Abstract
Quantitative magnetic resonance imaging (MRI) studies in children with fetal alcohol spectrum disorders (FASDs) have shown regional patterns of dysmorphology, most prominent in parietal and posterior temporal cortices. Various methods of image analysis have been employed in these studies, but abnormalities in cortical thickness have not yet been mapped over the entire cortical surface in individuals with FASD. Further, relationships between cognitive dysfunction and cortical thickness measures have not yet been explored. We applied cortical pattern matching algorithms and techniques for measuring cortical thickness in millimeters to the structural brain MRI images of 21 subjects with heavy prenatal alcohol exposure (8–22 years, mean age 12.6 years), and 21 normally developing control subjects (8–25 years, mean age 13.5 years). Dissociable cognitive measures, of verbal recall and visuospatial functioning, were correlated with cortical thickness, and group by test score interactions were evaluated for predicting cortical thickness. Significant cortical thickness excesses of up to 1.2 mm were observed in the FASD subjects in large areas of bilateral temporal, bilateral inferior parietal, and right frontal regions. Significant group by test score interactions were found in right dorsal frontal regions for the verbal recall measure and in left occipital regions for the visuospatial measure. These results are consistent with earlier analyses from our own and other research groups, but for the first time, we show that cortical thickness is also increased in right lateral frontal regions in children with prenatal alcohol exposure. Further, the significant interactions show for the first time that brain-behavior relationships are altered as a function of heavy prenatal alcohol exposure.
Keywords: FAS, frontal lobe, prenatal alcohol exposure, verbal learning, visuospatial
Introduction
Excessive prenatal alcohol exposure can lead to neurological, cognitive, and growth impairments (Jones and Smith 1975). Specific facial dysmorphology is required for a diagnosis of fetal alcohol syndrome (FAS) (National Center on Birth Defects and Developmental Disabilities Centers for Disease Control and Prevention Department of Health and Human Services 2004; Bertrand et al. 2005), but frequently, children with excessive prenatal exposure to alcohol (PEA) without the facial characteristics of FAS suffer cognitive and behavioral deficits as well. These children tend to be diagnosed with partial FAS, alcohol-related neurodevelopmental disorder (ARND), or alcohol-related birth defects (ARBD). Collectively, children with FAS, partial FAS, ARND, or ARBD have what is now referred to as a fetal alcohol spectrum disorders (FASDs) (Bertrand et al. 2005), although this term is not used in any diagnostic capacity.
We and others have recently spent considerable effort measuring brain morphological abnormalities in children with FASDs using in vivo imaging techniques (Johnson et al. 1996; Mattson, Riley, Sowell, et al. 1996; Sowell et al. 1996; Swayze et al. 1997; Archibald et al. 2001; Bookstein et al. 2001; Sowell, Mattson, et al. 2001; Sowell, Thompson, Mattson, et al. 2001; Autti-Ramo et al. 2002; Sowell, Thompson, Mattson, et al. 2002; Sowell, Thompson, Peterson, et al. 2002; O’Hare 2005). Among those with FAS diagnoses, microcephaly is common, as first described in the early 1970s (Jones 1975), and these findings have been corroborated in vivo in larger samples (Johnson et al. 1996; Swayze et al. 1997; Archibald et al. 2001; Autti-Ramo et al. 2002; Sowell, Thompson, Mattson, et al. 2002). Abnormalities of the corpus callosum (Riley et al. 1995; Bookstein et al. 2001; Sowell, Mattson, et al. 2001), basal ganglia (Mattson, Riley, Sowell, et al. 1996; Archibald et al. 2001), and cerebellar vermis (Sowell et al. 1996; O’Hare et al. 2005) have been reported in subcortical and cerebellar structures. Volumetric studies of cortical structures have shown that white matter may be more affected than gray matter and that parietal cortices may be particularly vulnerable to the teratogenic effects of prenatal alcohol exposure (Archibald et al. 2001). Our most recent work has focused on whole-brain voxel and surface-based analyses of cortical structures that allow localization of cortical abnormalities on a finer scale than the volumetric studies. Using various methods, we have localized gray matter concentration increases to posterior temporal and inferior parietal cortices bilaterally (Sowell, Thompson, Mattson, et al. 2001; Sowell, Thompson, Mattson, et al. 2002). Gray matter asymmetries are also abnormal in FASD individuals in the same temporal lobe regions where increased gray matter is observed (Sowell, Thompson, Peterson, et al. 2002).
A range of cognitive impairments have also been observed in children with prenatal alcohol exposure including overall intellectual functioning, learning, language, attention, visuospatial, and executive functioning (reviewed in Mattson and Riley 1998; Riley and McGee 2005). We have shown relationships between vermal (O’Hare et al. 2005) and callosal (Sowell, Mattson, et al. 2001) dysmorphology and cognitive function in children with FASD in which greater brain dysmorphology was associated with greater impairment on tests of verbal learning. To date, however, there are no reports in the literature describing relationships between cortical dysmorphology and cognitive function in FASD.
