Abstract
Objective
The purpose of the BalanceWise-hemodialysis study is to determine the efficacy of a dietary intervention to reduce dietary sodium intake in patients receiving maintenance, in-center hemodialysis (HD). Personal digital assistant (PDA)-based dietary self-monitoring is paired with behavioral counseling. The purpose of this report is to present a case study of one participant’s progression through the intervention.
Methods
The PDA was individually programmed with the nutritional requirements of the participant. With 25 minutes of personalized instruction, the participant was able to enter his meals into the PDA using BalanceLog® software. Nutritional counseling was provided based on dietary sodium intake reports generated by BalanceLog®.
Results
: At initiation of the study the participant required 4 HD treatments per week. The participant entered 342 meals over 16 weeks (≥3 meals per day). BalanceLog® revealed that the participant consumed restaurant/fast food on a regular basis, and consumed significant amounts of corned beef as well as canned foods high in sodium. The study dietitian worked with the participant and his wife to identify food alternatives lower in sodium. Baseline sodium consumption was 4,692 mg, and decreased at a rate of 192 mg/week on average. After 11 weeks of intervention, interdialytic weight gains were reduced sufficiently to permit the participant to reduce HD treatments from 4 to 3 per week. Because of a low serum albumin at baseline (2.9 g/dL) the study dietitian encouraged the participant to increase his intake of high quality protein. Serum albumin level at 16 weeks was unchanged (2.9 g/dL). Because of intense pruritis and a high baseline serum phosphorus (6.5 mg/dL) BalanceLog® electronic logs were reviewed to identify sources of dietary phosphorus and counsel the participant regarding food alternatives. At 16 weeks the participant’s serum phosphorus fell to 5.5 mg/dL.
Conclusions
Self-monitoring rates were excellent. In a HD patient who was willing to self-monitor his dietary intake, BalanceLog® allowed the dietitian to target problematic foods and provide counseling that appeared to be effective in reducing sodium intake, reducing interdialytic weight gain, and alleviating hyperphosphatemia and hyperkalemia. Additional research is needed to evaluate the efficacy of the intervention.
Keywords: hemodialysis, self care, computers, handheld, personal digital assistant, randomized clinical trial, behavioral research, adherence
Introduction
In advanced kidney failure, the only available treatment options are home or in-center hemodialysis (HD), home peritoneal dialysis, or kidney transplant. More than 93% of individuals with end-stage renal disease (ESRD) begin treatment with HD, and almost all patients (99.6%) on HD receive in-center treatment (USRDS 2007). In-center HD treatment consists of thrice weekly visits to a local dialysis unit, during which patients are dialyzed to remove toxins, maintain fluid balance, and normalize blood electrolytes. While kidney transplant yields the best outcomes in terms of morbidity, mortality, health care cost, and quality of life, some HD patients are not eligible for transplant due to the underlying disease state or the presence of other comorbid conditions. These individuals must remain on dialysis for the rest of their lives. For those who are eligible for a transplant, unless a living donor is available the waiting time for a kidney can be many years. Therefore, maintaining health while on HD is a key management goal. Remaining healthy is a challenge, as HD cannot entirely replace kidney function, and in-center HD treatments are associated with a variety of problems, in part due to the intermittent nature of this form of dialysis.
To minimize the problems associated with intermittent dialysis, in-center HD patients need to follow a complicated and fairly restrictive dietary regimen. Hyperphosphatemia develops in patients with ESRD because phosphorus is not adequately removed by dialysis and must be controlled through oral intake of phosphorus binders and/or reduced dietary intake of phosphates. HD patients also must limit dietary consumption of potassium by limiting their intake of fresh fruits and vegetables, to prevent hyperkalemia. Protein-energy malnutrition is common among HD patients (Rocco et al 2002), who must be vigilant to consume enough calories and high quality protein.
