duration and type of treatment), and current use of test strips (times per week) were collected by the physician from patients’ clinical records. Patients’ waist circumference was measured during this office visit. The study was approved by the ethics committee of the Fundació Jordi Gol i Gurina, Barcelona, Spain.
Treatment compliance
Treatment compliance was assessed using the Spanish version of the self-administered Morisky-Green questionnaire. The questionnaire has been shown to have good internal reliability (Morisky et al 1986) and consists of the following four items:
Do you ever forget to take your medicine?
Are you careless at times about taking your medication?
When you feel better, do you sometimes stop taking your medicine?
Sometimes if you feel worse when you take the medicine, do you stop taking it?
Response options are dichotomous (yes = 1, no = 0). The total score ranges from 0 to 4. Treatment compliance was categorized as high (score = 0), moderate (score between 1 and 2), and low (score ≥ 3) based on the overall Morisky-Green score. This questionnaire was administered only to patients receiving pharmacological treatment. Treatment compliance was analyzed descriptively and comparatively based on treatment type.
Analysis
Data analysis was performed using SAS 8.02 software (SAS Inc., Cary, NC, USA). A significance level of less than 0.05 was used as a criterion for statistical significance in all statistical comparisons. Prior to performing statistical analyses, data completeness and data quality were assessed. In general, analysis of variance (ANOVA) was used to analyze continuous variables and the χ2 test was used for categorical variables. Other statistical techniques were used as required.
A descriptive analysis of sociodemographic characteristics and T2DM history was performed. Current treatment patterns and the length of time the patient had been receiving pharmacological treatment were assessed. For patients on insulin treatment, the current number of insulin units per week and the type of insulin (brand name) were collected at 1, 6, 12, and 18 months after initiation of insulin treatment. In addition, the proportion of patients using glucose test strips for SMBG and the number of times per week they used them were analyzed for the sample as a whole and by treatment type. Patients were categorized by BMI as follows: overweight (25 ≤ BMI < 30), obese (30 ≤ BMI < 35) or severely obese (BMI ≥ 35).
A logistic regression was conducted to analyze factors associated with the level of compliance. The dependent variable was categorized as high, moderate or low compliance. The independent variables used in the model were: treatment type (treatment with oral antidiabetic medications [OAMs], and insulin treatment with or without OAM[s]), HbA1c value, sociodemographic variables, and complications of diabetes mellitus (no complications, only microvascular complications, only macrovascular complications, and both microvascular and macrovascular complications).
A second logistic regression was conducted to analyze factors associated with the level of HbA1c control. The dependent variable was defined as a dichotomous variable, which categorized an HbA1c of less than or equal to 6.5% as good glycemic control and an HbA1c of greater than 6.5% as poor glycemic control (IDF 2005). The independent variables introduced were: patient education level (completed at least primary education and less than primary education), number of years since T2DM diagnosis, patient treatment (no pharmacological treatment, treatment with OAM[s], and insulin treatment with or without OAM[s]), BMI (nonobese versus obese/severely obese), vascular complications of diabetes mellitus (no complications, only microvascular complications, only macrovascular complications, and both microvascular and macrovascular complications), and treatment compliance (high compliance versus low/moderate compliance).
Results
A total of 339 patients with T2DM visited their physicians during the study period. Of these, 294 (86.7%) were selected to take part in the study. Of the 45 patients that did not participate, the main reasons for nonparticipation were: no available data (35.7%), did not meet inclusion criteria (30.8%), withheld consent to take part in the study (11.5%), and exclusion for other reasons (22%). Because the study consisted of only one visit, there were no withdrawals during the study.