Although we are evaluating the same individuals studied previously with voxel-based morphometry (Sowell, Thompson, Mattson, et al. 2001) and surface-based gray matter concentration analyses (Sowell, Thompson, Mattson, et al. 2002), these new analyses of gray matter thickness in millimeters (Sowell et al. 2004, 2006; Thompson et al. 2004) may shed new light on brain abnormalities in FASD given that gray matter “concentration” and cortical thickness in millimeters are not identical measures and provide unique and partially independent information (Narr et al. 2005). Specifically, measures of gray matter concentration, defined based on signal intensity thresholds, essentially measure the proportion of tissue that has gray matter signal value on magnetic resonance imaging (MRI) relative to other tissue types. Cortical thickness measures, on the other hand, are more specific, and perhaps more intuitive, measures of cortical anatomy, that reflect intrinsic alterations in the gray matter and are unaffected by sulcal widening or other cerebrospinal fluid (CSF)-related effects. Thus, in addition to evaluating relationships between dissociable cognitive functions, namely, verbal recall and visuospatial functioning, and cortical thickness for the first time in FASD, we also report group differences in cortical thickness in millimeters for the first time.
Given our previous studies of gray matter concentration, we predicted cortical thickness increases in posterior temporal and inferior parietal regions in the FASD subjects, including those with histories of heavy prenatal alcohol exposure but without the facial dysmorphology necessary for a diagnosis of FAS (Sowell, Thompson, Mattson, et al. 2001). We also predicted that regional patterns of correlations between cortical thickness and behavioral scores would vary depending on the cognitive function evaluated (Lu et al. 2007). Our earlier volumetric study in typically developing children found that frontal lobe gray matter maturation was predictive of delayed verbal recall functioning, and this relationship did not appear to be mediated by chronological age (Sowell, Delis, et al. 2001). Similar, but less regionally specific relationships were observed for measures of visuospatial abilities and frontal lobe maturation (Sowell, Delis, et al. 2001). As would be expected, given that more mature cortical gray matter is “ thinner” (i.e., more myelinated, Yakovlev and Lecours 1967, with fewer synapses, Huttenlocher 1979), typically developing individuals with lower gray matter volumes relative to brain size performed better on the verbal recall and visuospatial tests (Sowell, Delis, et al. 2001). Given these results, we predicted that the visuospatial measure would correlate more strongly with structural differences in right frontal regions and that the verbal recall measure would correlate more with left frontal regions in the control subjects. Relationships between cortical structures and cognitive function have not, to our knowledge, been evaluated in FASD, but recent functional imaging studies indicate increased frontal lobe activation during verbal learning and recall in FASD subjects relative to controls (Sowell et al. forthcoming). Thus, we predicted that correlations between cortical thickness and cognitive function in frontal cortices would differ between FASD and control subjects.
Methods
Subjects
Twenty-one children, adolescents, and young adults with heavy prenatal alcohol exposure who were between the ages of 8 and 22 years (mean age 13 years; 11 females, 2 left handed) were studied with MRI and surface-based cortical thickness analyses. All of the children and young adults had histories of behavioral problems, cognitive impairment, and heavy prenatal alcohol exposure. Fourteen of them had the characteristic facial appearance (e.g., short palpebral fissures, thin vermillion, indistinct philtrum, maxillary hypoplasia; Jones and Smith 1975) that allowed for a diagnosis of FAS (mean age 12.6 years, 8 females, 1 left handed). Seven other subjects did not have the facial features to warrant a diagnosis of FAS and are instead referred to as subjects with PEA (mean age 13 years, 3 females, 1 left handed). Both alcohol-exposed groups were evaluated by Kenneth Lyons Jones, MD, a pediatric dysmorphologist at the University of California, San Diego. Children in the FASD group (both FAS and PEA) were referred by Dr Jones, by other professionals, or were self-referred by parents and were born to women known to drink heavily during pregnancy, in either a binge fashion or more regularly. As in most retrospective studies involving FAS and PEA, exact amounts and patterns of maternal drinking were typically not available. All children were screened for prenatal exposure through caregiver self-report. Additionally, for children in the alcohol-exposed groups, maternal self-report, medical, social, and/or legal records were reviewed to confirm prenatal alcohol exposure and to rule out heavy use of other drugs. Both alcohol-exposed groups exhibited neuropsychological deficits relative to the controls but did not differ significantly from each other. Details of these data are presented elsewhere (Mattson, Riley, Delis, et al. 1996; Mattson and Riley 1999; Mattson et al. 1999), but full-scale intelligence quotient scores are similar for both alcohol-exposed groups (FAS mean = 77, standard deviation [SD] = 15, range 49–92; PEA mean = 86, SD = 14, range 64–101).
