Abstract
Objective
To examine the relationship between bilateral oophorectomy (BSO) and risk of coronary heart disease (CHD).
Study Design
We searched PubMed, EMBASE, meeting abstracts, and reference lists for studies that compared women with BSO at the time of hysterectomy to 1) women with hysterectomy and ovarian conservation, 2) naturally menopausal women, 3) premenopausal women or, 4) women with no history of hysterectomy or BSO but unreported menopausal status. The primary outcome was fatal or nonfatal CHD.
Results
We reviewed 1,956 citations. Seven observational studies met inclusion criteria. Heterogeneity among studies precluded formal meta-analysis. Four studies reported BSO increases risk for CHD but only in some subgroups of women or not in fully adjusted multivariate models. Three studies found no increased risk of CHD following BSO but these studies had significant limitations.
Conclusion
The existing evidence is inconclusive to determine the effect of BSO on risk of CHD.
Keywords: oophorectomy, surgical menopause, coronary events
INTRODUCTION
Hysterectomy is the most common major surgery among nonpregnant women in the United States and half of these procedures include bilateral salpingo-oophorectomy.1, 2 The rate of BSO has increased dramatically over the last 40 years though the most common age range for hysterectomy has remained stable at 40–44 years; in 1965, 25% of all hysterectomies included BSO compared to 54% of the 600,000 hysterectomies performed annually from 2000–2004.1–4 Although BSO is common, the potential adverse consequences of this procedure have not been fully explored.
BSO is routinely offered concomitant with hysterectomy as a prophylactic procedure to prevent ovarian cancer and additional surgery for benign ovarian masses, and as treatment for pelvic pain, premenstrual syndrome, and symptomatic endometriosis.5–10 However, the absence of ovarian sex steroids following BSO has been associated with decreased sexual function, poorer mental health, and an increased risk of fractures compared to women who undergo ovarian conservation.11–15 Most concerning, some studies have reported that BSO increases risk for coronary heart disease (CHD), the leading cause of death among women.16, 17
The proposed mechanism whereby BSO increases risk for CHD is a surgically induced premature menopause. Younger age at natural menopause has been reported to increase risk for CHD and death due to cardiovascular disease.18–20 Van der Schouw and colleagues reported a 2% decrease in total cardiovascular mortality for each year of increasing age at natural menopause.21 CHD risk with early natural menopause is thought to result from additional years of endogenous estrogen deficiency which may accelerate the development of atherosclerosis. A similar mechanism may apply to BSO because most women are premenopausal at the time of surgery. Consequently, BSO results in a surgical menopause at an earlier age than would occur naturally.
Although there is a plausible biologic mechanism for BSO as a risk factor for CHD, the epidemiologic evidence for this association is conflicting. Several commonly cited cohort studies have reported higher rates of fatal and nonfatal coronary events among women with a history of BSO,16, 17 but others have found no association.22, 23 In addition, a decision analysis found that women who undergo BSO have an increased risk of total mortality compared to those who elect ovarian conservation due to an excess risk of CHD.24 However, these findings were primarily based on the CHD risk reported in a single cohort study among women who had never used estrogen.
We performed a systematic review of the medical literature to clarify the association of BSO and subsequent risk for CHD. We aim to provide an accurate summary of the existing evidence to assist in preoperative counseling for women facing the decision of whether or not to undergo an elective BSO at the time of hysterectomy.
MATERIALS AND METHODS
We searched PubMed (1966–2007) and EMBASE (1966–2007) to identify relevant citations. In PubMed, MeSH terms for the predictor (“ovariectomy”, “oophorectomy”, “castration”, “hysterectomy”, and “menopause”) were combined with MeSH terms for the outcome (“coronary diseases”, “myocardial infarction”, and “cardiovascular disease”). The outcome terms were restricted to subcategories epidemiology, etiology, or mortality. The term “hysterectomy” was restricted to the “adverse effects” subcategory. To further focus the search we used the following limits: humans, female, adult, and middle aged. In EMBASE, EMTREE key words that matched the selected MeSH terms were applied to the search. No language restriction was used. If multiple publications from the same cohort were identified, the publication with the latest date or largest study population was included.
In addition, we reviewed all abstracts presented at the Annual Clinical Meeting of the American College of Obstetricians and Gynecologists (ACOG) from 1996–2006 and the bibliography of all retrieved articles. Finally, we consulted a cardiologist with expertise in women’s health for recommendations of relevant articles, meetings, or scientific presentations to evaluate.
Inclusion criteria included cohort, case-control, cross-sectional, or clinical trial designs with predictor variable BSO at the time of hysterectomy. Comparison groups could include: 1) women with hysterectomy and ovarian conservation, 2) naturally menopausal women, 3) premenopausal women, or 4) women with no prior hysterectomy or BSO whose menopausal status was unknown or unreported. Studies in which the comparison was to population-based CHD rates were excluded because the possibility that the comparison group had undergone BSO could not be excluded.