Patient characteristics
Patient sociodemographic data are shown in Table 2. Equal numbers of men and women were included. Mean age was 67.5 (standard deviation [SD] = 10.2) years and more than 58% of patients were over 65 years of age. Most patients reported having completed at least primary education. Approximately 95% of the study population was European. Approximately 12% of patients were smokers (about half of these individuals smoked between 10 and 20 cigarettes per day) and 20% of patients reported consuming alcohol. Almost half of the patients had a family history of diabetes and the average time since diagnosis was 9.9 years (SD = 8.7). Table 3 shows patients’ clinical characteristics. Glycemic control was considered to be good (HbA1c less than or equal to 6.5%) in fewer than half of the patients. Almost half of patients in this study (47.4%) had microvascular complications, mac-rovascular complications, or both micro- and macrovascular complications. More specifically, cardiovascular disease was present in 38.1% of the patients, renal complications in 28.9%, retinopathy complications in 17.7%, neuropathy in 12.2%, and foot ulcer complications in 4.1%. In terms of body weight, only 16.1% of patients had a BMI that was considered normal or underweight (BMI less than 25). More than 45% were overweight (25 ≤ BMI < 30), and 38% were obese (30 ≤ BMI < 35) or severely obese (BMI ≥ 35). The mean BMI was 28.9 kg/m2 (SD = 4.5), and the mean waist circumference was 99.2 cm (SD = 16.2). With regard to duration of T2DM, 34.5% had had T2DM for <6 years; 43% between 6 and 14 years, and 22.5% > 15 years. As expected, the mean HbA1c was higher for patients with diabetes complications. HbA1c was also higher for patients with a higher BMI and was generally higher in patients who had been diabetic for longer.
Table 2.
Description of sociodemographic characteristics of T2DM patients included in this study
| Sex (n, %) | |
|---|---|
| Male | 147 (50%) |
| Female | 147 (50%) |
| Age (years) | |
| Mean (SD) | 67.5 (10.2) |
| Range | 24–91 |
| ≤ 65 years old | 123 (41.8%) |
| > 65 years old | 171 (58.2%) |
| Level of education (n, %) | |
| Below primary studies | 99 (33.9%) |
| Completion of primary studies | 136 (46.6%) |
| Completion of secondary studies | 39 (13.4%) |
| At least completion of university studies | 18 (6.1%) |
| Ethnicity (n, %) | |
| European | 280 (95.2%) |
| Other | 14 (4.8%) |
| Smoking habit (n, %) | 35 (11.9%) |
| Alcohol consumption (n, %) | 59 (20.6%) |
| Family history of diabetes | 136 (46.4%) |
| Time since T2DM diagnosis (years) | |
| Mean (SD) | 9.9 (8.7) |
Abbreviations: SD, standard deviation; T2DM, type 2 diabetes mellitus.
Table 3.
Clinical characteristics of T2DM patients and mean HbA1c per group
| Clinical characteristic | n, % | Mean (SD) HbA1c | P value* |
|---|---|---|---|
| Level of glycemic control | |||
| HbA1c ≤ 6.5 | 123 (41.8%) | ||
| HbA1c > 6.5 and ≤ 7 | 62 (21.1%) | ||
| HbA1c > 7 and ≤ 8 | 64 (21.8%) | ||
| HbA1c > 8 | 45 (15.3%) | ||
| Diabetes complications | |||
| No complications | 150 (52.6%) | 6.69 (1.48) | (P < 0.001) |
| Microvascular complications | 60 (21.1%) | 6.92 (1.17) | |
| Macrovascular complications | 33 (11.6%) | 6.61 (1.21) | |
| Microvascular and macrovascular complications | 42 (14.7%) | 7.68 (1.75) | |
| BMI | |||
| Mean (SD) 28.9 (4.5) | |||
| Normal or underweight (BMI < 25) | 45 (16.1%) | 6.58 (1.51) | (P < 0.05) |
| Overweight (25 ≤ BMI < 30) | 128 (45.9%) | 6.65 (1.30) | |
| Obese (30 ≤ BMI < 35) | 75 (26.9%) | 7.16 (1.67) | |
| Severely obese (BMI ≥ 35) | 31 (11.1%) | 7.45 (1.34) | |
| Duration of T2DM | |||
| Mean (SD) 9.9 years (8.7) | |||
| < 2 years | 48 (16.4%) | 6.28 (1.38) | (P < 0.001) |
| 3–5 years | 53 (18.1%) | 6.70 (1.48) | |
| 6–9 years | 81 (27.6%) | 6.88 (1.46) | |
| 10–14 years | 45 (15.4%) | 7.40 (1.83) | |
| > 15 years | 66 (22.5%) | 7.08 (1.09) | |
| Treatment | |||
| No pharmacological treatment | 18 (6.1%) | 6.04 (0.69) | (P < 0.05) |
| Oral monotherapy | 105 (35.7%) | 6.52 (1.10) | |
| Oral combination therapy | 102 (34.7%) | 7.05 (1.65) | |
| Insulin monotherapy | 24 (8.2%) | 7.19 (1.31) | |
| Insulin combination therapy | 8 (2.7%) | 8.00 (1.27) | |
| Insulin and oral treatment | 37 (12.6%) | 7.72 (1.97) | |
Notes:p values indicate level of significance in differences of HbA1c values among categories in ANOVA test.