Twenty-one typically developing children, adolescents, and young adults between 8 and 25 years were studied as a comparison group (mean age 13.5, 12 females, all right handed). All child and adolescent subjects were recruited as normal controls for a large, multidisciplinary neurodevelopmental research center or for the Center for Behavioral Teratology, both in San Diego. Each subject was screened for neurological impairments and for any history of learning disability or developmental delay. Informed consent was obtained from all children and their parents. The young adult subjects were recruited as normal controls for neuropsychiatric studies of adult patient populations. These subjects were thoroughly screened for medical, neurological, and psychiatric disorders, and again informed consent was obtained from each subject.
Imaging Protocol
Whole-brain image series were collected for each subject at one of 2 locations: either University of California, San Diego, Magnetic Resonance Institute or Scripps Clinic and Research Foundation. Both locations utilized a 1.5-T superconducting magnet (Signa; General Electric, Milwaukee, WI) and the same image acquisition protocol with the following parameters: gradient-echo (spoiled gradient-echo) T1-weighted sagittally acquired series with time repetition = 24 ms, time echo = 5 ms, number of excitations = 2, flip angle = 45 degrees, field of view = 24 cm, section thickness = 1.2 mm (0.9375 × 0.9375 mm within plane), no gaps.
Image Processing
Details of the image analysis procedures have been described previously (Sowell, Thompson, Tessner, et al. 2001; Sowell, Thompson, Mattson, et al. 2002; Sowell, Thompson, et al. 2003; Sowell et al. 2004; Thompson et al. 2004; Sowell et al. 2006). Briefly, the MRI data from each individual were analyzed with a series of manual and automated procedures that were applied in the following order: 1) transforming brain volumes into a standardized 3-dimensional (3D) coordinate space (Mazziotta et al. 1995) using a 12-parameter, linear, automated image registration algorithm (Woods et al. 1998); 2) Semiautomated tissue segmentation for each volume data set to classify voxels based on signal intensity as most representative of gray matter, white matter, or CSF (Sowell et al. 1999); 3) removing nonbrain tissue (i.e., scalp, orbits) and cerebellum; 4) automatically extracting the cortical surface of the brain, which was represented as a high-resolution mesh of 131 072 triangulated elements spanning 65 536 surface points (MacDonald et al. 1994); 5) tracing 17 sulcal and gyral landmarks on the lateral surface of each hemisphere along with 6 landmark curves surrounding the interhemispheric surface (Sowell, Thompson, Rex, et al. 2002; Sowell, Peterson, et al. 2003); 6) transforming image volumes back into their own native image acquisition space by mathematically inverting the transformation which took them into standard space (step 1 above); 7) spatially registering with a rigid-body 6-parameter transformation all segmented images and brain surfaces for each individual by defining 40 standardized, manually defined anatomical landmarks (the first and last points on each of 20 of the sulcal lines drawn in each hemisphere) (Sowell, Thompson, Rex, et al. 2002; Sowell, Peterson, et al. 2003); and 8) measuring cortical thickness in millimeters averaged within a 15-mm sphere attached to each point on the cortical surface (see below).
Gray matter thickness was calculated using the Eikonal Fire Equation (Sapiro 2001; Thompson et al. 2004). Although various other approaches have been used (Fischl and Dale 2000; Jones et al. 2000; Miller et al. 2000; Kabani et al. 2001), our approach seemed a natural and intuitive measure given that it defines thickness as the distance from the inner gray-white boundary to the closest point on the outer gray matter surface. Given that laminar structure is not visible with MRI, we could not rely on laminar structure to define thickness, as we have done using other automated approaches in histological data (Annese et al. 2004). Although the brain image volumes acquired for this study had voxel dimensions of approximately 1 × 1 × 1.2 mm, we supersampled the image data to create voxel dimensions of 0.33 mm cubed. The 3D Eikonal equation was applied only to voxels that segmented as gray matter, and a smoothing kernel was used to average gray matter thickness values within a 15-mm sphere at each point on the cortical surface. This allowed us to calculate cortical thickness for each subject with an effective resolution much finer than the original voxel size given that the error associated with localizing anatomy on the inner and outer cortical surfaces was averaged with the unbiased error of all other voxels within the smoothing kernel (i.e., approximately 42 000 0.33-mm cubed voxels). Points on the cortical surfaces surrounding and between the sulcal contours drawn on each individual’s brain surface were calculated using the averaged sulcal contours as anchors to drive 3D cortical surface mesh models from each subject into correspondence using elastic warping parameters described in more detail in another report (Thompson et al. 2004). This allows the creation of average surface models and the creation of maps of group differences on gray matter thickness or correlations between cortical thickness and cognitive test scores. To map gray matter thickness onto the surface rendering of each subject, the coordinate for each point on the brain surface for each individual (anatomically matched across individuals) was mapped to the same anatomical location in their individual “thickness” volume, and the average gray matter thickness value within the 15-mm sphere for each subject was calculated. In a previous report, we helped to establish the validity of these methods by showing close regional correspondence between the cortical thickness maps created for normally developing children in vivo (Sowell et al. 2004) and for the 1929 postmortem data of Von Economo (1929). In our earlier report (Sowell et al. 2004), we also assessed test-retest reliability of cortical thickness measures among individuals with 2 image volumes acquired at short intervals and showed maximal error estimates of 0.15 mm.
