Abstract
Myotonic dystrophy type 2 (DM2) is an autosomal dominantly inherited multisystemic disorder and a common cause of muscular dystrophy in adults. Although neuromuscular symptoms predominate, there is clinical and imaging evidence of cerebral involvement. We used voxel-based morphometry (VBM) based on T1-weighted magnetic resonance images to investigate brain morphology in 13 DM2 patients in comparison to 13 sex- and age-matched controls. Further, we employed novel computational surface-based methods that specifically assess callosal thickness. We found grey and white matter loss along cerebral midline structures in our patient group. Grey matter reductions were present in brainstem and adjacent hypothalamic and thalamic regions, while white matter was mainly reduced in corpus callosum. The reduced callosal size was highly significant and independently confirmed by different methods. Our data provide first evidence for grey and white matter loss along brain midline structures in DM2 patients. The reduced size of the corpus callosum further extends the spectrum of white matter changes in DM2 and may represent the morphological substrate of neuropsychological abnormalities previously described in this disorder.
Keywords: DM2, brainstem, corpus callosum, VBM, morphometry
Introduction
Myotonic dystrophy type 2 (DM2) is an autosomal dominantly inherited multisystemic disorder that shares RNA pathogenesis with myotonic dystrophy type 1 (DM1). Although neuromuscular symptoms predominate, there is clinical, neuropathological, and imaging evidence of cerebral involvement in DM2 [9–11, 17, 19, 24].
Several magnetic resonance imaging (MRI)-based morphometric methods have been used to study structural brain abnormalities in neurodegenerative disorders. Initial morphometric MRI studies used a region-of-interest (ROI)-guided approach measuring areas or volumes of manually segmented brain regions. In these studies, an inherent bias is introduced by selecting a limited number of brain regions for study. In addition, the segmentation procedure is often arbitrary and poorly reproducible. Therefore, new techniques for a voxel-wise comparison of anatomical data were developed including voxel-based morphometry (VBM) [2, 6]. These techniques allow an unbiased and comprehensive assessment of brain morphology. VBM is based on high-resolution T1-weighted images and automated segmentation of brain tissue into grey and white matter as it compares the density (or regional volume) of grey or white matter between different groups of individuals.
Recently, VBM was employed in DM1 patients revealing grey matter reduction in various cortical regions. In addition, diffusion tensor imaging(DTI)showed changes in corpus callosum subregions associated with cortical volume loss in corresponding regions [1, 18].
In the present study, we used VBM to analyse brain morphology in DM2 patients. Since hypersomnia is a prominent sign of cerebral involvement in DM2, we additionally compared patients with and without hypersomnia. Furthermore, we applied a novel computational surface-based method to specifically evaluate the thickness of the corpus callosum.
Patients and methods
The study was performed in 13 consecutive genetically confirmed DM2 patients (5 women, 8 men, age 53.3 ± 12.0 years, disease duration 12.0 ± 8.8 years) that were compared to 13 age- and sex-matched healthy controls (5 women, 8 men, age 53.5 ± 10.2 years). None of the patients had a past medical history of other neuromuscular or central nervous system disorders. One male patient harboured caryotype XYY and showed mild cognitive impairment. Importantly, previous morphometric analyses did not reveal differences between XYY subjects and healthy male controls [25]. Five patients complained about excessive daytime sleepiness. Eleven patients showed patchy to confluent subcortical and periventricular white matter lesions in brain MRI T2-multi-echo and fluid-attenuated inversion recovery (FLAIR) sequence analysis as described previously [11].
The study protocol was approved by the local ethics committee. Informed written consent was obtained from all participants.
Data acquisition
Brain MRI measurements were performed using a 1.5-T scanner (Siemens Symphony, Siemens AG, Erlangen, Germany) with the standard head coil. The MRI protocol consisted of a T1-weighted, MPRAGE sequence (TR 11.08 ms, TE 4.3 ms, FA 15°, FOV 230 mm, 256 × 256 acquisition matrix), yielding 200 sagittal slices and a voxel size of 0.9 × 0.9 × 0.9 mm3.