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Treatment patterns
Almost all (93.9%) study participants were receiving some form of pharmacological treatment for their diabetes (Table 3). On average, patients had been receiving treatment for 8.2 years (SD = 7.5). The most common treatments were oral monotherapy (35.7%) and therapy with more than one OAM (34.7%). With regard to the type of treatment, 43.8% of the patients who received oral monotherapy used metformin and 40% used sulfonylureas. Of those receiving oral combination therapy, 60.8% took a combination of metformin and sulfonylureas. A total of 23.5% of the study participants were on insulin therapy: 8.2% of the total sample used only one insulin, 2.7% used a combination of more than one type of insulin, and 12.6% of patients were treated with a combination of insulin and OAM(s). The most commonly prescribed type of insulin among these patients was intermediate-acting (49.3%), followed by the combination of intermediate-acting and fast-acting (30.4%) insulins. The least prescribed type of insulin was fast-acting insulin alone (14.5%). The mean number of insulin units per week was 262.37 (SD = 124. 17), or about 37 insulin units daily. The mean HbA1c generally increased in patients on more intensive therapy, which is likely due to the longer duration of disease for patients taking insulin. The mean HbA1c was lowest in patients on no pharmacological treatment (mean = 6.04, SD = 0.69) and highest in patients taking more than one type of insulin therapy (mean = 8.00, SD = 1.27).
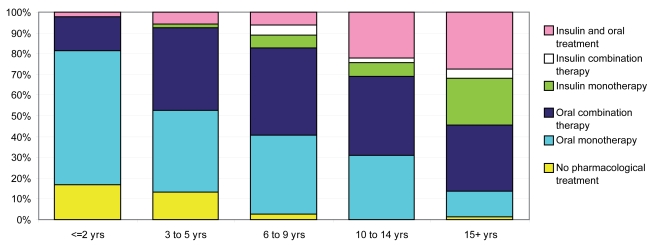
Analysis of treatment patterns according to time since diagnosis showed that oral monotherapy was the most common treatment in patients with ≤ 2 years since diagnosis (Figure 1). For all other patients (ie, patients with more than 2 years since diagnosis), the most common treatment was an oral combination therapy. Nevertheless, insulin treatment became more frequent the longer the duration of diabetes. In patients with a time since diagnosis over 15 years, 55% were treated with insulin, either alone or in combination with an OAM(s).
Figure 1.
Distribution of current treatment patterns of T2DM patients according to number of years since T2DM diagnosis. Abbreviation: T2DM, type 2 diabetes mellitus.
A total of 66% of patients used test strips to monitor their blood glucose levels (Table 4). There were statistically significant differences among groups regarding test-strips usage according to their T2DM treatment type (p < 0.05). Among patients treated with insulin, >90% used test strips, whereas among patients with no treatment or who were only taking oral treatment, the corresponding percentages were 33.3% and 59.4%, respectively. The number of test strips used per week was higher in patients taking insulin (ranging from 6.3 to 7.7) than in those with no treatment or only oral treatment (5.2 and 4.4, respectively). The number of strips used was numerically higher in the group who were not receiving pharmacological treatment compared with the group on oral therapy but the difference was not statistically significant.
Table 4.