Sodium and fluid control are perhaps the most important components of the HD diet. Excessive intake of sodium that is not excreted by failed kidneys results in intense thirst and, excessive fluid intake. Extracellular volume expansion, which is controlled primarily through dietary intake of sodium, is the main pathophysiologic determinant of hypertension in HD patients (D’Amico and Locatelli 2002). Hypertension occurs in an estimated 72%–90% of HD patients (Salem 1995; Rocco et al 2001; Agarwal 2000). An increase in pulse pressure, due to an increase in systolic blood pressure (BP), is an important predictor of survival in HD (Klassen et al 2002). Recent small studies suggest that salt restriction and strict volume control can reduce interdialytic weight gain (IDWG) (Rigby-Matthews et al 1999; Ozkahya et al 2002) and, in turn, improve patient outcomes (Ozkahya et al 2002; Saran et al 2003).
The National Kidney Foundation recently released clinical practice recommendations for HD adequacy (NKF 2006). These guidelines highlight the importance of moderate dietary sodium restrictions (2–2.4 g/day) for controlling fluid volume and BP, and ultimately reducing cardiovascular disease. These guidelines recommend that weight gain between dialysis treatments should not exceed 1 kg during the week and 1.5 to 2 kg during the weekend. Adhering to such a complicated dietary regimen is likely to be difficult for even the most motivated HD patient.
New dietary self-monitoring technologies may be useful for helping HD patients make good dietary decisions. The purpose of the BalanceWise-HD study was to pilot test a dietary intervention to reduce sodium intake in in-center HD patients. The intervention involves behavioral counseling based on social cognitive theory (SCT), paired with personal digital assistant (PDA)-based dietary self-monitoring with BalanceLog® software. In this case report we describe the implementation of the intervention in a study participant who experienced a reduction in IDWGs over the course of the study.
Case history
Participant (ppt) #03 is an African American male who began in-center HD in July of 2006 when he was 57 years old. His nephrotic syndrome and chronic kidney disease were due to biopsy-proven amyloidosis and focal segmental glomerulosclerosis. He presented to the emergency department complaining of general malaise and poor oral intake. His creatinine was 9.8 mg/dl. The acute component of his kidney failure was believed to be multifactorial from partial obstruction as well as prerenal, with perhaps some degree of acute tubular necrosis. While in the emergency department, ppt #03 was hydrated and a Foley catheter was placed. Despite these interventions his creatinine levels did not improve and HD was initiated in the inpatient setting via a temporary catheter. He was transferred subsequently to a skilled nursing facility and thrice weekly HD continued on an outpatient basis.
Ppt #03 was discharged home in November of 2006. Although his appetite was poor at the time of his discharge, it gradually improved over the subsequent 3 months. As his appetite increased, serum potassium and phosphorus levels increased. Hyperphosphatemia became a significant problem, resulting in intense pruritis, and phosphate binders were added to his regimen. Although his serum albumin was low, a request for interdialytic parenteral nutrition was denied by his insurance carrier. He was advised to drink protein powder supplements but refused to do so. His increasing IDWGs necessitated the intensification of his dialysis treatment regimen from 3 to 4 days/week. Despite intensive dietary counseling as well as interest and willingness on the part of the ppt to learn more about the HD diet, laboratory data and IDWGs suggested non-compliance to a difficult and complicated dietary regimen.
Methods
In January 2007, ppt #03 enrolled in the 16-week Balance-Wise Study. The BalanceWise-HD study is a randomized clinical trial to pilot test a dietary intervention to reduce dietary sodium intake. Hemodialysis patients are approached by a dialysis center staff person to solicit their interest in the study. Those signing a Health Insurance Portability and Accountability Act (HIPAA) release are approached by a study staff person who obtains a signed informed consent. Participants are randomized to either intervention or to an attention control group.
The intervention involves dietary counseling based on SCT (Bandura 1986, 1997). Table 1 presents a list of common diet-related problems experienced by HD patients and examples of SCT-based behavioral solutions used in the study to enhance adherence to the dialysis dietary regimen. Intervention participants also receive a PDA with BalanceLog® software. BalanceLog® allows participants to evaluate the content of various foods, track dietary intake, and evaluate the percent of dietary targets achieved by meal and by day. A description of BalanceLog® software when used within the context of a behavioral self-management intervention appears elsewhere (Sevick et al 2008).
Table 1.