Studies were included if the primary outcome was CHD events defined as nonfatal myocardial infarction or fatal CHD. Because the results of observational studies are susceptible to bias due to confounding factors, we excluded studies that did not use matching, stratification, or multivariate models in the analysis to address the effect of confounding. We included all multivariate models irrespective of the number or types of variables included.
One author (V.J.) reviewed the title and abstract for all citations identified in the search. The full text of relevant studies as well as those with no abstract, indeterminate content based on the citation, or published in a foreign language were obtained. Among these studies, potentially eligible manuscripts were evaluated by two authors (V.J. and G.S.) who abstracted complete data from all studies that met inclusion criteria. If disagreement occurred with data abstraction or eligibility criteria, consensus was obtained through discussion and re-evaluation. If disagreement persisted, the third author (D.G.) arbitrated the final decision. This systematic review was exempt from Institutional Review Board review.
Each study was categorized as high, intermediate, or poor quality based on study design, years of follow-up, percent lost to follow-up, and the method of outcome ascertainment.25 We defined a high quality study as one with a prospective cohort design, ≥10 years of follow-up, <20% losses to follow-up, and adjudicated, blinded assessment of all reported CHD events. Intermediate quality was defined as a retrospective cohort or case-control design, <10 years of follow-up, >20% losses to follow-up, and/or unblinded assessment of the outcome or adjudication of a subsample of events. Poor quality was defined as a cross-sectional design with outcomes determined by self-report of the subject or health care provider, not confirmed through review of the medical record.
We planned to perform formal meta-analyses to calculate mean variance-weighted odds ratios for CHD comparing women with BSO at the time of hysterectomy to those without BSO. However, heterogeneity of comparison groups and analysis strategies precluded combining the data using meta-analytic techniques and applying statistical tests to assess publication bias. In two studies that did not include tests of statistical significance17, 26, we used the published raw data to perform chi-squared tests for the comparisons of interest.
RESULTS
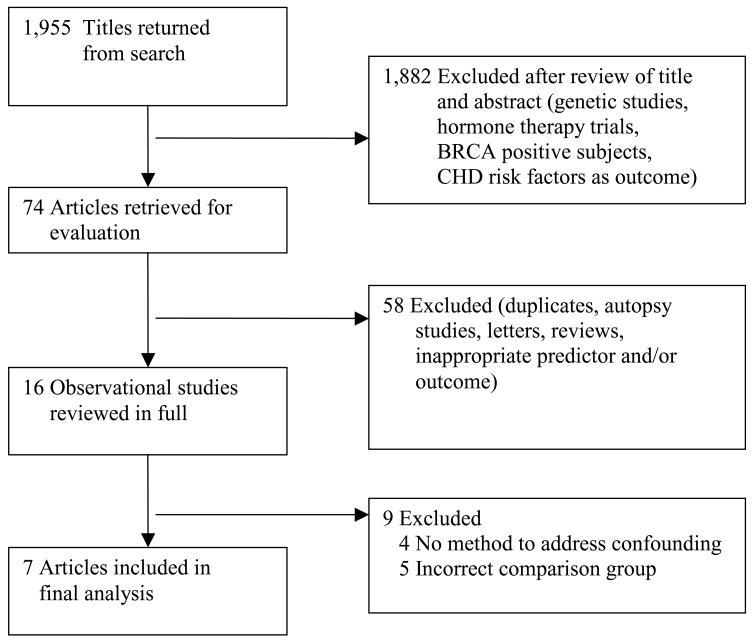
The searches identified 1,956 citations; 1,551 from PubMed, 399 from EMBASE, and 6 from bibliographies of retrieved articles. There were no additional citations obtained from ACOG meeting abstracts or consultation with the cardiologist. Fourteen articles required translation into English. Based on review of the title and abstract, 1,882 citations were excluded due to content or study design that did not meet inclusion criteria (Figure 1). Seventy four articles were retrieved for further review. Of these, 58 studies were excluded because they were duplicate publications, autopsy studies, letters to the editor, review articles, age at menopause was the predictor variable, atherosclerosis diagnosed on angiogram was the outcome, or review of the abstract in English precluded further consideration.
Figure 1.
Study selection
Sixteen potentially eligible studies were reviewed in full; nine were excluded from the final analysis (Table 1). The most common reason for exclusion was use of a comparison group that did not meet our inclusion criteria. Three studies compared CHD rates among women with BSO to population-based CHD rates.27–29 Two studies compared CHD rates in women who underwent BSO at different ages, but did not evaluate women with BSO compared to ovarian conservation.30, 31 Four observational studies were excluded because they did not use multivariate models or other methods to account for the potential effects of confounding in the analysis.32–35
Table 1.