Proportion of use of test strips and test strip usage per week of type of pharmacological treatment
| No treatment | Oral treatment | Insulin | Insulin and oral treatment | Total | |
|---|---|---|---|---|---|
| Use of test strips n (%) | |||||
| Yes | 6 (33.3%)A,B,C | 123 (59.4%)A,B,C | 31 (96.9%)B | 34 (91.9%)C | 194 (66%) |
| No | 12 (66.7%) | 84 (40.6%) | 1 (3.1%) | 3 (8.1%) | 100 (34%) |
| Test strips per week | |||||
| Mean (SD) | 5.2 (2.9) | 4.4 (3.8) | 7.7 (6.4) | 6.3 (5.8) | 5.3 (4.8) |
| Range | 1–7 | 1–18 | 1–21 | 1–21 | 1–21 |
Notes: In each row, statistically significant differences (p < 0.05) were obtained between values with the same letter ( A, B, or C). A compares “oral treatment” with “no treatment”; B compares “no treatment” with “oral treatment” and with “insulin treatment,”; and C compares “no treatment” with “oral treatment” and with “insulin and oral treatment.”
Table 5 reports patient compliance by treatment type. Using the self-administered Morisky-Green questionnaire, 50 % of patients taking a pharmacological treatment reported high compliance with treatment. The highest proportion of patients reporting good compliance (67%) was found in the group treated with insulin monotherapy, and the lowest proportion (39%) was in patients treated with insulin in combination with OAM(s). Treatment type and HbA1c level were the only significant factors related to compliance in the multivariate logistic regression analysis that examined factors related to levels of compliance after controlling for socio-demographic variables and complications. More specifically, the results showed that the probability of having a high level of compliance is significantly higher in patients receiving only insulin (OR: 2.7) than in patients on OAM(s). Moreover, the likelihood of better compliance increases with lower HbA1c values (OR of 1.3 for improved compliance given a one percentage point reduction in HbA1c values).
Table 5.
Percentage of patients with reporting various levels of treatment compliance by type of pharmacological treatment
| Compliance (%) | No pharmacological treatment | All treatments | Oral treatment | Insulin treatment | |||
|---|---|---|---|---|---|---|---|
| Oral monotherapy | Oral treatment combination | Insulin monotherapy | Insulin combination | Insulin and oral treatment | |||
| High compliance | n.a. | 50 | 49 | 50 | 67 | 57 | 39 |
| Moderate compliance | n.a. | 41 | 41 | 42 | 29 | 29 | 47 |
| Low compliance | n.a. | 9 | 10 | 8 | 4 | 14 | 14 |
Abbreviation: n.a., not applicable.
Table 6 shows the outcomes of the logistic regression to examine factors associated with poor glycemic control. Level of education, T2DM duration, treatment type, and presence and type of complications were not individually significant. The only significant variables (p < 0.05) were obesity and treatment compliance (ORs of 2.193 and 1.789, respectively, for patients with a BMI greater than 30 and for patients with low to moderate compliance).
Table 6.
Logistic regression of HbA1c control
| Parameter | Pr > ChiSq | OR | CI OR 95% |
|---|---|---|---|
| Intercept | 0.3856 | ||
| At least completed primary studies (vs below than primary studies) | 0.2692 | 0.714 | 0.392–1.298 |
| Number of years since T2DM diagnosis | 0.1075 | 1.038 | 0.992–1.085 |
| Insulin treatment (vs no drug treatment) | 0.4418 | 2.273 | 0.103–5.076 |
| Oral treatment (vs no drug treatment) | 0.7447 | 1.382 | 0.759–3.564 |
| Obese/severely obese (vs nonobese) | 0.0092 | 2.193 | 1.214–3.959 |
| Macrovascular complications (vs no complications) | 0.8684 | 0.929 | 0.389–2.219 |
| Microvascular complications (vs no complications) | 0.1485 | 1.729 | 0.823–3.633 |
| Micro and macrovascular (vs no complications) | 0.1678 | 1.932 | 0.758–4.925 |
| Low/moderate compliance (vs high compliance) | 0.0402 | 1.789 | 1.026–3.119 |
Notes: Dependent variable HbA1c scored as 0 for values ≤ 6.5% and 1 for values > 6.5%.
Abbreviations: CI, confidence interval; OR, odds ratio; T2DM, type 2 diabetes mellitus.