VBM analyses
Data were preprocessed as described elsewhere [23] applying the “optimized VBM protocol” [6] and using SPM2 (statistical parametric mapping; http://www.fil.ion.ucl.ac.uk/spm/software/spm2). This procedure optimizes the normalization for the explored tissue type by the use of tissue-specific templates. After defining the anterior commissure in each image as the origin of the individual stereotaxic space, we reoriented all images into the axial view. Reoriented T1-weighted images were segmented into grey matter and white matter probability maps. These maps were normalised to a tissue-specific template and the thereby estimated transformations were applied to the original T1 dataset, which was then normalised, resampled to an 1.5 × 1.5 × 1.5 mm3 voxel-size, segmented and smoothed with a 12-mm Gaussian kernel. Unmodulated data, rather than modulated data, were used to be more sensitive with respect to density changes rather than to pure volume changes.
The preprocessed unmodulated grey and whiter matter components were compared between DM2 patients and healthy controls using a 2-sample t-test. The statistical analysis was controlled for global differences by including the overall brain volume as a confounding covariate in the design matrix. All results are based on the contrast between the two groups at a FDR-corrected voxel threshold of p < 0.05 and a corrected cluster threshold of p < 0.05. Given that 38 % of our DM2 patients complained about increased daytime sleepiness, we additionally compared grey and white matter components between five patients with hypersomnia and eight patients without hypersomnia using statistical criteria as described above.
Brain volume quantification
As a widely used quantitative marker for global brain atrophy [10, 20], the brain parenchymal fraction (BPF) was determined using an automated analysis technique written in MATLAB 7.0.1® (The MathWorks Inc., Natick, MA, USA) based on the segmented images, obtained by VBM procedure. BPF is defined as the ratio of brain parenchymal tissue volume (e.g., grey and white matter) to the intracranial volume (e.g., the total volume within the brain surface contour). The ratios of grey and white matter fractions to the intracranial volume were calculated separately and compared between DM2 patients and healthy controls using a 2-sample t-test.
Callosal thickness analysis
The corpus callosum was outlined in normalised data. A systematic overview of the basic steps in the measurement of callosal thickness is provided elsewhere [12]. Briefly, one rater (E.L.) manually outlined upper and lower callosal boundaries in the midsagittal section of each brain. Subsequently, the spatial average from 100 equidistant surface points representing the upper and lower traces was calculated by creating a new midline segment, also consisting of 100 equidistant points. Finally, the distances between 100 corresponding surface points from this new midline to callosal upper and lower segments were quantified. Since this is a highly operator dependent technique, the corpus callosum was countered twice in six different randomly selected brains by the same rater, achieving an intra-rater reliability of r = 0.99.
Callosal thickness values were compared between patients with DM2 and healthy controls using 2-sample t-tests. Given that statistical tests were made at hundreds of callosal surface points and adjacent data points are highly correlated, permutation testing was employed to control for multiple comparisons, using a threshold of p = 0.05. For this purpose, callosal sections were randomly assigned to either patient or control group 100,000 times, and a new statistical test was performed at each callosal surface point for each random assignment. The number of significant results from these randomizations was compared to the number of significant results in the true assignment to produce a corrected overall significance value for the uncorrected statistical maps.
To evaluate a possible influence of the inclusion of one patient with XYY caryotype on our examination results, we performed additional VBM and callosal thickness analyses as well as brain volume quantification excluding this patient from our patient group.