Characteristics of Excluded Studies
| Source | Study Design | Outcome Measures | Comparison group | Subject Numbers | Reason for Exclusion | Population details |
|---|---|---|---|---|---|---|
| Beard et al25 1995 | Retrospective cohort | MIa, sudden unexplaine d death, or angina | Age-matched women living in same geographic area | BSOb: 457 ComparisonN/A |
Comparison group | Cohort from Rochester, Minnesota Years of BSO 1950–1979 30–55 years at time of BSO |
| Broeders26 1969 | Cross-sectional | Fatal coronary artery disease | General population of Dutch women | BSO: 906 | Comparison group | Dutch women Data collected 1940–62 |
| Casiglia et al29 2000 | Prospective cohort | Coronary artery disease: angina or MI | Natural menopause | BSO: 56 Natural menopause:205 |
No strategy for confounding | Italian cohort Recruited 1978 18–60 years at start of study |
| Dringoli et al30 1965 | Prospective cohort | Cardio-vascular disease | Natural menopause | BSO: 36 Natural menopause:24 |
No strategy for confounding | Cohort from Sienna hospital, Italy 27–58 years at enrollment |
| Falkeborn et al24 2000 | Case-cohort | MI | General population in local geographic region | BSO: 17,126 | Comparison group | Subjects from central Sweden Years of BSO: 1965–83 Mean age 45.9 years |
| Lokkegaard et al27 2005 | Prospective cohort | First ischemic heart disease | BSO at age>45 years | BSO: 504 | Comparison group | Cohort of Danish nurses Recruitment 1993 |
| Novotny et al31 1979 | Cross- sectional | Coronary disease | No BSO | BSO 18 No BSO 195 |
No strategy for confounding | German women identified by hospital records |
| Ossewaarde et al28 2005 | Prospective cohort | Fatal stroke, ischemic heart disease, or cerebro-vascular disease | BSO at age 50–54 | BSO: 12,134 | Comparison group | Dutch women enrolled in breast cancer screening study Recruitment 1974–1977 48–68 years at enrollment |
| Robinson et al32 1959 | Cross-sectional | Coronary heart disease: Angina or history of MI | Hysterectomy with ovarian conservation | BSO: 102 Hysterectomy with ovarian conservation:112 |
No strategy for confounding | Women in USA Data reviewed from medical records 1936–55 Age <45 at time of surgery |
Myocardial infarction
Bilateral salpingo-oophorectomy
Characteristics of the seven studies that met our complete inclusion criteria are outlined in Table 2. Five cohort studies, 1 cross-sectional study, and 1 case-control study were included. Self-reported BSO status was confirmed by formal review of medical records in all but 2 studies.23, 36 Six of the studies incorporated nonfatal myocardial infarction into a composite cardiovascular outcome with angina, heart failure, or fatal CHD 16, 17, 22, 26, 36, 37 One study used a cardiovascular disease index with 3 cardiac-related endpoints and stroke as the primary outcome.36
Table 2.
Characteristics of Included Studies
| Source | Study design | Ascertainment of BSOa |
Outcome measures |
Comparison group |
Subject numbers |
Population details | Follow-up | Outcome ascertainment |
Quality Rating |
|---|---|---|---|---|---|---|---|---|---|
| Colditz et al14 1987 | Prospective cohort | Self-report and review of medical records for subset of subjects | CHDb: Nonfatal MIc and death due to CHD | Premenopausal women | Total cohort: 116,258 For nonfatal MI outcomes:112,387 |
Nurses Health Study cohort, USA Recruited 1976 Excluded: history of angina or MI 98% Caucasian 30–55 years at enrollment |
6 years 5% lost to follow-up |
Review of medical records attempted for all subjects For death: autopsy or death certificate |
Intermediate |
| Gordon et al15 1978 | Prospective cohort | Self-report Medical records when available |
CHD: MI, angina, or death due to CHD | Hysterectomy with ovarian conservation, premenopausal and naturally menopausal women | Total cohort: 2,873 BSO: 398 |
Framingham cohort, USA Recruitment started 1948 29–62 years at enrollment |
24 years “Essentially complete follow-up” |
Self-report, physical examination, EKG, review of medical records for all cases | High |
| Howard et al33 2005 | Prospective cohort | Self-report | Cardiovascular disease: MI, stroke, CABG/PTCAd, or coronary death | Naturally menopausal women | Total cohort: 89,914 Hysterectomy: 36,865 Hysterectomy/BSO: 18,543 |
Women’s Health Initiative Observational Study, USA Recruited 1994–98 Excluded subjects: BSO alone, baseline CVDe, angina, CHFf, unknown ethnicity or BSO/hysterectomy status 50–79 years at enrollment |
Mean follow-up: 5.1 years | Self-report and review of all medical records | Intermediate |
| Luoto et al20 1995 | Cross-sectional | Self-report Medical records if available (78% of subjects) |
Angina and MI, or heart failure | No history of hysterectomy or BSO | BSO: 55 No history of gynecologic surgery: 3,562 |
Finnish women, random population sample Recruitment 1977–80 Year of hysterectomy 1944–79 30–95 years |
N/A | Self-report and exam by physician at entry to study | Poor |
| Palmer et al21 1993 | Case-control | Self-report | First nonfatal MI | Natural menopause | Cases: 858 Controls: 858 |