Discussion
This study provides a general overview of the management of T2DM in Spain from both a physician and a patient perspective. Physicians who took part in the study were selected so as to provide a representative sample based on population distribution in Spain. Typical of studies in T2DM, the population included in the study had a high mean age. The population was equally distributed in terms of sex. A relatively low percentage of patients in this study had completed secondary or university studies (about 20%); however, education level did not explain glycemic control in the multivariate analysis. With regard to lifestyle, the percentage of smokers and drinkers is similar to that of other studies (Benito et al 2004; Arroyo et al 2005), but less than that reported for the Spanish population as a whole (Clemente et al 1999). This low percentage could be explained by the fact that it relied on patient self-reports. Over 80% of patients had a BMI of > 25 kg/m2, which is similar to other studies indicating that up to 90% of the T2DM population is overweight (Tremble and Donaldson 1999). The percentage of patients with a family history of T2DM and the mean duration of the disease (about 10 years) were also comparable to figures in other Spanish studies (González-Clemente 1997; Zorrilla Torras et al 1997; Clua Espuny et al 1999; Arroyo et al 2005).
The ADA (ADA 2006) recommends that HbA1c in diabetes patients should be under 7% as these levels of HbA1c are associated with a lower risk of long-term microvascular complications. Nevertheless, a 7% threshold does not completely rule out the risk of complications and other medical societies have proposed a lower level of 6.5% (BCS et al 2005; IDF 2005). Of the patients in this study, 42% had an HbA1c less than or equal to 6.5%, and 63% had an HbA1c of less than or equal to 7%. These values are similar to those from other studies carried out in Spain: Mata Cases and colleagues (2003) reported 62% of patients with an HbA1c < 7.5% and Sender Palacios and colleagues (2002) reported a similar percentage (66%). Different estimates of the proportion of individuals with good glycemic control have been obtained in other settings. For example, in a study carried out in the United States, Spann and colleagues (2006) reported that only 40% of patients had an HbA1c level less than or equal to 7%. These data were similar to those observed in the Third National Health and Nutrition Examination Survey (Harris 2000), which was conducted between 1991 and 1994 and which reported 42.3% of patients with an HbA1c under 7%. A recent retrospective study (Grant et al 2005) of general practice and endocrine specialists in the United States demonstrated even poorer levels of control, with only 34% of patients achieving glycemic control targets. Although this may indicate that glycemic control is better in Spain, it may also be due to different methodological characteristics. For example, in our study, only patients treated by general practitioners were included. By not including patients treated by diabetes specialists we may have biased our sample towards patients with better glycemic control.
Regardless of the comparisons, the HbA1c values are clearly suboptimal. One possible reason for this is that patients are noncompliant with their prescribed treatment regimen. Another possibility is that in real clinical practice, physicians prescribe insufficient hypoglycemic drugs. Although we found that patients receiving more than one hypoglycemic drug and/or insulin had worse glucose control than patients on none or only one oral hypoglycemic drug, this is probably because the most severe patients are usually treated with a greater number of drugs. In similar studies of primary care patients, a significant correlation was found to exist between levels of HbA1c and diabetes duration (Fernández Herraez et al 1999). This illustrates how difficult it is for physicians and patients to manage T2DM and to achieve control objectives when the disease progresses and multiple medications are subsequently needed (UKPDS 1998).
It is worth noting that, despite a mean duration with the disease of almost 10 years and, therefore, progressive beta-cell failure, only 34.7% of patients were receiving oral combination therapy and 23.5% received insulin alone or in combination. The relatively low use of drug combinations or insulin in clinical practice is surprising given the well-known findings of landmark studies, which have demonstrated that a greater use of these therapies lead to better HbA1c control. For example, the United Kingdom Prospective Diabetes Study (UKPDS) showed that only 24% of patients treated with sulfonylureas in monotherapy and 13% of those treated with metformin maintained levels of HbA1c lower than 7% after 9 years of treatment. In more than 50% of the cases, combined therapy was needed to achieve better HbA1c control (Turner et al 1999). Nevertheless, the prescription of hypoglycemic drugs in actual clinical practice appears to be suboptimal (Mata Cases et al 2003; Spann et al 2006).