Common problems experienced by hemodialysis patients and examples of SCT-based behavioral solutions to enhance self-efficacy
| Common problems | Counseling approach |
|---|---|
| Knowledge deficits, nonadherence to dietary restrictions. The HD diet is complex, requiring patients to be knowledgeable, and aware of their intake of several key nutrients. | The intervention fosters a sense of mastery in adopting the HD diet. PDA self-monitoring permits the patient to become self-aware of their dietary patterns. In collaboration with the dietician the patient sets short-term, achievable goals that permit them to experience success in adhering to their dietary regimen. |
| Lifestyle change. Giving up favorite foods and otherwise changing dietary patterns require significant motivation and self-discipline. | SCT interventions acknowledge that lifestyle changes do not occur overnight. Using individual counseling, patient values and goals regarding treatment are clarified, and behavior changes required to meet those goals are defined with the patient. “Stepped”, achievable goals are negotiated with the patient, allowing the patient to develop a sense that they are able to succeed in adhering to their diet (mastery). Verbal persuasion is used to reinforce to the patient that they are capable of making dietary changes. Stimulus control methods are use to help patients avoid cues to unhealthy food choices (eg, avoiding the chips and soda aisle when shopping) or change their response to cues by building healthier habits (eg, eating unsalted pretzels instead of potato chips). Involving the patient’s social support system is employed to encourage sustained healthy food choices. |
| Disruption of meals by dialysis. Our preliminary data and our clinical observations support the notion that dialysis treatments disrupt normal meals (either because of the timing of dialysis, or because patients feel “washed-out” after dialysis and do not feel like cooking or eating). | The dietician works with the patient to problem solve around meal disruptions. Example solutions could be drinking a can of Boost when the patient does not feel like eating, packing a meal the night before to take to the dialysis center, preparing meals in advance, cooking in volume and freezing single serving size meals. |
| Intra- and post-dialytic symptoms. Intradialytic hypotension and post-dialysis symptoms are associated with the rapid removal of fluid during dialysis and are particularly problematic in patients with large interdialytic weight gains. During dialysis patients may experience malaise, muscle cramping, nausea, diarrhea, diaphoresis, chest pain, and visual changes. After dialysis, those who have experienced the removal of large amounts of fluid are likely to feel extreme fatigue. | The dietitian works with the patient to identify the physiologic symptoms associated with high interdialytic weight gains, and recognize improvements that occur as a result of successes in reducing dietary sodium intake. |
| Anorexia, change in sense of taste. As a result of these changes, many HD patients do not eat properly. | Similar to giving-up favorite foods, eating a sufficient amount of food may be a challenge to HD patients; motivation and self-discipline are required. As described in the “lifestyle” section above, verbal persuasion, mastery performance, stimulus control, and use of social support systems will be useful in assisting patients in making healthy food choices. Problem solving methods are used to identify and maximize incorporation of food preferences into the daily diet. |
| Dietary intolerances, diarrhea | The dietician will work with the patients to problem solve around dietary intolerances, eg, suggesting alternative foods, gradual increases in serving sizes, etc. |
| Limited economic resources. Patients may have difficulty with the cost of healthy food choices. | The dietician will problem solve with the patient to identify inexpensive food choices, and will refer to the social worker as necessary to obtain assistance whenever possible. |
| Periodic illness and hospitalization. Progress toward achieving a healthier diet may be interrupted by illness, hospitalization, or dietary lapses. | Patients will be counseled with regard to how they can deal with lapses and relapses. They will be told that lapses and relapses are common, and that they should not be discouraged by these. When lapses occur they are counseled to reformulate goals, as appropriate, to get back “on-track” toward achieving a healthier diet. |
Intervention participants are exposed to a 16-week intervention with contacts performed during regularly scheduled inpatient dialysis treatments. The study dietitian reviews PDA electronic dietary records and provides counseling. Intervention contacts occur twice a week during weeks 1–6, weekly during weeks 7–12, and every other week for weeks 13–16. (The gradual reduction in frequency of contacts is intended to assist the participant in independently maintaining the newly acquired self-management behaviors after the intervention period has ended). Ppt #03 was one of the first participants to enroll in this ongoing study, which anticipates a final recruitment of 25–30 participants.