Participants from Massachusetts, community age-matched controls Data collected 1986–90 45–69 years at time of MI |
N/A | Inpatient hospital records confirmed by physician report for all cases | Intermediate |
| Ritterband et al34 1963 | Retrospective cohort | Medical records | Arteriosclerotic heart disease: MI, positive EKG, or angina | Hysterectomy with ovarian conservation | BSO: 267 Hysterectomy only: 385 |
New York, USA Subjects identified through hospital records All subjects ≥10 years from surgery |
N/A | Self-report And Physical examination | Intermediate |
| Svanberg35 1982 | Double-cohort study | Medical records | MI and angina, other CHD | No history of hysterectomy or BSO | BSO: 32 No history of hysterectomy or BSO: 32 |
Swedish women Comparison group: age-matched admitted to hospital for uterine prolapse surgery Outcome data collected 1978 15–30 years at BSO 52–84 years at data collection |
N/A | Self-report and exam by study physician | Intermediate |
Bilateral salpingo-oophorectomy
CHD
Myocardial infarction
Coronary artery bypass graft/percutaneous coronary angioplasty
Cardiovascular disease
Congestive heart failure
Only one study was determined to be of high quality.17 The two largest cohort studies were categorized as intermediate quality due to <10 years of follow-up time.16, 36 Three other studies were rated intermediate quality due to a study design other than a prospective cohort analysis.23, 26, 37 One study was deemed poor quality due to a cross-sectional design in which conclusions about causal inference are limited.22
Table 3 illustrates the diversity of analysis strategies used in the included studies. Three studies report CHD risk stratified by age alone or age at the time of surgery. 17, 26, 37. Four studies used multivariate models with a diverse range of covariates and measures of association across studies.16, 17, 22, 23 One study provided relative risks for myocardial infarction among women with BSO in each of three age strata compared to women with natural menopause at age ≥50 years. To control for age in the comparison, we include only the results for women with a history of BSO at age ≥50 years.23
Table 3.
Summary of Results from Included Studies
| Source | Strategy to Address Confounding | Results | |||
|---|---|---|---|---|---|
|
Comparison group: Hysterectomy with ovarian conservation | |||||
| Ritterband et al34 1963 | Stratification by multiple characteristics (age at surgery and exam, parity, history of estrogen use, or indication for surgery) | Percent of subjects (n) with arteriosclerotic heart disease | |||
| BSOa | Hysterectomy | p-value | |||
| Age 16–40 at surgery | |||||
| Age ≤50 at exam | |||||
| 5.8 (4) | 5.9 (7) | .98 | |||
| Age 51–65 at exam | |||||
| 8.7(9) | 9.3 (16) | .85 | |||
| Age 41–45 at surgery | |||||
| Age 513–65 at exam | |||||
| 9.6 (9) | 8.5 (8) | .79 | |||
|
| |||||
| Gordon et al15 1978 | Stratification by age | Cases/Person-years | |||
| Hysterectomy/BSO | Hysterectomy | p-value | |||
| Age 40–44 | |||||
| 3/636 | 1/316 | .79 | |||
| Age 45–49 | |||||
| 3/1176 | 3/500 | .32 | |||
| Age 50–54 | |||||
| 3/580 | 8/1702 | .85 | |||
|
| |||||
|
Comparison group: Natural menopause | |||||
| Multivariate model | Cases | Controls | RR (95% CI) | ||
| Palmer et al21 1993 | Covariates: age, smoking, drugs needed to treat diabetes, hypertension, or cholesterol, family history MIb <age 60, physical activity, BMI, history MIb <age 60, physical activity, BMI, coffee intake, alcohol use, education, spouse education, estrogen use, occupation, age at menarche, parity, age at first birth | Natural menopause age ≥50 | |||
| 237 | 294 | reference | |||
| BSO, age ≥50 | |||||
| 24 | 30 | 1.1 (.6,2,3) | |||
|
| |||||
| Howard et al34 | Multivariate models | HRc | 95% C.I. | ||
| Model 1: unadjusted | Model 1 | ||||
| 1.28 | 1.16, 1.42 | ||||
| Model 2 covariates: age, ethnicity, family history early MI, education, income | Model 2 | ||||
| 1.19 | 1.07,1.33 | ||||
| Model 3 covariates: model 2 covariates and waist, BMId, physical activity, dietary saturated fat | Model 3 | ||||
| 1.16 | 1.04, 1.30 | ||||
| Model 4: model 3 covariates and smoking, hypertension, diabetes, hypercholesteremia, DVTe ever, peripheral arterial disease ever | Model 4 | ||||
| 1.11 | 0.99, 1.24 | ||||
|
| |||||
| Gordon et al15 1978 | Stratification by age | Cases/Person-years | |||
| Hysterectomy/BSO | Natural menopause | p-value | |||
| Age 40–44 | |||||
| 3/636 | 5/1374 | .71 | |||
| Age 45–49 | |||||
| 3/1176 | 7/2336 | .85 | |||
| Age 50–54 | |||||
| 3/580 | 14/3138 | .89 | |||
|
| |||||
|
Comparison group: Premenopausal women | |||||
| Colditz14 1987 | Multivariate models Model 1 covariates: age, smoking Model 2 covariates: model 1 covariates and hypertension, hypercholesteremia, diabetes, parental history of MI‡ at age≤60, Quetelet’s index | RR | 95%C.I. | ||
| Never use of estrogen | |||||
| Model 1 | |||||
| 2.2 | 1.2,4.2 | ||||
| Model 2 | |||||
| 1.7 | 0.9, 8.6 | ||||
| Ever use of estrogen | |||||
| Model 1 | |||||
| 0.9 | 0.6, 1.6 | ||||
| Model 2 | |||||
| 0.7 | 0.4, 1.2 | ||||