Although some oral hypoglycemic drugs can produce hypoglycemia, it is a more frequent problem in patients treated with insulin. Therefore, blood glucose monitoring is suggested to help optimize dosing and to avoid hypoglycemia. Consistent with these guidelines, the results of this study suggest that patients taking insulin regularly test their blood glucose levels. The generally accepted diabetes guidelines in Spain do not recommend self-monitoring in patients who are treated with diet only (García Soidán et al 2005). The same is true of the ADA guidelines, which also do not establish an optimum frequency or regularity of self-monitoring in patients treated with OAMs (ADA 2006). Our study showed a high use of test strips in patients treated only with diet or OAMs. Other studies conducted in Spain also show test strip usage which are above the recommended levels (Olveira et al 1998; Clua Espuny et al 1999). The high use of test strips produces significant costs to the Spanish health system yet there is still some debate as to whether these higher monitoring costs lead to improved outcomes (Oliva et al 2004; Guerci et al 2003; Franciosi et al 2005; Martín et al 2006). Some critics even suggest that such monitoring could worsen a patient’s metabolic control and cause greater psychological problems (Oliva et al 2004). Given the controversy regarding the effectiveness of SMBG in patients treated with diet or with an OAM, it is surprising that patients on these treatments make such frequent use of test strips.
According to the results of this study, fewer than 50% of patients on pharmacological treatment reported high compliance. The proportion of good compliers was higher in patients treated in insulin than in those treated with OAMs and much lower in those treated with a combination of insulin and OAMs. Although it might be expected that compliance would be lower for injectable treatments, our results suggest that compliance is related more to the severity of diabetes than to the type of drug administration. Insulin treatment may also have a psychological effect on the patient in the sense that initiating treatment with insulin forces the patient to think seriously about the disease and possibly makes the patient more conscientious about taking his or her medication. Our results are consistent with a systematic review of compliance to diabetes treatment, which concluded that many diabetic patients complied poorly with treatment that included both OAMs and insulin and indicated that, as with other chronic diseases, treatment compliance was not related to the complexity of the treatment regimen, the severity of the disease, or the possible consequences of the forgotten doses (Cramer 2004).
Some of the most interesting contributions of the current study may be the conclusions derived from the logistic regression analysis conducted to determine the factors associated with glycemic control. The results show that treatment compliance and BMI are the best predictors for not achieving optimal glycemic control. In our model, patient education level, treatment type, disease progression, and the presence and type of complications were not significantly associated with poor HbA1c control. While other studies have also examined predictors of glycemic control, our study includes an important variable that is often missing in these types of analyses, namely, compliance (Sender Palacios et al 2002; Díaz Grávalos et al 2006; Spann et al 2006). The fact that the two most important variables – BMI and compliance – are largely dependent on the patient’s behavior, demonstrates the importance of the patient’s role in achieving target clinical outcomes. Clinicians should continue to help their patients to understand the importance of weight management and treatment compliance and how they are associated with clinical outcomes, such as HbA1c control.
This study provides a comprehensive examination of actual clinical practice for patients with type 2 diabetes in Spain; however, the study had several limitations. First, the recruitment of patients from among those consulting their physician within a three-month period infl uenced selection. Although all patients with diabetes, including those with different treatment patterns and at different stages of the disease, should consult their physicians, the three-month enrolment period could have led to the recruitment of patients who consult more regularly with their physicians. The direction of this bias is unknown as patients in good health may be in good health because they see their physicians more often and our sample would therefore tend to include patients with better outcomes. On the other hand, patients with poor health may visit their physicians more often and in that case our sample would be biased towards patients with a poorer health status. Another source of potential bias for this study was recruiting only patients who were treated by a general practitioner. As more patients with poor glycemic control are likely treated by diabetes specialists, the results from our study are not likely to be representative of the overall management and outcomes of patients with type 2 diabetes in Spain. Finally, an indirect method which relies on patient self-report (ie, the Morisky-Green questionnaire) was used to measure treatment compliance. This might be expected to produce infl ated compliance scores; however, the proportion of patients reporting good compliance in our study was actually lower than that found in other recent studies on compliance (Grant et al 2003; Mino-Leon et al 2005).