Behavioral Measures
As part of a battery of tests administered by a large multidisciplinary neurodevelopmental research center (controls) or as part of a larger project studying the effects of prenatal alcohol exposure, most children were given the California Verbal Learning Test for Children (CVLT-C; Delis et al. 1994) and the Rey-Osterrieth Complex Figure Test (ROCF; Rey 1941; Osterrieth 1944) within 4 months of scanning. We chose these 2 tests (from a larger battery of tests), in part, because they tapped somewhat dissociable cognitive functions, and were administered by both the neurodevelopmental research group who tested most of the controls, and the research group who studied the FASD subjects and some controls. Eighteen of the 21 controls were administered the CVLT-C, and the same 18 were administered the ROCF. Three subjects were excluded from the administration of the ROCF due to neuropsychological test administration time constraints. Eighteen of the 21 FASD subjects were administered the CVLT-C and 15 of them the ROCF. Again, neuropsychological test administration time constraints resulted in the exclusion of 3 FASD subjects from the brain-behavior analyses for the CVLT-C and 6 FASD subjects from the analyses for the ROCF.
Briefly, the CVLT-C involves the oral presentation of a supraspan list of words (5 words in each of 3 semantic categories for a total 15 words). Each subject was given 5 consecutive trials to learn the list with a test of immediate recall after each administration. The 5 learning trials were followed by the presentation of a new word list, and recall (free recall, semantically cued recall, and recognition) was tested for the first list after a 20-min delay. Although many measures can be obtained from the CVLT-C, the only measure used here was the long-delay free recall (LDF) condition because it was thought to be the best measure of verbal recall functioning, which has been shown to be impaired in FASD (Mattson, Riley, Delis, et al. 1996).
The ROCF test was individually administered to each subject. First, the subject was asked to copy the ROCF from a stimulus card using colored pens. The examiner handed the subject a new colored pen each minute in a prescribed order, and there was no time limit. On completion of the copy, the examiner removed the stimulus card and the subject’s copy and placed a blank sheet of paper on the desk. The subject was then asked to draw as much of the figure as he or she could remember, and again, the examiner gave the subject a new colored pen each minute in the same order as in the copy presentation. After approximately 20–30 min in which the subject performed other neuropsychological tests, each subject was asked to draw the figure again from memory using the same procedure as in the immediate recall condition. For these brain-behavior analyses, we used only the copy condition as it was thought to best tap visuospatial functioning, which is impaired in FASD (Mattson and Riley 1998). The Boston Qualitative Scoring System (Stern et al. 1994) was used providing a detailed analysis of accuracy in reproducing the ROCF for the copy condition evaluated in these analyses.
Statistical Analyses
Uncorrected statistical maps of differences between groups were created for gray matter thickness for the entire FASD sample versus controls and for the PEA and FAS groups separately, both compared with controls. In these analyses, the correlation (Pearson’s r) between group membership and gray matter thickness at each point on the brain surface was calculated for the comparison of FASD (FAS and PEA combined) and controls, PEA and controls, and FAS and controls. A surface-point significance threshold of P = 0.05 was used to visualize local changes in gray matter thickness at each point on the cortical surface.
Statistical maps were also generated to evaluate correlations (Pearson’s r) between gray matter thickness and each of 2 cognitive test scores, ROCF copy, and CVLT-C LDF for the control and FASD groups separately. Subgroups (i.e., PEA or FAS) were not evaluated separately for the brain-behavior analyses given the relatively small sample sizes for within-group brain-behavior correlations. Group (FASD or control) by test score interaction maps for each cognitive measure were also created. These maps allowed for the statistical evaluation of differences between the FASD and control groups in their degree of correlation between within-group test scores and thickness. To evaluate the group-by-score interactions, analysis of variance was used to compare a full model, which included group-by-score interactions, with a reduced model that did not include these interactions. The main effects of group and score were included in both of these linear models. F ratios were computed at each point on the cortical surface and were converted to uncorrected P values. An uncorrected threshold of P = 0.05 was used to illustrate the regions where interactions between group membership and test score should be considered. Subjects without both behavioral measures were eliminated from the analyses resulting in a sample size of 32 subjects including FASD and controls for the interaction analyses. While groups were matched for age, we also conducted analyses where age was statistically controlled (for both group differences in thickness and brain-behavior analyses) given the wide age range of the samples. Results were virtually identical for all statistical maps, and thus, the results from the simpler model without age correction are presented.
