Abstract
The authors used surface-based anatomic mapping to detect features of hippocampal anatomy that correlated with surgical outcomes in patients undergoing surgery for mesial temporal lobe epilepsy with hippocampal sclerosis. Compared with a seizure-free group, hippocampal profiles for the non–seizure-free group had greater diffuse ipsilateral atrophy and more region-specific contralateral atrophy in the anterior, lateral hippocampus. These atrophic regions may indicate areas of increased epileptogenicity, contributing to poorer surgical outcomes.
Even though patients with mesial temporal lobe epilepsy (MTLE) and hippocampal sclerosis (HS) are more likely to gain seizure control with anteromesial temporal resection than continued antiepileptic drug treatment, not all patients are rendered seizure free after surgery.1 Many favorable surgical prognostic factors actually predict HS. Only a personal history of status epilepticus has been associated with poorer surgical outcome.2 Therefore, few clinical characteristics have surgical prognostic value.
MRI volumetric analyses of HS cannot be used to predict surgical outcome consistently.3 We recently developed an anatomic surface modeling approach combined with surface-based statistics to compare hippocampal anatomy in patients seizure free (SF) postoperatively with those having continued seizures (NSF). These mapping techniques were applied to isolate profiles of hippocampal deficits associated with better or poorer outcomes after surgery.
Methods
Using the UCLA adult epilepsy surgical database (1993 to 2002), we reviewed 40 patients who met all of the following criteria: 1) preoperative MRI showing unilateral or asymmetric hippocampal atrophy; 2) history and semiology compatible with MTLE; 3) noninvasive ictal EEG revealing at least three seizures with unilateral temporal lobe onset, concordant to the side of hippocampal atrophy; and 4) surgical pathology showing HS. Patients with MRI or pathologic abnormalities other than HS were excluded. No patient required invasive intracranial EEG monitoring because the epileptogenic zone was well localized noninvasively. All patients underwent the same en bloc anteromesial temporal resection with same resection volume, as described by Spencer et al.,4 by a single neurosurgeon. Intraoperative electrocorticography was not used to tailor the extent of resection. A nurse conducted postoperative telephone interviews every 2 to 3 months for at least 2 years to determine patients’ seizure frequency. Thirty patients were free of disabling seizures except for auras in isolation, and 10 patients continued to have seizures. Three NSF patients had 1 to 2 seizures per year, and seven had 0.5 to 3 seizures per month. All patients received maintenance antiepileptic drug therapy during the follow-up period.
Statistical analysis of clinical data and hippocampal volumes
Fisher’s exact test and unpaired t test (two-tailed p values) were used to compare clinical characteristics between SF and NSF patients. Differences in hippocampal volumes between the two surgical groups and published norms from our laboratory were analyzed with unpaired t tests.5 The clinical characteristics listed in the table were assessed as potential predictors of hippocampal volumes simultaneously with multiple linear regression.
MRI scans
High-resolution MRI images were acquired on a GE Signa 1.5-T clinical scanner (Milwaukee, WI) at UCLA. Three-dimensional T1-weighted coronal images were acquired using spoiled gradient recalled acquisition in the steady state with the following acquisition parameters: acquisition matrix 256 × 256, TR/TE 40/9 milliseconds, flip angle 35 degrees, number of excitations 1, field of view 24 cm, and contiguous 1.8-mm-thick slices covering the entire brain. We applied an anatomic surface modeling approach to MR images to visualize regions of hippocampal atrophy. These procedures are described in detail in other reports5,6 and summarized in figure 1.
Figure 1.