In conclusion, we observed that fewer than half of all patients with T2DM being treated by primary care physicians in Spain have a good level of glycemic control. The worst levels of glycemic control were observed in patients on insulin therapy. Although this may be due to the fact that these patients have a more advanced form of diabetes, the results may also indicate that insulin treatment should be better managed in this population. We also found that fewer than half of the patients included who were using pharmacological treatment reported having high compliance with their treatment. Patients on insulin monotherapy and insulin combination therapy reported the highest levels of compliance. This is perhaps surprising considering that injectable administration is typically considered more bothersome than oral administration. However, patients on insulin may recognize the severity of the disease more than patients on OAM(s). Patients also report a high frequency of blood glucose monitoring, including frequent use of test strips among patients who were not using insulin. This is not consistent with treatment guidelines in Spain and it is not clear whether or not such monitoring is warranted. Healthcare providers may need to consider health education programs to help patients determine how frequently they should self-monitor their blood glucose. Finally, given the results of the analysis of the factors most strongly associated with glycemic control, special attention should be paid to treatment compliance and BMI. In the course of managing diabetes, physicians and patients should attempt to improve compliance and lower BMI, which could lead to better glycemic control.
Footnotes
Disclosure
Funding for this study was provided by Eli Lilly and Company.
References
- [ADA] American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28:S37–S42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- [ADA] American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2006;29(Suppl 1):S4–42. [PubMed] [Google Scholar]
- Arroyo J, Badía X, de la Calle H, et al. Tratamiento de los pacien-tes con disbetes mellitus tipo 2 en españa. Medicina Clínica (Barc) 2005;125:166–72. doi: 10.1157/13077139. [DOI] [PubMed] [Google Scholar]
- Bayo J, Latorre PM, García F, et al. Factores de riesgo asociados a la prevalencia de diabetes mellitus no insulinodependiente en Lejona (Vizaya) Medicina Clínica (Barc) 1996;107:572–7. [PubMed] [Google Scholar]
- Benach J, Yasui Y, Borrell C, et al. Material deprivation and leading causes of death by gender: evidence from a nationwide small area study. J Epidemiol Community Health. 2001;55:239–45. doi: 10.1136/jech.55.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito LP, Garcia MR, Puig DM, et al. Pathological characteristics of patients with diabetes mellitus type 2 in Spanish primary care. Revista Clínica Española. 2004;204:18–24. doi: 10.1157/13056787. [DOI] [PubMed] [Google Scholar]
- [BCS] British Cardiac Society, British Hypertension Society, Diabetes UK. JBS2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente ML, Alonso JA, Córdoba R, et al. Descripción de las guías disponibles en España para el abordaje del tabaquismo en Atención Primaria. Atención Primaria. 1999;24:101–8. [PubMed] [Google Scholar]
- Clua Espuny JL, Puig Junoy J, Ciuranan Roca E, et al. Self-monitoring of blood glucose: evaluation of its prescription and results in type-2 diabetes. Atención Primaria. 1999;24:316–25. [PubMed] [Google Scholar]
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–24. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- Díaz Grávalos GK, Palmeiro Fernández G, Casado Górriz I, et al. Cumplimiento de los objetivos de control metabólico en diabetes mellitus en el medio rural de Ourense. Revista Española de Salud Pública. 2006;80:67–75. doi: 10.1590/s1135-57272006000100007. [DOI] [PubMed] [Google Scholar]
- Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Herraez E, Ferré Larrosa F, Jiménez Alfonso L, et al. Valoración de los factores relacionados con el control de la glucemia en la diabetes mellitus tipo 2. Atención Primaria. 1999;24:39–43. [PubMed] [Google Scholar]
- Franciosi M, Pellegrini F, De Berardis G, et al. Self-monitoring of blood glucose in non-insulin treated diabetic patients: a longitudinal evaluation of its impact on metabolic control. Diabetic Med. 2005;22:900–6. doi: 10.1111/j.1464-5491.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314:964–7. doi: 10.1136/bmj.314.7085.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Soidán FJ, Novo Rodríguez JM, Vázquez Troitiño F, et al. Diabetes Mellitus tipo 2 [online] [Accessed on March 3, 2007];Guías Clínicas. 2005 5(15) URL: http://www.fisterra.com/guias2/diabetes_mellitus.asp. [Google Scholar]
- González-Clemente JM. Non-insulin dependent diabetes mellitus: care in an area of Barcelona. Medicina Clínica (Barc) 1997;108:91–7. [PubMed] [Google Scholar]
- Grant RW, Buse JB, Meigs JB. Quality of diabetes care in US academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RW, Devita NG, Singer DE, et al. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26:1408–12. doi: 10.2337/diacare.26.5.1408. [DOI] [PubMed] [Google Scholar]
- Guerci B, Drouin P, Grange V, et al. Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active Study. Diabet Metabol. 2003;29:587–94. doi: 10.1016/s1262-3636(07)70073-3. [DOI] [PubMed] [Google Scholar]
- Harris MI. Health care and health status and outcomes for patients with type 2 diabetes. Diabetes Care. 2000;23:754–8. doi: 10.2337/diacare.23.6.754. [DOI] [PubMed] [Google Scholar]
- [IDF] International Diabetes Federation. Global guideline for type 2 diabetes [online] 2005. [Accessed March 3, 2007]. URL: http://www.idf.org/webdata/docs/IDF%20GGT2D.pdf.