The statistical maps (uncorrected) are crucial for allowing us to visualize the spatial patterns of group differences or group-by-score interactions in gray matter thickness. However, permutation methods (Bullmore et al. 1999) were used to assess the significance of the statistical maps of the main effects of group on cortical thickness and to correct for multiple comparisons (i.e., for the statistical tests at each of 65 536 surface points) as follows. In order to localize effects at a lobar level, 10 coarse regions of interest (ROIs; 5 in each hemisphere) were created from a probabilistic atlas (Evans et al. 1996) for the frontal lobe (ventral and dorsal regions separated by an axial plane passing through the intersection of the posterior extent of the inferior frontal sulcus and the precentral sulcus in each hemisphere), parietal lobe, temporal lobe, and occipital lobe. In the permutation analyses, subjects were randomly assigned to groups for 10 000 new correlation analyses at each surface point in each ROI, and the number of significant results (i.e., gray matter thickness at any surface point that significantly differed between groups at the threshold of P = 0.05) that occurred in the real test for group differences was compared with the null distribution of significant results that occurred by chance. In other words, the threshold for assessing the significance of statistical maps based on the permutation tests (within each of the ROIs) was determined objectively by calculating the surface area (number of surface points) of significant effects in the real test of group differences. That surface area within any tested ROI was used as the threshold for comparison with the random tests for that ROI, and if fewer than 5% (i.e., P < 0.05) of the results from random tests reached or exceeded the surface area of the real test, the statistical map (within ROIs) was considered significant.
It is not possible to perform an exact permutation test that separates the effects of group-by-score interactions from the main effects of group or test score (given that by definition, the main effects of group and test score must be held constant). However, approximate permutation tests, based on permutation of the residuals of these statistical models, have been described and validated (Freedman and Lane 1983; Anderson and Legendre 1999; Anderson and Ter Braak 2003). While holding constant the portions of variance in cortical thickness that are attributed by the reduced model to group and test score, the associated residual variances in thickness not attributable to these factors were permuted randomly across all subjects irrespective or group or test score. The new set of observations generated by combining the permuted contribution to cortical thickness with the nonpermuted contribution associated with the main effects of group and test score was then analyzed using the same statistical model as was used to analyze the original data. By combining a large number of such permutations (N = 10 000), a distribution for the number of brain locations where the uncorrected P value was less than 0.05 was created. Based on this distribution and the number of such points observed in the original data, a corrected overall P value was assigned for each of the same 5 ROIs (per hemisphere) described above for permutation of the main effects of group. Despite the fact that only residuals were permuted, this analysis was analogous to the exact permutation testing performed for the main effects of group under the assumption of no significant group-by-score interactions. Similar methods have been used to evaluate the effects of gender and age-by-gender interactions on cortical thickness (Sowell et al. 2006).
Results
Gray Matter Thickness
FASD versus Control
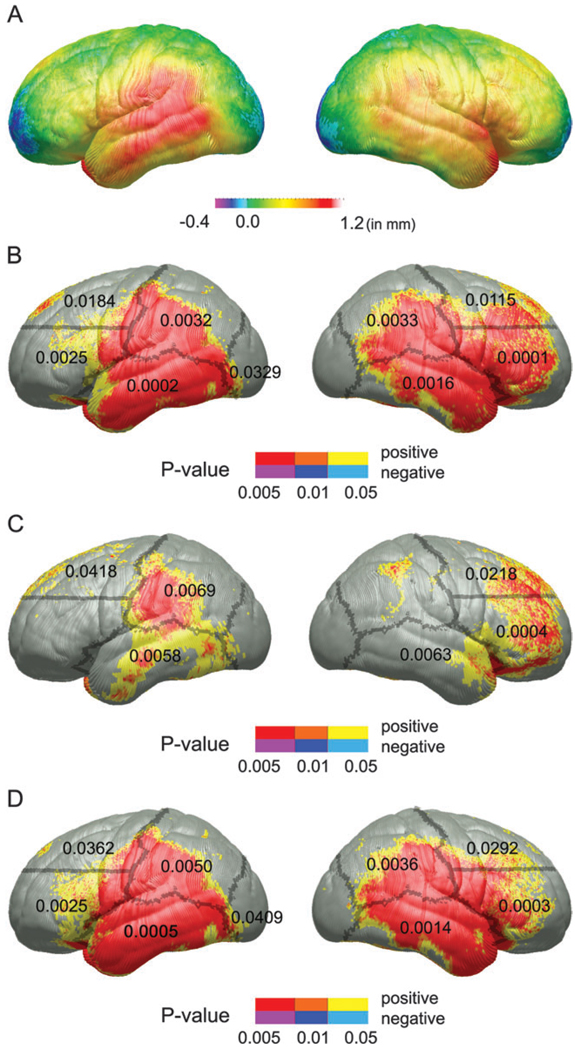
Maps of the mean difference in gray matter thickness in millimeters between FASD (FAS and PEA combined) and control groups can be seen in Figure 1A. Most of the lateral brain surface in frontal, temporal, occipital, and parietal cortices exhibit thickness increases of up to 1.2 mm in the FASD subjects. More dorsal cortices in the right frontal lobe were also thicker in the FASD group. Uncorrected statistical maps of the group effect can be seen in Figure 1B. Permutation analyses were significant in all but the right occipital ROI. The FASD group did not exhibit thinner cortex than controls in any region evaluated.