|
| |||||
| Gordon et al15 1978 | Stratification by age | Cases/Person-years | |||
| Hysterectomy/BSO | Premenopausal | p-value | |||
| Age 40–44 | |||||
| 3/636 | 1/4518 | <.01 | |||
| Age 45–49 | |||||
| 3/1176 | 4/3266 | .36 | |||
| Age 50–54 | |||||
| 3/580 | 1/600 | .34 | |||
|
| |||||
|
Comparison group: No history of hysterectomy, menopausal status unknown | |||||
| Svanberg35 1982 | Stratification by age | Number of subjects with MI and angina | |||
| BSO | No previous surgery | ||||
| Age ≥60 | 2 | 0 (P<.05) | |||
| Age ≥65 | 5 | 0 (P<.05) | |||
| Age≥70 | 9 | 1 (P>.05) | |||
| Age≥75 | 11 | 3 (P>.05) | |||
|
| |||||
| Luoto et al20 1995 | Multivariate model | OR | 95% C.I. | ||
| Model 1 covariate: age | Model 1 | ||||
| 1.9 | 0.92, 3.92 | ||||
| Model 2 covariates: age, BMI, hormone use, total cholesterol, HDLf, triglycerides, glucose, smoking, alcohol use, education, interaction age/BMI | Model 2 | ||||
| 2.02 | 0.95, 4.26 | ||||
Bilateral salpingo-oophorectomy
Myocardial infarction
Hazard ration
Body mass index
Deep vein thrombosis
High density lipoprotein
Risk of CHD following BSO
Among the 7 included studies, 4 comparison groups were used to examine risk of CHD following BSO: 1) women who underwent hysterectomy and ovarian conservation, 2) naturally menopausal women, 3) premenopausal women, and 4) women with no history of hysterectomy or BSO but menopausal status was not reported in the study. Because each of these groups likely has a unique set of risk factors for CHD, we present results separately for each comparison group (Table 3).
Comparison group: Hysterectomy and ovarian conservation
Two studies report the incidence of CHD among women with BSO compared to ovarian conservation concomitant with hysterectomy (Table 3).17, 26 Ritterband et al reported no significant difference in the percent of subjects with arteriosclerotic heart disease by BSO status in multiple subgroup analyses by age at surgery, age at exam, parity, history of estrogen use, or indication for surgery.26 In the Framingham cohort study, there was no statistically significant increase in the annual incidence of CHD among women with a history of hysterectomy and BSO in all age categories from 40–54 years.
Comparison group: Naturally menopausal women
Three studies evaluated the risk of CHD following BSO compared to natural menopause (Table 3). In a case-control study of women with a first nonfatal MI, BSO at age ≥50 years was not identified as a significant predictor of CHD after controlling for cardiovascular risk factors and the use of estrogen.23 In a large observational study of nearly 90,000 women, there was a small increase in risk of cardiovascular disease in the BSO group in both unadjusted analysis and multivariate models (hazard ratios 1.11–1.23).36 There was no statistically significant difference in the incidence of CHD between groups in the Framingham cohort study.17
Comparison group: Premenopausal women
Two studies examined the risk of CHD among women who underwent hysterectomy and BSO compared to premenopausal women. In a large cohort of nurses who had never used estrogen, the relative risk for CHD was 2.2 (95% C.I. 1.2,4.2) after adjusting for age and smoking, 16 This risk was attenuated to 1.7 (95% CI 0.9, 8.6) after adjustment for additional CHD risk factors including hypertension and hyperlipidemia. There was not a statistically significant increased risk of CHD with BSO among women who had ever used estrogen. In the Framingham cohort study, the annual incidence of CHD was higher among women with a history of BSO compared to premenopausal women in the age strata 40–44 years, but not among women 45–54 years.17
Comparison group: Women with no history of hysterectomy or BSO, menopausal status unknown
In two studies, the comparison group was women with no history of hysterectomy or BSO but the menopausal status of the comparison group is not explicitly stated in the manuscript. In unadjusted analyses, Svanberg et al found a higher incidence of myocardial infarction and angina among women age 52–65 years with a history of BSO but this risk was not statistically significant among women age 66–75 years. Luoto et al reported no statistically significant increase in the odds ratio for angina, myocardial infarction, or heart failure for women with a BSO in multivariate models that controlled for multiple CHD risk factors..22
COMMENT
In this systematic review of the medical literature, there was inconclusive evidence to determine if BSO is an independent risk factor for CHD. There were no randomized trials of BSO and only 7 observational studies that met our inclusion criteria. Although 4 of these studies suggested some increased risk of CHD following BSO16, 17, 36, 37, the risk was statistically significant in only certain subgroups of women or in some multivariate models, but not the fully adjusted models. Three studies found no statistically significant increased risk of CHD among women who underwent BSO22, 23, 26 but these studies had several shortcomings in study design and/or statistical analysis.