- [INE] Instituto Nacional de Estadistica [National Institute of Statistics] Demographics and Population. INEbase [online] Madrid: National Institute of Statistics; 2006. [Accessed on December 14, 2006]. Population referring to January 1, 2005 by autonomous communities and sex. URL: http://www.ine.es. [Google Scholar]
- King H, Aubert RE, Herman WH. Global burden of diabetes 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- Laing W, Williams R. 1996. Diabetes, a model for health care management, Office of Health Economics, London, 1989 (Paper 92). Cited by: Marks L. Counting the cost: the real impact of non-insulin-dependent diabetes. A King’s Fund report commissioned by the British Diabetic Association.
- Martín S, Schneider B, Heinemann L, et al. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49:271–8. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- Mata Cases M, Roset Gamisans M, Badia Llach X, et al. Impacto de la disbetes mellitus tipo 2 en la calidad de vida de los pacientes tratados en las consultas de atención primaria en España. Atención Primaria. 2003;31:493–9. doi: 10.1016/S0212-6567(03)70722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino-Leon D, Figueras A, Amato D, et al. Treatment of type 2 diabetes in primary health care: a drug utilization study. Ann Pharmacother. 2005;39:441–5. doi: 10.1345/aph.1E273. [DOI] [PubMed] [Google Scholar]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Oliva J, Lobo F, Molina B, et al. Direct health care costs of diabetic patients in Spain. Diabetes Care. 2004;27:2616–21. doi: 10.2337/diacare.27.11.2616. [DOI] [PubMed] [Google Scholar]
- Olveira G, Soriguer F, Ortega C, et al. Use of reagent materials for self-monitoring in the metropolitan area of Malaga (1994–1996) Atención Primaria. 1998;21:75–80. [PubMed] [Google Scholar]
- Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28:2130–35. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- Sender Palacios MJ, Vernet Vernet M, Larrosa Sáez P, et al. Carac-terísticas sociodemográficas y clínicas de una población de pacientes con diabetes mellitus. Atención Primaria. 2002;29:474–80. doi: 10.1016/S0212-6567(02)70616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann SJ, Nutting PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice-based research network study. Ann Fam Med. 2006;4:23–31. doi: 10.1370/afm.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo-Marco B, Faure-Nogueras E, Roche-Asensio MJ, et al. Prevalence of diabetes and impaired glucose tolerance in Aragon, Spain. Diabetes Care. 1997;20:534–6. doi: 10.2337/diacare.20.4.534. [DOI] [PubMed] [Google Scholar]
- Tremble JM, Donaldson D. Is continued weight gain inevitable in type 2 diabetes mellitus? J Royal Soc Health. 1999;119:235–9. doi: 10.1177/146642409911900406. [DOI] [PubMed] [Google Scholar]
- Turner R, Cull C, Frighi V, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- [UKPDS] UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- [WHO] World Health Organization. Prevalence of diabetes in the WHO European region [online] 2007. [Accessed March 3, 2007]. URL: http://www.who.int/diabetes/facts/world_figures/en/print.html.
- World Health Organization Regional Office for Europe. European mortality database. 2007. [Accessed March 3, 2007]. URL: http://data.euro.who.int/hfamdb/
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Zorrilla Torras B, Cantero Real JL, Martínez Cortés M. Study of non-insulin-dependent diabetes mellitus in primary care in the community of Madrid using network of sentinel physicians. Atención Primaria. 1997;20:543–8. [PubMed] [Google Scholar]