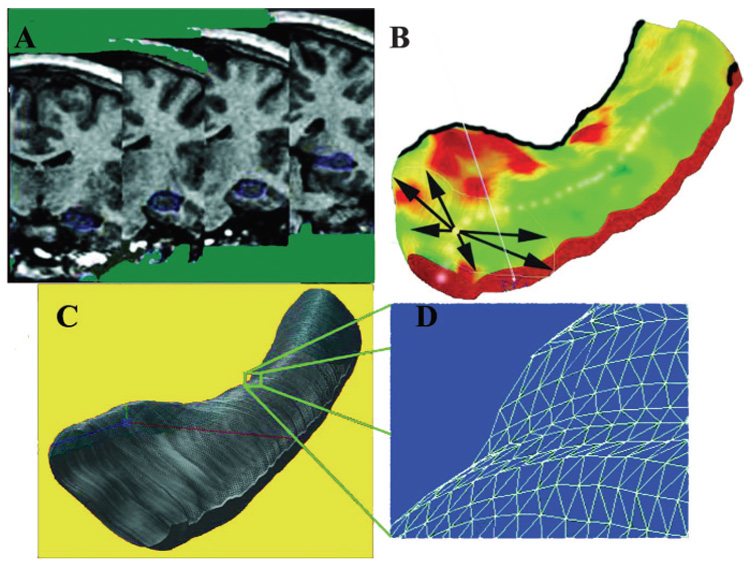
Steps involved in three-dimensional hippocampal modeling. Each individual’s hippocampus is traced in consecutive coronal MRI sections (A) and converted to a three-dimensional parametric surface (B) in which the radial size of the hippocampus is measured from a centerline and plotted in color on the surface, to index radial atrophy. These meshes are averaged across subjects (C) and atrophy relative to the contralateral hippocampus in each surgical outcome group (seizure free and not seizure free) and across the two surgical outcome groups is computed at each surface grid point (D)
Results
Clinical characteristics and hippocampal volume
There was no significant difference in clinical characteristics between the two surgical outcome groups (see table). Simultaneous multiple linear regression of the eight clinical predictors listed in the table and ipsilateral hippocampal volume showed that only duration of epilepsy was negatively correlated with ipsilateral hippocampal volume (p < 0.05), and none correlated with contralateral hippocampal volume (figure E-1, available on the Neurology Web site at www.neurology.org).
Table.
Clinical characteristics and hippocampal volume
| Clinical features and hippocampal volume | Seizure free, n = 30 | Not seizure free, n = 10 | p Value |
|---|---|---|---|
| Age at MRI scan, years | 31.4 ± 1.6 | 37.0 ± 4.2 | 0.23§ |
| Sex, M/F | 14/16 | 1/9 | 0.06║ |
| Side of seizure onset, R/L | 15/15 | 6/4 | 0.72║ |
| Age at first seizure onset, years | 12.3 ± 1.6 | 11.8 ± 4.3 | 0.92§ |
| Duration of epilepsy, years | 19.6 ± 1.8 | 25.0 ± 3.2 | 0.16§ |
| Seizure frequency per month | 9.0 ± 1.1 | 12.4 ± 3.9 | 0.43§ |
| History of febrile seizures, %‡ | 40 | 20 | 0.44║ |
| History of CNS infection, %‡ | 13 | 20 | 0.63║ |
| Ipsilateral hippocampal volume† | 1,348 ± 282 mm3 | 889 ± 305 mm3 | <0.0009§ |
| Contralateral hippocampal volume† | 2,293 ± 454 mm3 | 1,470 ± 181 mm3 | <0.00001§ |
Data are shown as mean ± SEM.
Data are shown as mean ± SD of the mean.
Percent of total subjects.
Student’s t test.
Fisher exact test.
R = right; L = left.
Compared with the SF group, the NSF group had significantly smaller bilateral mean hippocampal volumes (see table). Greater ipsilateral hippocampal atrophy pattern in the NSF group was consistent in our population. Using the same anatomic boundary and image analysis technique, we recently showed that a healthy adult control population —with similar age and sex to this epilepsy group—had no hippocampal volume asymmetry (L = 2,563 ± 327 mm3 and R = 2,702 ± 425 mm3; paired t test, p > 0.1).5 In the current study, the degree of asymmetry between the two hippocampi in both outcome groups was on average much greater than these normal controls (SF vs normal, p < 0.0001; NSF vs normal, p = 0.005). The NSF group showed bilateral volume deficits compared with these norms (average normal = 2,632 ± 392 mm3; ipsilateral NSF = 889 ± 305 mm3; contralateral NSF = 1,470 ± 181 mm3; p < 0.0001).