The Framingham cohort was the only study that we rated high quality based on long term follow-up of a prospective cohort using adjudicated CHD outcomes. However, no tests of statistical significance are presented for the comparisons of interest; we calculated the p values in Table 3 based on raw data in the manuscript.17 The 6 other studies were rated intermediate or poor quality based on limited follow-up time,16, 36, or a weak study design.22, 23, 26, 37 These observational studies are susceptible to bias due to confounding that is best addressed through multivariate models that include baseline predictors of CHD risk. We only included studies that utilized one of three accepted method to address confounding (matching, stratification, or multivariate models). However, 2 studies were significantly limited because they stratified participants only by age, not according to other risk factors for CHD.17, 37 Only 4 studies used rigorous multivariate models to adjust for common cardiovascular risk factors such as age, smoking, and hypertension. Two of these studies found no statistically significant increased risk of CHD following BSO22, 23 and the other 2 studies reported a modest increased risk in the least adjusted models with relative risks from 1.19 to 2.2, but not in the fully adjusted models.16, 36 Although the most adjusted models may include variables in the causal pathway between BSO and CHD and thus hinder the ability of the study to detect BSO as a significant risk factor, exploring the potential for confounding with a diverse range of variables is critical given the inherent limitations of observational data.
A significant limitation of the majority of studies is the selected comparison group of women with no prior hysterectomy who were either naturally menopausal or premenopausal. Women who undergo hysterectomy have an increased prevalence of multiple cardiovascular risk factors and an increase risk of CHD events compared to women who do not undergo hysterectomy.22, 36, 38 Several mechanisms have been proposed to explain this association including postoperative changes in prostaglandin levels,39 hemoglobin and iron storage,40–42 insulin levels,43 or the demographic, socioeconomic, and lifestyle characteristics of women with high rates of hysterectomy.36 For instance, in a large cohort study, African-American and Hispanic women had higher rates of hysterectomy compared to white women, and women with a history of hysterectomy reported lower socioeconomic status, less physical activity, higher saturated fat intake, and higher rates of hypertension, diabetes, and hyperchloesteremia.36 Therefore, to assess the independent contribution of BSO to CHD risk, the appropriate comparison group is women who undergo hysterectomy with ovarian conservation rather than women who have not had a hysterectomy. In our review, only 2 studies used this comparison group; Neither of them found a statistically significant difference in the rate of CHD compared to hysterectomy with BSO. 26,17
Three additional issues limit the interpretation of the 7 included studies. First, age and menopausal status at the time of BSO likely contribute to the risk of CHD following surgery. Premature cessation of ovarian estrogen production following BSO in a young woman remote from menopause will have different cardiovascular effects then BSO in an older postmenopausal woman. However, only 2 studies stratify participants by age at BSO and none report menopausal status at the time of surgery. Ritterband reported equal rates of CHD between women with a history of BSO between age 16 and 40 years compared to those who underwent hysterectomy and ovarian conservation. However, this study had limited power to detect a difference in CHD rates due to a relatively small sample size. Second, postoperative estrogen use may influence the risk of CHD following BSO, but only 4 studies16, 22, 23, 26 included hormone use in the analysis and 3 of them did not report the duration of use. In the last 25 years, estrogen has frequently been prescribed for postoperative patients with rates as high as 85% among premenopausal women following BSO in some studies.44 Estrogen deficiency due to BSO has been postulated to increase risk of CHD. Therefore, understanding the effect of exogenous estrogen on the relationship between BSO and CHD is critical for counseling women regarding the risks and benefits of BSO. Finally, 2 studies did not validate self-report of hysterectomy and/or BSO using medical records which may lead to misclassification of these groups.23, 36 However, several studies have found high accuracy for the self-report of hysterectomy and/or BSO among various cohorts of women so the effect of these misclassifications is unlikely to be highly significant.45, 46
To our knowledge, this is the first systematic review that examines the association between BSO and risk of CHD events. Although a recent meta-analysis of multiple outcomes following BSO presented a summary relative risk of 2.62 for cardiovascular disease (95% CI, 1.15–1.35) among women who had a BSO,47 half of the six included studies presented risk factors for cardiovascular disease as the primary outcome including aortic calcification48 and stenotic vessels diagnosed at autopsy49 rather than CHD events. In addition, this meta-analysis includes two reports from the same cohort of women16, 50 and presents only the least-adjusted model from a large cohort study.16 Our review focuses on actual coronary events to avoid the limitation of surrogate markers that may not be appropriately validated for a population of middle-aged women following hysterectomy. In addition, we present data from all multivariate models within each study to demonstrate the effect of confounding variables on relevant measures of association.