Figure 1.
(A) FASD (PEA and FAS combined) versus control maps of the group difference in cortical thickness in millimeters according to the color bar. (B) Uncorrected P maps representing the significance of group differences in cortical thickness for the FASD versus control comparison. Positive correlations (i.e., FASD > control) are shown in hot colors, and negative correlations are shown in cool colors. There were no regions where the FASD subjects had thinner cortex than the controls. Regions in red are significant at an uncorrected P value of 0.005 or less; in orange, P values range between 0.01 and 0.005; and in yellow, P values between range between 0.05 and 0.01. (C) Uncorrected statistical P maps of the PEA versus control comparison. (D) Uncorrected tatistical P maps of the FAS versus control comparison. The color coding for (C) and (D) are identical to those described for Figure 1B. The outline of gross ROI boundaries used in permutation analyses are shown superimposed over the left and right lateral surfaces of each of the uncorrected maps, and permutation P values, where significant, are displayed within each ROI.
PEA versus Control
Uncorrected statistical maps of the PEA group versus control difference can be seen in Figure 1C. Thickness increases were also observed in the PEA group in left parietal, right ventral frontal, bilateral temporal, and dorsal frontal ROIs. Permutation test results indicated that these group differences did not occur by chance, despite the significantly reduced power with the smaller group. PEA subjects did not exhibit thinner cortex than controls in any region evaluated.
FAS versus Control
Uncorrected statistical maps for the FAS group versus control difference can be seen in Figure 1D. Like the FASD versus control maps, these maps were highly significant in all regions on the lateral brain surface except the right occipital ROI, confirmed with permutation analyses. FAS subjects did not exhibit thinner cortex than controls in any region evaluated.
Thickness and Behavior
Verbal Recall
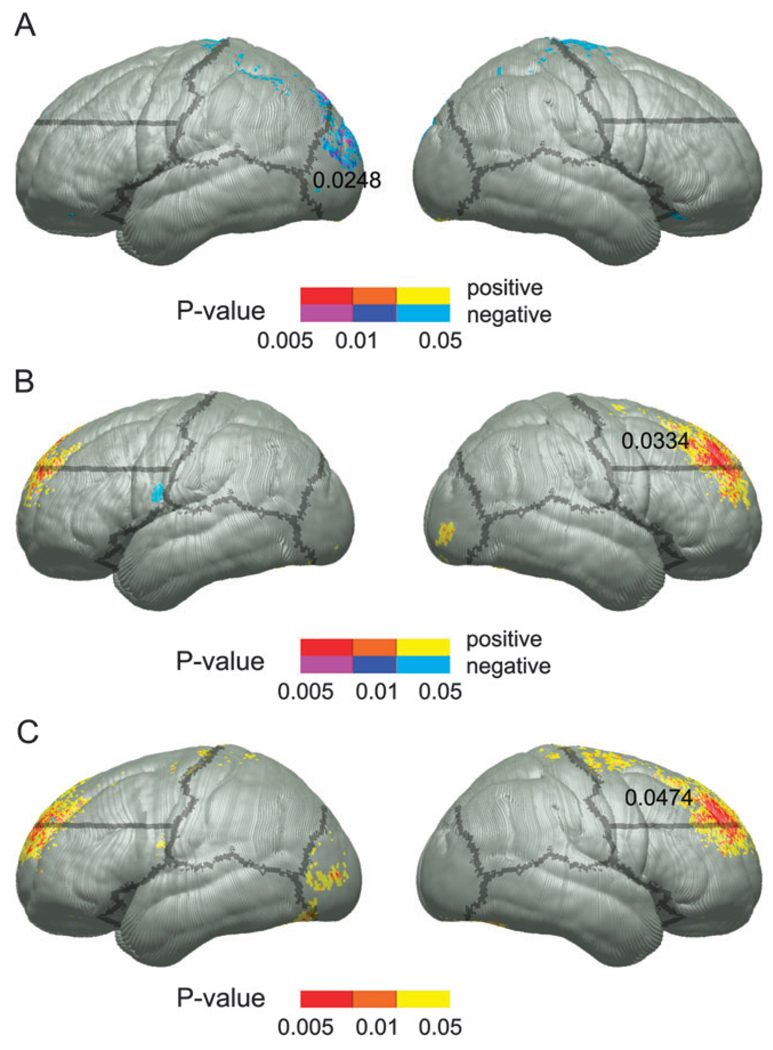
Descriptive statistics for the CVLT-C LDF measure for FASD, PEA, FAS, and control groups can be seen in Table 1. The FASD group performed significantly more poorly than the controls on the verbal recall measure. When divided into FAS and PEA subgroups, only the FAS group performed significantly worse than the controls. Uncorrected statistical maps of the simple correlation between performance on the CVLT-C LDF condition and cortical thickness within the control and FASD groups can be seen in Figure 2A,B, respectively. Within the control group, negative correlations were observed at the superior extent of the motor strip bilaterally and in the left occipital region. These were locations in which thinner cortex was associated with improved performance. Permutation results were significant only in the left occipital ROI (P = 0.0248). Within the FASD group, bilateral dorsal prefrontal regions showed positive correlations such that FASD children with poorer performance had thinner cortex in these regions. While we predict correlations between left frontal regions and performance on the CVLT-C LDF condition, consistent with the uncorrected statistical maps shown here, permutations were only significant in the right dorsal frontal ROI (P = 0.033). Group by LDF score interactions (Fig. 2C), however, did yield interesting results with significant interactions in bilateral dorsal prefrontal regions in the uncorrected maps. Permutation analyses confirmed the significance of these effects only in the right hemisphere (permutation P value 0.047). The individual within-group maps suggested that thinner cortex in frontal regions was associated with worse performance within the FASD but not the control groups.