Results
VBM
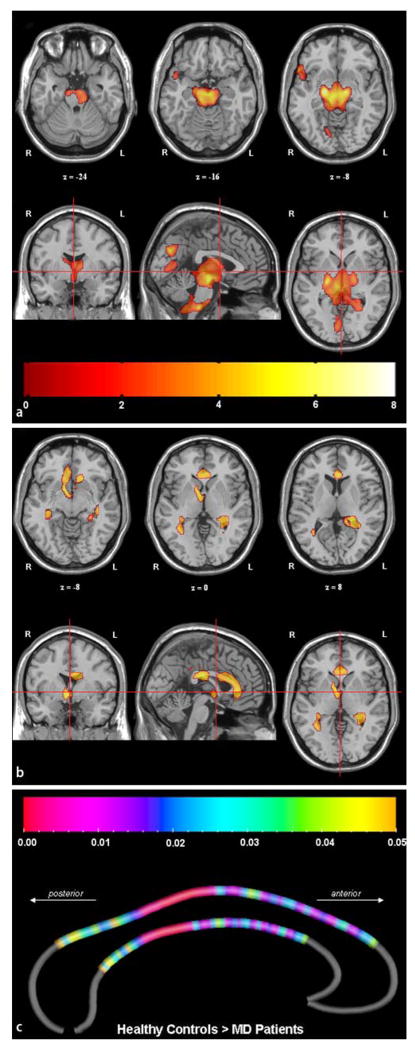
Compared to controls, grey matter density in DM2 patients was bilaterally reduced in mesencephalon and adjacent midline brain regions. This reduction extended caudally into pons and lower brainstem and cranially into hypothalamus, thalamus, and internal pallidum. Additional smaller clusters were found in the right lingual gyrus and inferior frontal gyrus, extending into the anterior part of the superior temporal gyrus (Table 1, Fig. 1a). The inverted comparison revealed no increase of grey matter density in DM2 patients.
Table 1.
Voxel size 1.5 × 1.5 × 1.5 mm3. An FDR-corrected voxel threshold of p < 0.05, with a cluster threshold of p < 0.05 was used. The coordinates refer to the Talairach reference space (Transformation of coordinates from the MNI to the Talairach reference space was performed using www.mrc-cbu.cam.ac.uk)
| cluster-level size | voxel-level |
Coordinates |
Side | Area | |||
|---|---|---|---|---|---|---|---|
| p (FDR-cor) | T-value | X | Y | Z | |||
| Grey matter (decrease) | |||||||
| 22472 | 0.004 | 7.68 | 18 | −12 | −7 | Right | midbrain (thalamus, int. pallidum) |
| 0.004 | 6.68 | −14 | −15 | −7 | Left | midbrain (thalamus, int. pallidum) | |
| 2430 | 0.004 | 5.62 | 2 | −70 | 33 | Right | lingual gyrus |
| 579 | 0.007 | 4.82 | 56 | 20 | −8 | Right | inferior frontal gyrus |
| 0.020 | 3.80 | 47 | 11 | −12 | Right | superior temporal gyrus | |
| White matter (decrease) | |||||||
| 999 | 0.042 | 5.56 | 36 | −23 | −22 | Right | subcortical to fusiform gyrus |
| 11745 | 0.042 | 5.35 | 8 | 7 | −7 | Right | internal capsule |
| 0.042 | 4.89 | −9 | −19 | 25 | Medial | corpus callosum | |
Fig. 1.
All coloured regions reflect grey matter loss (a), white matter loss (b), and callosal thickness reduction (c) in 13 DM2 patients compared to 13 healthy individuals. In a, b, images are in the neuroradiological orientation (the left side of the images refers to the right side of the brain; L = left, R = right). The z-coordinates refer to the Talairach reference space, the cross hairs to the anterior commissure. The colour bar in a and b represents the corresponding T-values. In c the colour bar represents the corresponding p-values
White matter density in DM2 patients was reduced in corpus callosum, right internal capsule, and bilaterally in regions subcortical to the right fusiform gyrus (Table 1, Fig. 1b). The inverted comparison revealed no increase of white matter density in DM2 patients. We did not detect any significant differences with respect to grey and white matter density when comparing DM2 patients with and without hypersomnia (data not shown).
Results of grey matter VBM analyses were not influenced by including the XYY caryotype patient in our patient group. White matter changes had a similar pattern but results were less significant after excluding this patient. However, excluding the XYY caryotype patient had no more impact on our results than excluding any other subject of our patient group (data not shown).