Hippocampal atrophy profiles
The average radial volumes of both hippocampi were compared between SF and NSF groups. Importantly, ipsilateral and contralateral maps were both able to distinguish between SF and NSF outcome patients. The NSF group had greater ipsilateral hippocampal atrophy along the lateral and medial surface, with relatively uniform involvement of the head, body, and tail (p < 0.01 by permutation; see figure 2, A), and greater contralateral atrophy involving the head and lateral body of the hippocampus, with maximal atrophy in the anterior hippocampus (p < 0.002 by permutation; see figure 2, B). Only the contralateral hippocampi showed more regionspecific differences between the outcome groups.
Figure 2.
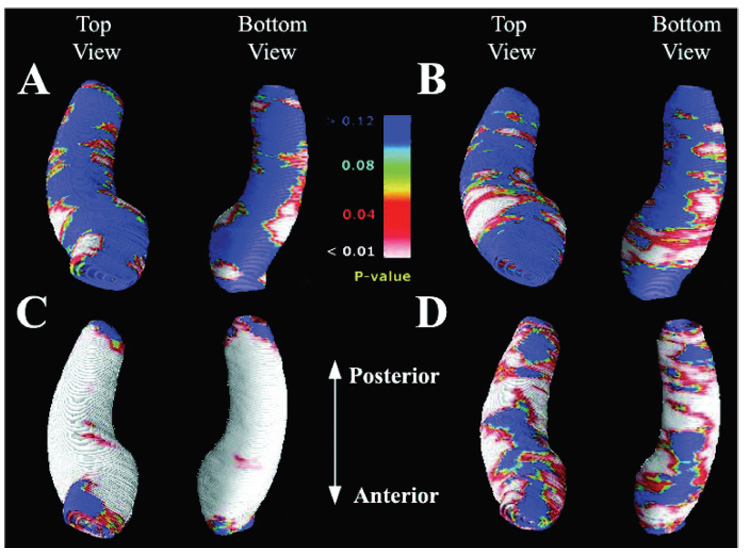
Maps identifying regions where seizure-free (SF) and non– seizure-free (NSF) surgical outcome groups differ in their degree of atrophy (A and B) and regions of hippocampal asymmetry in each surgical outcome group (C and D). Group difference maps show mean hippocampal volume differences ipsilateral (A) and contralateral (B) to the side of seizure onset. Areas of significant atrophy between the two surgical outcome groups are plotted as a map of p values. The NSF groups show significantly greater diffuse atrophy in the ipsilateral hippocampus (A), whereas the contralateral side shows more region-specific atrophy pattern (B). Maximal atrophy is seen in the anterior and lateral aspects of the contralateral hippocampus. The average distribution of atrophy was computed for patients with seizure-free postsurgical outcome (C) and those who continued to have seizures (D) by directly comparing the ipsilateral to the contralateral hippocampus. Areas of significant asymmetry are visualized as a map of p values. Both SF and NSF groups showed severe diffuse deficits along the entire hippocampus. The overall asymmetry pattern suggests that the NSF group (D) had a lesser degree of asymmetry when compared with the SF group (C). However, the anterior to posterior distribution of the asymmetry pattern does not differentiate the two surgical groups.
Hippocampal asymmetry maps compared the distribution of ipsilateral hippocampal atrophy relative to the contralateral side in each surgical outcome group. In both the SF and NSF groups, the hippocampal asymmetry profile showed severe diffuse deficits involving the head, body, and tail of the hippocampus without regional specificity (SF and NSF, p < 0.0001 by permutation; see figure 2, C and D). The overall asymmetry pattern suggests that compared with the SF group, the NSF group had a lesser degree of asymmetry.