Table 1.
Neurocognitive test results: presented are sample sizes, means, and SD for each of the 3 main contrasts for the FASD subjects and the PEA and FAS subgroups
| Behavioral results | ||||||
|---|---|---|---|---|---|---|
| CVLT-C LDF | ROCF copy | |||||
| Group | N | Mean (SD) | Alcohol-exposed versus contol group difference, t value (P value) |
N | Mean (SD) | Alcohol-exposed versus control group difference, t value (P value) |
| FASD | 18 | 8.7 (3.6) | 2.2 (0.03) | 15 | 14.8 (3.4) | 2.3 (0.03) |
| PEA | 6 | 9.8 (3.3) | 1.0 (NS) | 6 | 15.3 (4.2) | 1.0 (NS) |
| FAS | 12 | 8.2 (3.8) | 2.4 (0.03) | 9 | 14.4 (2.9) | 2.4 (0.03) |
| Control | 18 | 11.4 (3.5) | NA | 18 | 17.2 (2.4) | NA |
Note: Two-sample t-tests were conducted to evaluate group differences for test performance. NS, not significant; NA, not applicable.
Figure 2.
Uncorrected P maps representing the significance of correlations between cortical thickness and test scores for the CVLT-C LDF measure within the control group (A) and the FASD group (B). The P values for positive and negative correlations are color coded according to the color bar with positive correlations (i.e., thinner cortex, worse performance) shown in red, orange, and yellow and negative correlations (i.e., thinner cortex, better performance) in purple, dark blue, and light blue. Statistical P maps of the interaction between group and CVLT-C LDF scores in predicting cortical thickness are shown in (C). Note that these P values were converted from F ratios in analyses of variance, thus positive and negative values are not color coded separately. Boundaries of ROIs used in permutation analyses are overlaid on the left and right surfaces, and the permutation P values, where significant, are displayed within the ROI from which they came.
Visuospatial Functioning
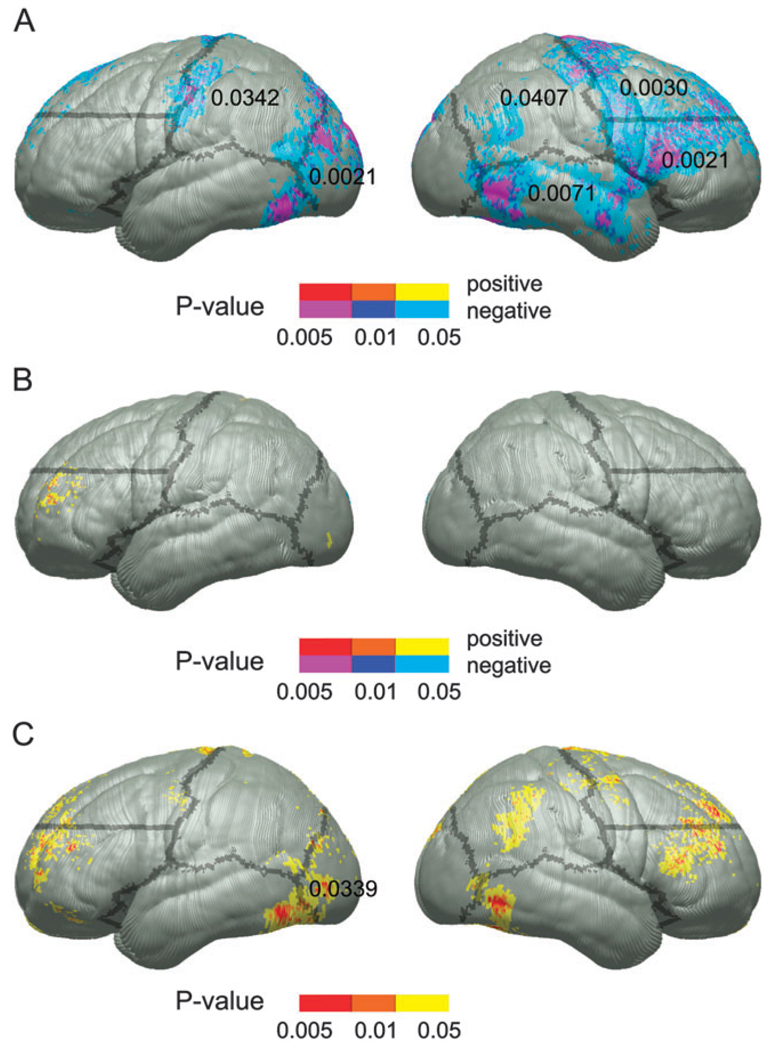
Descriptive statistics for the ROCF copy measure for FASD, PEA, FAS, and control groups can be seen in Table 1. The FASD group performed significantly more poorly than the controls on the visuospatial measure. When divided into FAS and PEA subgroups, only the FAS group performed significantly worse than the controls (P = 0.03). Uncorrected statistical maps of the simple correlation between performance on the ROCF copy condition and cortical thickness within the control and FASD groups can be seen in Figure 3A,B, respectively. Within the control group, negative correlations were prominent in bilateral parietal, left occipital, right dorsal and ventral frontal, and temporal cortices. These predominantly right hemisphere regions were locations in which thinner cortex was associated with improved performance. Permutation analyses supported the significance of the effects in all of these regions (permutation P values from 0.04 in the right parietal region to 0.002 in the right ventral frontal and left occipital regions). Within the FASD group, cortical thickness was positively associated with ROCF copy performance only in small, circumscribed regions within the left frontal lobe where thinner cortex was associated with worse performance. However, permutation results were not significant in any region in this FASD within-group analysis. Interactions (Fig. 3C) were significant (uncorrected) in all regions where negative correlations between thickness and performance were observed in the control but not the FASD group. Only the left occipital region surpassed correction for multiple comparisons with permutation (p = 0.047), but results at trend level significance were also observed in the right dorsal frontal ROI (permutation P value 0.062).