Brain volume quantification
Compared to controls (mean BPF 0.66 ± 0.04), the BPF in DM2 patients (0.62 ± 0.04) was significantly decreased (p = 0.03). This difference was mainly caused by differences in the white matter fraction in patients compared to controls (0.25 ± 0.03 vs. 0.28 ± 0.02; p = 0.01), whereas the grey matter fraction did not differ significantly (0.37 ± 0.02 vs. 0.38 ± 0.03; p = 0.17).
Callosal thickness analysis
Callosal regions were significantly thinner in DM2 patients across the whole callosal body, most pronounced in the posterior half of the callosal middle third and sparing only the most extreme anterior and posterior callosal section (Fig. 1c). We detected no region in which callosal thickness was increased in DM2 patients compared to controls (map not shown). Permutation tests were highly significant for the comparison of callosal thickness between DM2 patients and healthy controls (p = 0.005), indicating that the observed disease effects did not occur by chance.
The exclusion of the XYY caryotype patient did not change the results of brain volume quantification and callosal thickness analysis.
Discussion
Our imaging data provide first evidence for grey and white matter loss along brain midline structures in DM2 patients. By demonstrating changes in brainstem and corpus callosum our results extend the previously described cerebral involvement in DM2 [9–11, 15–17, 19, 24]. Earlier findings of global atrophy in DM2 patients, as estimated with the BPF, were confirmed by our data [10].
It is intriguing to speculate that structural brainstem, thalamic, and hypothalamic changes may be associated with increased daytime sleepiness and apathy previously observed in DM2 and reported by five of our patients. We did not detect differences between our patients with hypersomnia and those without, albeit these subgroups were too small to allow a valid and reliable conclusion. While systematic examination of fatigue is still missing in DM2, existing data on DM1 points towards a high prevalence of sleep abnormalities [5]. An association with the hypocretin neurotransmission system was suggested; however data regarding levels of hypocretin-1, a hypothalamic neuropeptide essential in the regulation of the sleep/wakefulness cycle and vigilance, are still discrepant at present [3, 14]. For ethical reasons, lumbar puncture and analysis of cerebrospinal fluid levels of hypocretin-1 could not be performed in our DM2 patients.
Interestingly, intraneuronal accumulation of micro-tubuli-associated tau protein present in DM1 brains was recently described in a DM2 brain [15, 22]. The tau pathology in this case was not only observed in the hippocampus, entorhinal cortex, and prefrontal areas, but also in several infratentorial regions including locus coeruleus, substantia nigra, periaqueductal grey substance, and oculomotor nuclei. These tau-positive regions partly overlap with the areas of grey matter loss identified in the present study.
White matter alterations, mostly depicted on T2- or FLAIR-weighted images, are well known in DM2 patients [9, 11, 19]. The reductions, seen in the corpus callosum on T1-weighted images, further extend the spectrum of white matter involvement in DM2. Cortical laterality interacts with the size of corpus callosum and vice versa [26]. A putative role of age and sex on callosal size is still debated in healthy controls. However, those studies are usually performed in larger samples [7, 13]. Nevertheless, our patient and control group were matched for age and sex. This ensures that our observations are not biased by these factors. Thus, the reduced callosal size in DM2 patients cannot be attributed to these factors. Up to now, atrophy or hypoplasia of the corpus callosum have only been described in DM1 [8]. As in the congenital form of DM1, the reduced callosal size in DM2 may be due to a developmental defect. Alternatively, it may be a consequence of Wallerian degeneration secondary to subcortical and periventricular white matter lesions as previously described in multiple sclerosis, where a correlation between the axonal loss in the corpus callosum and the volume of white matter lesions was found [4]. Interestingly, a recent DTI analysis in DM1 described a correlation between reduced fractional anisotropy along the corpus callosum and cortical volume loss in corresponding regions [18]. However, analogous data in DM2 is missing to date.