Interrater reliabilities
To evaluate interrater reliabilities for the manual tracing, 10 brains were randomly chosen (5 SF and 5 NSF). Working independently but using the same anatomic protocol, the two raters traced out the left hippocampus. There was a high interrater reliability for the hippocampal volumes (intraclass correlation coefficient, r = 0.9188; figure E-2).
Discussion
Our data show that even in a rigorously selected group of MTLE patients with HS, there are few clinical features that reliably predict outcome. However, using surface-based anatomic mapping, we were able to detect prognostic features of hippocampal anatomy. It is important to recognize potential limitations of our study. The frequency of secondary generalized seizures was not consistently documented in the clinical assessments before surgery. We cannot reliably compare the neuropsychological test scores because during this time period, two neuropsychologists performed the tests with different test batteries. Quantitative hippocampal volumes were not computed at UCLA before surgery, and visual assessment of hippocampal atrophy may have missed bilateral disease. Because the MRI scans were initially collected in patients for clinical purposes and because of upgrades in equipment and software, normal control MRI scans with the same protocol were not available for comparison.
We were able to isolate certain anatomic correlates of surgical outcome. The spatial pattern of bilateral hippocampal damage in the NSF group may suggest a progression of the disease. In our study, the NSF ipsilateral hippocampus, compared to the SF group, showed greater diffuse atrophy, whereas the contralateral hippocampus exhibited greater but restricted damage in the anterior and lateral hippocampus. We suggest that with disease progression, the sclerotic ipsilateral hippocampus may already be diffusely damaged (i.e., a floor effect), whereas the contralateral hippocampus may exhibit damage only in regions with the greatest susceptibility for epileptogenesis. In animal studies, the ventral hippocampus, analogous to the human anterior hippocampus, has greater seizure susceptibility.7 In human surgical specimens, the anterior hippocampus had more neuronal loss than the posterior hippocampus.8 Seizure propagation from one hippocampus to the other over time may lead to damage first in the most vulnerable areas of the contralateral hippocampus.
Acknowledgment
The authors thank Sandra Dewar, RN, MS, for conducting patient telephone interviews.
Supported by grants from the Epilepsy Foundation Clinical Research Training Fellowship, National EpiFellows Foundation Fritz E. Dreifuss Award (to J.J.L. and J.E.), National Institute for Biomedical Imaging and Bioengineering, the National Center for Research Resources, and the National Institute on Aging (to P.M.T.: R21 EB01651, R21 RR019771, P50 AG016570; to A.W.T.: P41 RR13642 and M01 RR00865 [GCRC]).
Footnotes
Disclosure: The authors report no conflicts of interest.
Additional material related to this article can be found on the Neurology Web site. Go to www.neurology.org and scroll down the Table of Contents for the October 11 issue to find the title link for this article.
References
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 2.Hardy SG, Miller JW, Holmes MD, et al. Factors predicting outcome of surgery for intractable epilepsy with pathologically verified mesial temporal sclerosis. Epilepsia. 2003;44:565–568. doi: 10.1046/j.1528-1157.2003.39202.x. [DOI] [PubMed] [Google Scholar]
- 3.Quigg M, Bertram EH, Jackson T, Laws E. Volumetric magnetic resonance imaging evidence of bilateral hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsia. 1997;38:588–594. doi: 10.1111/j.1528-1157.1997.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 4.Spencer DD, Spencer SS, Mattson RH, et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15:667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Akaike K, Tanaka S, Tojo H, et al. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res. 2001;900:65–71. doi: 10.1016/s0006-8993(01)02252-1. [DOI] [PubMed] [Google Scholar]
- 8.Babb TL, Lieb JP, Brown WJ, et al. Distribution of pyramidal cell density and hyperexcitability in the epileptic human hippocampal formation. Epilepsia. 1984;25:721–728. doi: 10.1111/j.1528-1157.1984.tb03483.x. [DOI] [PubMed] [Google Scholar]