At the time of enrollment, ppt #03 was receiving 4 dialysis treatments per week to address his high IDWGs. His serum laboratory values on enrollment are shown in Table 2.
Table 2.
Serum laboratory values of participant 03 on enrollment
| Serum laboratory test | Value | Normal range |
|---|---|---|
| Albumin | 2.9 g/dL | ≥3.8 |
| Potassium | 6.0 Eq/L | 3.5–5.5 |
| Phosphorus | 6.5 mg/dL | 3.5–5.5 |
Ppt #03 was randomized to the intervention and provided with a PDA with BalanceLog® software. BalanceLog® was used to log dietary intake and provide graphical feedback to the ppt on the percent of target nutrients (sodium, protein, calories) that he consumed by meal and by day. The ppt also was shown how to track fluid intake in the program. BalanceLog® was individually programmed for the nutritional requirements of ppt #03 (Table 3).
Table 3.
Nutritional requirements of participant 03 programmed into PDA
| Target calories | 2,175 kcal |
| Target protein | 130 g/day |
| Sodium (upper limit) | 2,000 mg/da |
Ppt #03 also was prescribed the upper limits of 2,000 mg/ day of potassium, and 1,000 mg/day of phosphorus. Although the software does not contain information on the potassium or phosphorus content of the foods in the BalanceLog® database, when monthly labs revealed hyperphosphatemia or hyperkalemia the electronic log was reviewed by the dietitian to identify high potassium and high phosphorus foods. The ppt then was counseled regarding approaches for staying within the prescribed targets.
Ppt #03 received individualized instruction on entering foods into BalanceLog®. He was shown a PowerPoint slide demonstration of how to enter foods into the program, and followed along on his individually programmed PDA. The ppt was instructed to record his meals and snacks as soon as possible after eating so that recalling the food several hours later would not be an issue, a phenomenon that is common among individuals who self-monitor dietary intake (Burke et al 2006, 2008).
Instruction of ppt #03 required approximately 25 minutes of staff time. After initial training, the study dietitian met with ppt #03 according to the schedule described above. Using the dietary logs, routinely schedule laboratory test data (serum albumin, potassium, phosphorus), and information on IDWG, the study dietitian worked with ppt #03 to identify potential problems, assist him in setting realistic goals for dietary change, evaluate goal achievement, and provide the feedback and encouragement important to developing his sense of mastery over the dietary regimen. Whenever possible, the participant’s wife was included in counseling sessions.
Results
After a single instruction session, ppt #03 entered meals every day for the duration of the 16-week intervention (including 7 days during which he was hospitalized). The participant entered 342 meals over 16 weeks (≥3 meals per day). Although the focus of the intervention was the reduction of dietary sodium intake, ppt #03 was highly motivated to reduce his dialysis treatments from a 4 day/week regimen to 3 days/week. The study dietitian noted on his dietary logs that ppt #03 was eating frequently at restaurants, including fast food establishments. He also was consuming a significant amount of corned beef as well as canned foods high in sodium. The dietitian worked with the ppt and his wife to identify food alternatives lower in sodium. In particular the dietitian advised them to minimize their consumption of restaurant/fast food. When consuming protein, they were advised to eat fresh, unprocessed meats. They also were advised to minimize the consumption of canned vegetables. When this was not possible, they were advised to rinse the vegetables to eliminate as much sodium as possible.
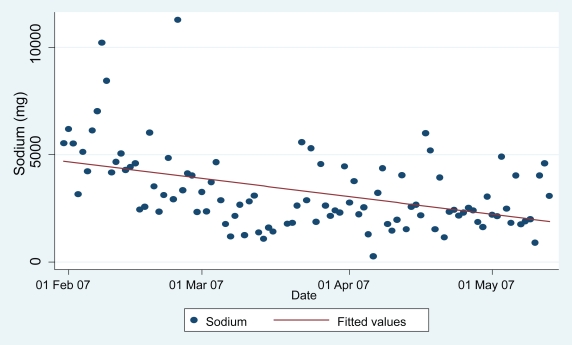
Figure 1 shows the daily sodium consumption for ppt #03 over the 16-week study period, uploaded from his PDA electronic record, with a super-imposed linear regression line. Based on the fitted regression line, the estimated baseline daily sodium consumption was 4,692 mg and decreased at a rate of 192 mg per week on average. By the end of the study period, his daily sodium consumption decreased by an estimated 3,077 mg from the baseline level, with a final average below the ceiling recommended by the National Kidney Foundation. Furthermore, the extremely high values (>6,000 mg sodium) did not occur after the first few weeks of the study.