In DM1, a clinical association between a reduced size of the anterior part of the corpus callosum and excessive daytime sleepiness was previously suggested [5]. It is conceivable that white matter changes and reduced callosal size represent a morphological substrate of fatigue, cognitive dysfunction, and neuropsychological abnormalities in DM2 as well [16, 19, 21].
Further studies are already in progress to elucidate the temporal evolution of cerebral structural abnormalities and to clarify the relationship between brain morphological changes and the clinical phenotype in DM2. The additional implementation of DTI may further increase the sensitivity to detect white matter changes in DM1 and DM2 patients.
Acknowledgments
The authors thank Wolfram Kress, Institute of Human Molecular Genetics, University of Würzburg, Germany, for performing the genetic tests. The scientific work of E.L., P.M.T., and A.W.T. was made possible by Grant Numbers P41 RR013642 and M01 RR000865 from the National Center for Research Resources (NCRR). Algorithm development was partially supported by grants from the National Institute for Biomedical Imaging and Bioengineering, the National Center for Research Resources, and the National Institute on Aging (EB01651, RR019771, AG016570 to PMT). Additional support was provided by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB). Information on the National Centers for Biomedical Computing can be obtained from http://nihroad-map.nih.gov/bioinformatics.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Antonini G, Mainero C, Romano A, Giubilei F, Ceschin V, Gragnani F, Morino S, Fiorelli M, Soscia F, Di Pasquale A, Caramia F. Cerebral atrophy in myotonic dystrophy: a voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2004;75:1611–1613. doi: 10.1136/jnnp.2003.032417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 3.Ciafaloni E, Mignot E, Sansone V, Hilbert JE, Lin L, Lin X, Liu LC, Pigeon WR, Perlis ML, Thornton CA. The hypocretin neurotransmission system in myotonic dystrophy type 1. Neurology. 2008;70:226–230. doi: 10.1212/01.wnl.0000296827.20167.98. [DOI] [PubMed] [Google Scholar]
- 4.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain. 2000;123:1845–1849. doi: 10.1093/brain/123.9.1845. [DOI] [PubMed] [Google Scholar]
- 5.Giubilei F, Antonini G, Bastianello S, Morino S, Paolillo A, Fiorelli M, Ferretti C, Fieschi C. Excessive daytime sleepiness in myotonic dystrophy. J Neurol Sci. 1999;164:60–63. doi: 10.1016/s0022-510x(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 6.Good CD, Johnsrude IS, Ashburner J, Henson N, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 7.Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T, Taxama M, Miyazaki M, Miyazaki M, Murakawa K, Kawai H, Nishitani H, Kuroda Y. Neuroimaging study of myotonic dystrophy. I. Magnetic resonance imaging of the brain. Brain Dev. 1995;17:24–27. doi: 10.1016/0387-7604(94)00096-g. [DOI] [PubMed] [Google Scholar]
- 9.Hund E, Jansen O, Koch MC, Ricker K, Fogel W, Niedermaier N, Otto M, Kuhn E, Meinck HM. Proximal myotonic myopathy with MRI white matter abnormalities of the brain. Neurology. 1997;48:33–37. doi: 10.1212/wnl.48.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Kassubek J, Juengling FD, Hoffmann S, Rosenbohm A, Kurt A, Jurkat-Rott K, Steinbach P, Wolf M, Ludolph AC, Lehmann-Horn F, Lerche H, Weber YG. Quantification of brain atrophy in patients with myotonic dystrophy and proximal myotonic myopathy: a controlled 3-dimensional magnetic resonance imaging study. Neurosci Lett. 2003;348:73–76. doi: 10.1016/s0304-3940(03)00740-7. [DOI] [PubMed] [Google Scholar]