The goal of this review is to provide accurate data to inform clinical practice for women considering the option of elective BSO concomitant with hysterectomy. Unfortunately, the current evidence on BSO and CHD precludes a definitive recommendation. We did not encounter any randomized trials that address this topic and, as described, the observational data had significant limitations. Nearly all of the studies used a comparison group that does not address the primary question of whether hysterectomy with BSO confers additional CHD risk compared to hysterectomy alone. In addition, the significant heterogeneity of multivariate models among the studies prohibited the formulation of a unified conclusion on the risk of BSO. While further observational research may enrich our understanding of CHD risk following BSO with use of appropriate comparison groups and statistically rigorous analyses, randomized trials will provide the highest quality data to answer this common clinical question.
Acknowledgments
Source of support: Dr. Jacoby is funded by the Women’s Reproductive Health Research Career Development Program (Grant K12 HD001262)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kesharvarz HKBMP. Hysterectomy surveillance-United States, 1994–99. MMWR CDC Surveill Summ. 2002;51:1–8. [Google Scholar]
- 2.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2007 doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Lepine LM, Hillis SP, Koonin LM, MPH, Morrow B, MA, Kierke BM, Wilcox LM., MPH Hysterectomy Surveillance-United States, 1980–1993. NNWRm Syrveukkabce Synnarues. 1997;46(SS4):1–15. [PubMed] [Google Scholar]
- 4.Pokras MA, Vicki Georges Hufnagel M. Hysterectomy in the United States, 1965–84. Am Jo of Public Health. 1988;78(7):852–53. doi: 10.2105/ajph.78.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Averette HE, Nguyen HN. The role of prophylactic oophorectomy in cancer prevention. Gynecol Oncol. 1994;55(3 Pt 2):S38–41. doi: 10.1006/gyno.1994.1339. [DOI] [PubMed] [Google Scholar]
- 6.Beard RW, Kennedy RG, Gangar KF, et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol. 1991;98(10):988–92. doi: 10.1111/j.1471-0528.1991.tb15336.x. [DOI] [PubMed] [Google Scholar]
- 7.Cronje WH, Vashisht A, Studd JW. Hysterectomy and bilateral oophorectomy for severe premenstrual syndrome. Hum Reprod. 2004;19(9):2152–5. doi: 10.1093/humrep/deh354. [DOI] [PubMed] [Google Scholar]
- 8.Namnoum AB, Hickman TN, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64(5):898–902. doi: 10.1016/s0015-0282(16)57899-6. [DOI] [PubMed] [Google Scholar]
- 9.Piver MS. Prophylactic Oophorectomy: Reducing the U.S. Death Rate from Epithelial Ovarian Cancer. A Continuing Debate Oncologist. 1996;1(5):326–30. [PubMed] [Google Scholar]
- 10.ACOG. ACOG practice bulletin 7. Clinical mangaement guidelines for obstetrican-gynecologists. 1999 [Google Scholar]
- 11.Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol. 2003;85(2–5):363–6. doi: 10.1016/s0960-0760(03)00204-8. [DOI] [PubMed] [Google Scholar]
- 12.Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(Suppl 4):S42–8. doi: 10.1016/s0015-0282(02)03001-7. [DOI] [PubMed] [Google Scholar]
- 13.Nathorst-Boos J, von Schoultz B. Psychological reactions and sexual life after hysterectomy with and without oophorectomy. Gynecol Obstet Invest. 1992;34(2):97–101. doi: 10.1159/000292735. [DOI] [PubMed] [Google Scholar]
- 14.Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy--effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14(4):283–93. doi: 10.3109/01674829309084451. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ, 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18(5):900–5. doi: 10.1359/jbmr.2003.18.5.900. [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 17.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study Ann Intern Med. 1978;89(2):157–61. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 18.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155(4):339–45. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–6. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen BK, Nilssen S, Heuch I, Kvale G. Does age at natural menopause affect mortality from ischemic heart disease? J Clin Epidemiol. 1997;50(4):475–9. doi: 10.1016/s0895-4356(96)00425-8. [DOI] [PubMed] [Google Scholar]
- 21.van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–8. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 22.Luoto T, Kaprio J, Reunanen A, Rutanen EM. Cardiovascular morbidity in relation to ovarian function after hysterectomy. Obstetrics and Gynecology. 1995;85(4):515–22. doi: 10.1016/0029-7844(94)00456-N. [DOI] [PubMed] [Google Scholar]
- 23.Palmer JR, Rosenberg L, Shapiro S. Reproductive factors and risk of myocardial infarction. Am J Epidemiol. 1992;136(4):408–16. doi: 10.1093/oxfordjournals.aje.a116513. [DOI] [PubMed] [Google Scholar]