Figure 1.
Daily sodium consumption from PDA electronic record for participant 03.
Because of intense pruritis and a high baseline serum phosphorus level (6.5mg/dL) the study dietitian reviewed the BalanceLog® electronic logs to identify sources of dietary phosphorus. The study dietitian found that the patient was consuming large quantities of chili with cheese and beans almost daily and was not taking phosphorus binders with each meal. By 16 weeks the ppt’s phosphorus had fallen to 5.5 mg/dL. As noted above, the ppt was advised to maintain adequate dietary intake of protein by consuming high quality proteins, and by trying to eat the protein component of his meal first. Serum albumin at 16 weeks was unchanged.
The ppt had a serum K+ lab value of 6.0 mEq/L at the initiation of the study. The study dietitian reviewed with the ppt instructional materials on limiting dietary potassium. She also provided a USDA list of foods and their respective potassium content to him and his wife. After 1 month of dietary counseling his K+ dropped to 4.4 mEq/L and continued to remain in the normal range until the conclusion of the study, when his K+ was 5.1 mEq/L.
We also evaluated the impact of the intervention on IDWG. IDWGs are a function of the number of days that have elapsed between dialysis treatments. For example, in those participants who dialyze on the usual 3 day/week schedule, the interval between dialysis treatments will vary from 2–3 days. For those who dialyze on a 4 day/week schedule, the interval between dialysis treatments will vary from 1–3 days. To account for variation in IDWG by treatment interval, we calculated the average daily IDWG (IDWGA) for each dialysis treatment, as the total IDWG for each treatment observation divided by the number of days that had elapsed since the last HD treatment.
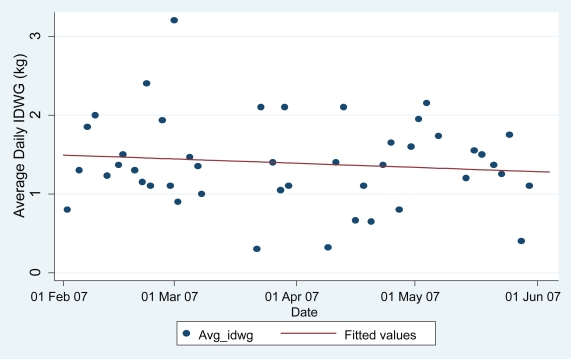
Figure 2 shows the IDWGAs for ppt #03 over the 16-week intervention with a superimposed linear regression line. Based on the fitted regression line, the estimated IDWGA for ppt #03 was 1.49.kg at baseline, and decreased at a rate of 0.012 kg per week on average. By the end of this 16 week study, his IDWGA had decreased from baseline by an estimated 0.19 kg, or his IDWG decreased by 0.38 kg between two weekday dialysis sessions (eg, Monday and Wednesday). After 11 weeks of intervention, IDWGs were reduced sufficiently to permit ppt #03 to decrease his dialysis regimen from 4 to 3 days/week.
Figure 2.
Average daily interdialytic weight gains for participant 03.
Discussion
Dietary change is widely recognized to be difficult to initiate and sustain in healthy populations (Burke et al 1997; Kumanyika et al 2000; Rothman 2000; Rothman et al 2004). Dietary change is likely to be even more difficult for patients on intermittent in-center HD because of the complexity of the dialysis diet and the numerous other nondietary demands of the treatment regimen. Sodium intake is particularly difficult to control because many foods are naturally high in sodium, and most prepared/prepackaged foods have significant amounts of sodium added to enhance taste and prolong shelf-life. Perhaps because the dietary regimen is so complex, most of the research surrounding management of fluid volume has focused on restricting fluid intake, or using medications and manipulating dialysis treatments. However, we believe that with advent of new information technologies, healthcare professionals now are positioned to help HD patients make better dietary decisions.