- 11.Kornblum C, Reul J, Kress W, Grothe C, Amanatidis N, Klockgether T, Schroder R. Cranial magnetic resonance imaging in genetically proven myotonic dystrophy type 1 and 2. J Neurol. 2004;251:710–714. doi: 10.1007/s00415-004-0408-1. [DOI] [PubMed] [Google Scholar]
- 12.Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- 13.Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, Mazziotta JC, Toga AW. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Rodriguez JE, Lin L, Iranzo A, Genis D, Marti MJ, Santamaria J, Mignot E. Decreased hypocretin-1 (Orexin-A) levels in the cerebrospinal fluid of patients with myotonic dystrophy and excessive daytime sleepiness. Sleep. 2003;26:287–290. doi: 10.1093/sleep/26.3.287. [DOI] [PubMed] [Google Scholar]
- 15.Maurage CA, Udd B, Ruchoux MM, Vermersch P, Kalimo H, Krahe R, Delacourte A, Sergeant N. Similar brain tau pathology in DM2/PROMM and DM1/Steinert disease. Neurology. 2005;65:1636–1638. doi: 10.1212/01.wnl.0000184585.93864.4e. [DOI] [PubMed] [Google Scholar]
- 16.Meola G, Sansone V, Perani D, Scarone S, Cappa S, Dragoni C, Cattaneo E, Cotelli M, Gobbo C, Fazio F, Siciliano G, Mancuso M, Vitelli E, Zhang S, Krahe R, Moxley RT. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2) Neuromuscul Disord. 2003;13:813–821. doi: 10.1016/s0960-8966(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 17.Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve. 2007;36:294–306. doi: 10.1002/mus.20800. [DOI] [PubMed] [Google Scholar]
- 18.Ota M, Sato N, Ohya Y, Aoki Y, Mizukami K, Mori T, Asada T. Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neurosci Lett. 2006;407:234–239. doi: 10.1016/j.neulet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 19.Romeo V, Palmieri A, Squarzanti F, Ferrati C, Zucchetta P, Manara R, Trevisan C, Pegoraro E, Angelini C. Brain involvement in myotonic dystrophy type 2. Neuromuscul Disord. 2007;17(9–10):858. [Google Scholar]
- 20.Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53:1698–1704. doi: 10.1212/wnl.53.8.1698. [DOI] [PubMed] [Google Scholar]
- 21.Sansone V, Gandossini S, Cotelli M, Calabria M, Zanetti O, Meola G. Cognitive impairment in adult myotonic dystrophies: a longitudinal study. Neurol Sci. 2007;28:9–15. doi: 10.1007/s10072-007-0742-z. [DOI] [PubMed] [Google Scholar]
- 22.Sergeant N, Sablonniere B, Schraen-Maschke S, Ghestem A, Maurage CA, Wattez A, Vermersch P, Delacourte A. Dysregulation of human brain microtubule-associated tau mRNA maturation in myotonic dystrophy type 1. Hum Mol Genet. 2001;10:2143–2155. doi: 10.1093/hmg/10.19.2143. [DOI] [PubMed] [Google Scholar]
- 23.Specht K, Minnerop M, Muller-Hubenthal J, Klockgether T. Voxel-based analysis of multiple-system atrophy of cerebellar type: complementary results by combining voxel-based morphometry and voxel-based relaxometry. NeuroImage. 2005;25:287–293. doi: 10.1016/j.neuroimage.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Vielhaber S, Jakubiczka S, Gaul C, Schoenfeld MA, Debska-Vielhaber G, Zierz S, Heinze HJ, Niessen HG, Kaufmann Jl. Brain (1)H magnetic resonance spectroscopic differences in myotonic dystrophy type 2 and type 1. Muscle Nerve. 2006;34:145–152. doi: 10.1002/mus.20565. [DOI] [PubMed] [Google Scholar]
- 25.Warwick MM, Doody GA, Lawrie SM, Kestelman JN, Best JJ, Johnstone EC. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J Neurol Neurosurg Psychiatry. 1999;66:628–632. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. The association of macro- and microstructure of the corpus callosum and language lateralisation. Brain Lang. 2006;97:80–90. doi: 10.1016/j.bandl.2005.07.133. [DOI] [PubMed] [Google Scholar]