- 24.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106(2):219–26. doi: 10.1097/01.AOG.0000167394.38215.56. [DOI] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Ritterband AB, Jaffe IA, Densen PM, Magagna JF, Reed E. Gonadal Function and the Development of Coronary Heart Disease. Circulation. 1963;27:237–51. doi: 10.1161/01.cir.27.2.237. [DOI] [PubMed] [Google Scholar]
- 27.Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53(8):832–7. doi: 10.1016/s0895-4356(00)00187-6. [DOI] [PubMed] [Google Scholar]
- 28.Beard CM, Crowson CS, Malkasian GD, O’Fallon WM, Melton LJ., III Cardiovascular disease and cancer risk following bilateral oophorectomy: A population-based study in Rochester, Minnesota. Journal of Women’s Health. 1995;4(2):133–41. [Google Scholar]
- 29.Broeders G. The significance of the endocrine function of the ovaries for the prevention of coronary sclerosis and the adverse effects of early castration. Ned Tijdschr Geneeskd. 1969;113(1):13–6. [PubMed] [Google Scholar]
- 30.Lokkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: influence of Hormone Therapy. Maturitas. 2006;53(2):226–33. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Ossewaarde ME, Bots ML, Verbeek AL, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–62. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 32.Casiglia E, Ginocchio G, Tikhonoff V, et al. Blood pressure and metabolic profile after surgical menopause: Comparison with fertile and naturally-menopausal women. Journal of Human Hypertension. 2000;14(12):799–805. doi: 10.1038/sj.jhh.1001113. [DOI] [PubMed] [Google Scholar]
- 33.Dringoli R, Cavallini F, Piccolomini A. Electrocardiographic and clinical study of ovariectomized women and natural menopause. Riv Ostet Ginecol. 1967;22(4):249–54. [PubMed] [Google Scholar]
- 34.Novotny A, Dvorak V, Kamarytova K. Late consequences of surgical castration in women (author’s transl) Schweiz Rundsch Med Prax. 1979;68(10):332–6. [PubMed] [Google Scholar]
- 35.Robinson RW, Higano N, Cohen WD. Increased incidence of coronary heart disease in women castrated prior to the menopause. Arch Intern Med. 1959;104:908–13. doi: 10.1001/archinte.1959.00270120064010. [DOI] [PubMed] [Google Scholar]
- 36.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111(12):1462–70. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 37.Svanberg L. Effects of estrogen deficiency in women castrated when young. Acta Obstet Gynecol Scand Suppl. 1981;106:11–5. doi: 10.3109/00016348209155324. [DOI] [PubMed] [Google Scholar]
- 38.Hsia J, Barad D, Margolis K, et al. Usefulness of prior hysterectomy as an independent predictor of Framingham risk score (The Women’s Health Initiative) Am J Cardiol. 2003;92(3):264–9. doi: 10.1016/s0002-9149(03)00621-0. [DOI] [PubMed] [Google Scholar]
- 39.Shelton JD. Prostacyclin from the uterus and woman’s cardiovascular advantage. Prostaglandins Leukot Med. 1982;8(5):459–66. [PubMed] [Google Scholar]
- 40.Kannel WB, Gordon T, Wolf PA, McNamara P. Hemoglobin and the risk of cerebral infarction: the Framingham Study. Stroke. 1972;3(4):409–20. doi: 10.1161/01.str.3.4.409. [DOI] [PubMed] [Google Scholar]
- 41.Naimark BJ, Ready AE, Sawatzky JA, et al. Serum ferritin and heart disease: the effect of moderate exercise on stored iron levels in postmenopausal women. Can J Cardiol. 1996;12(12):1253–7. [PubMed] [Google Scholar]
- 42.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):1293–4. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 43.Razay G, Heaton KW, Bolton CH. Coronary heart disease risk factors in relation to the menopause. Q J Med. 1992;85(307–308):889–96. [PubMed] [Google Scholar]
- 44.Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol. 1997;146(10):870–80. doi: 10.1093/oxfordjournals.aje.a009204. [DOI] [PubMed] [Google Scholar]
- 45.Brett KM, Madans JH. Hysterectomy use: the correspondence between self-reports and hospital records. Am J Public Health. 1994;84(10):1653–5. doi: 10.2105/ajph.84.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kritz-Silverstein D, Von Muhlen DG, Ganiats TG, Barrett-Connor E. Hysterectomy status, estrogen use and quality of life in older women: the Rancho Bernardo study. Qual Life Res. 2004;13(1):55–62. doi: 10.1023/B:QURE.0000015318.00707.53. [DOI] [PubMed] [Google Scholar]
- 47.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–79. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 48.Witteman JC, Grobbee DE, Kok FJ, Hofman A, Valkenburg HA. Increased risk of atherosclerosis in women after the menopause. Bmj. 1989;298(6674):642–4. doi: 10.1136/bmj.298.6674.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parrish HM, Carr CA, Hall DG, King TM. Time interval from castration in premenopausal women to development of excessive coronary atherosclerosis. Am J Obstet Gynecol. 1967;99(2):155–62. doi: 10.1016/0002-9378(67)90314-6. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol. 1981;139(1):47–51. doi: 10.1016/0002-9378(81)90410-5. [DOI] [PubMed] [Google Scholar]