Because HD dietary recommendations often conflict, use of dietary self-monitoring software such as BalanceLog® may be particularly useful for this patient population. For example, HD patients often experience a loss of appetite. Counseling them to reduce their dietary sodium intake could result in the elimination of many of their favorite foods. If acceptable alternatives are not identified, the patient may be placed at increased risk of malnutrition. Similarly, counseling HD patients who have a low serum albumin to consume more protein may result in hyperphosphatemia. Switching from canned to frozen or fresh foods (especially vegetables) may result in hyperkalemia. Use of programs such as BalanceLog® allows the dietitian to target dietary problems as they arise, and allows the patient to consider the real time dietary consequences of adherence to dietary recommendations. Given his high rate of self-monitoring, the use of BalanceLog® did not appear to be burdensome to ppt #03. This case study suggests that the intervention, which is currently being tested in a small randomized study, may be effective for reducing dietary sodium intake and IDWGA.
However, caution must be used in drawing firm conclusions from the data presented for several reasons. First, the case was not selected randomly. Ppt #03 was selected for this case study because of his high baseline IDWGs, his high level of adherence to PDA self-monitoring, and the possibility that the intervention resulted in dietary changes that could have led to the improvements described. Ppt #03 may not be typical of the average HD patient.
Second, it is not clear to what extent reductions in dietary sodium intake and IDWG were the result of PDA self-monitoring versus the additional attention the participant received from the dietitian. As the study progresses, we plan to discount the potential competing hypothesis that improvements seen could have been due to additional attention, by virtue of our design which employs randomization and an attention control group.
Third, we cannot be certain of whether the normalization of serum phosphorus was due to reduced dietary phosphorus or better compliance with phosphate binders, because the study dietitian counseled ppt #03 about both, and because adherence to phosphate binders was not measured. However, we do know that dietary recalls performed prior to the intervention had been unsuccessful in revealing the source of the participant’s hyperphosphatemia. A food log that is completed in real time may allow clinicians to identify and address dietary problems more accurately and efficiently than is possible with dietary recalls which are dependent on the memory of the patient. It is also possible that better phosphate management was due to increased dialysis dose or a change of dialyzer membrane, data that were not collected in this study.
Fourth, changes in IDWG can be influenced by a number of factors that were not measured in this small pilot study. For example, patients with very high IDWGs require removal of a large volume of fluid (referred to as ultrafiltration in the dialysis literature). High levels of ultrafiltration are associated with hypotension which may require fluid replacement during dialysis. This fluid replacement may, in turn, result in net inadequate fluid removal. For many patients, particularly for those with high IDWGs, fluid removal is accompanied by intradialytic symptoms such as cramping, nausea, vomiting, and diarrhea. To ease the discomfort and enhance tolerability of fluid removal, some physicians will prescribe sodium modeling. In sodium modeling, a dialysate solution with a higher concentration of sodium is used initially during treatment, with sodium concentration decreased over the course of dialysis. Although it alleviates intradialytic symptoms, sodium modeling increases the patient’s serum sodium and, thus, may increase postdialytic thirst and subsequent IDWG. Several other factors may impact IDWG, such as changes in cardiovascular conditions, residual renal function, Kt/V (dialysis dose), dry weight (eg, weight gain or loss in body fat or lean body mass), glycemic control (in those with diabetes), inflammation, and general nutritional status (Lopez-Gomez et al 2005).
Finally, PDA records provide a convenient source of information on possible changes in dietary patterns over time. However, the use of dietary records obtained from the PDA as a measure of dietary change potentially confounds independent and dependent variables. In future reports, we will be able to present the results of independent measures of dietary intake, including sodium consumption from dietary recalls obtained with unscheduled telephone calls to the participant’s home over a 2-week period, including one dialysis weekday, one nondialysis weekday, and one nondialysis weekend day.
Conclusion
The dietary self-monitoring intervention, which aims to reduce fluid volume overload by reducing dietary sodium intake appears to be feasible, and may be effective in reducing sodium intake, increasing dietary self-efficacy, and reducing IDWG.
Footnotes
Disclosure
This study was supported through a grant from the Paul Teschan Research Foundation. The authors report no conflicts of interest in this work.
References
- Agarwal R. Strategies and feasibility of hypertension control in a prevalent hemodialysis cohort. Clin Nephrol. 2000;53:344–53. [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- Bandura A. Self-Efficacy: The Exercise of Control. New York: Freeman and Co; 1997. [Google Scholar]
- Burke LE, Dunbar-Jacob J, Hill M, et al. Compliance to cardiovascular risk reduction strategies: Review of the research. Ann Behav Med. 1997;19:239–63. doi: 10.1007/BF02892289. [DOI] [PubMed] [Google Scholar]
- Burke LE, Sereika SM, Choo J, et al. Ancillary study to the PREFER trial: A descriptive study of participants’ patterns of self-monitoring: Rationale, design and preliminary experiences. Contemp Clin Trials. 2008;21:23–33. doi: 10.1016/j.cct.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Burke LE, Sereika SM, Music E, et al. Using instrumented paper diaries to document self monitoring patterns in weight loss. Contemp Clin Trials. 2008;29:182–93. doi: 10.1016/j.cct.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico M, Locatelli F. Hypertension in dialysis: pathophysiology and treatment. J Nephrol. 2002;15:438–45. [PubMed] [Google Scholar]
- Klassen PS, Lowrie EG, Reddan DN, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–55. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Van Horn L, Bowen D. Maintenance of dietary behavior change. Health Psychol. 2000;19(1 Suppl):42–56. doi: 10.1037/0278-6133.19.suppl1.42. [DOI] [PubMed] [Google Scholar]
- Lopez-Gomez JM, Villaverde M, Jofre R, et al. Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int Suppl. 2005;67:S63–S68. doi: 10.1111/j.1523-1755.2005.09314.x. [DOI] [PubMed] [Google Scholar]
- [NKF] National Kidney Foundation. Clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 2006;48:S13–97. [Google Scholar]
- Ozkahya M, Toz H, Ozerkan F, et al. Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol. 2002;15:655–60. [PubMed] [Google Scholar]
- Rigby-Mathews A, Scribner BH, Ahmad S. Control of interdialytic weight gain (IDWG) without water restriction in hemodialysis. J Am Soc Nephrol. 1999;10:A1348. [Google Scholar]
- Rocco MV, Paranandi L, Burrowes JD, et al. Nutritional status in the HEMO Study cohort at baseline. Hemodialysis. Am J Kidney Dis. 2002;38:245–56. doi: 10.1053/ajkd.2002.30543. [DOI] [PubMed] [Google Scholar]
- Rocco MV, Yan G, Heyka RJ, et al. Risk factors for hypertension in chronic hemodialysis patients: baseline data from the HEMO study. Am J Nephrol. 2001;21:280–8. doi: 10.1159/000046262. [DOI] [PubMed] [Google Scholar]
- Rothman AJ, Baldwin AS, Hertel AW. Self-regulation and behavior change: Disentangling behavioral initiation and behavioral maintenance. In: Vohs K, Baumeister R, editors. The Handbook of Self-Regulation Research. New York: Guilford Press; 2004. pp. 130–48. [Google Scholar]
- Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19:64–9. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- Salem MM. Hypertension in the hemodialysis population: a survey of 649 patients. Am J Kidney Dis. 1995;26:461–8. doi: 10.1016/0272-6386(95)90492-1. [DOI] [PubMed] [Google Scholar]
- Saran R, Bragg-Gresham JL, Rayner HC, et al. Nonadherence in hemodialysis: Associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254–62. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Sevick MA, Korytkowski M, Piraino B, et al. Design, feasibility, and acceptability of an intervention using personal digital assistant-based self-monitoring in managing type 2 diabetes. Contemp Clin Trials. 2008;29:396–409. doi: 10.1016/j.cct.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [USRDS] US Renal Data System. Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]