Figure 3.
The color-coding schemes are identical to those described for the CVLT-C LDF analyses described for Figure 2. Uncorrected P maps representing the significance of correlations between cortical thickness and test scores for the ROCF copy measure within the control group (A) and the FASD group (B). Statistical P maps of the interaction between group and ROCF copy scores in predicting cortical thickness are shown in (C). Note that as these P values were converted from F ratios in analyses of variance, positive and negative values are not color coded separately.
Discussion
In this report, we showed that cortical thickness was increased by up to 1.2 mm in individuals with FASD. These cortical thickness increases occurred over large areas of lateral temporal, parietal, and frontal cortices while the more dorsal cortices of the frontal and parietal lobes did not show significant group differences and may be relatively spared in FASD. These results were highly consistent with our own investigations of the same subjects where we showed gray matter concentration increases bilaterally in the posterior temporal and inferior parietal lobes (Sowell, Thompson, Mattson, et al. 2001; Sowell, Thompson, Mattson, et al. 2002). However, for the first time here we revealed cortical thickness abnormalities over large areas of dorsolateral prefrontal lobes, predominantly in the right hemisphere. These effects were previously unappreciated in our studies of gray matter concentration, perhaps suggesting that the new methods for measuring gray matter thickness in millimeters were more sensitive to changes that were intrinsic to the cortical gray matter, or offer better signal to noise for detecting group differences.
Alcohol-exposed individuals with and without facial dysmorphology exhibited statistically significant cortical thickness increases, making it clear that having facial dysmorphology is not a prerequisite for having brain dysmorphology. The animal literature also supports this conclusion. Recent work in the mouse model has demonstrated that even moderate alcohol exposure can result in neural tube midline defects without gross physical malformations near or after birth (Zhou et al. 2003). Similar phenomena may be occurring in the PEA children.
The increase in cortical thickness in the alcohol-exposed subjects may not seem intuitive given the overall microcephaly characteristic of prenatal alcohol exposure in general (Jones and Smith 1973) and reduced brain volume observed in this particular sample (Sowell, Thompson, Mattson, et al. 2002). Generally, it seems reasonable to speculate that measures of cortical thickness reflect regional functional integrity. For example, we have previously observed that normally developing children with thinner cortex in left frontal and parietal regions perform better on tests of verbal abilities (Sowell et al. 2004), and older children with thinner frontal and parietal cortices perform better in tests of general intellectual functioning (Shaw et al. 2006). This makes sense in light of the fact that the cortex in these regions has been shown to thin throughout the childhood and adolescent years (Sowell et al. 2004; Shaw et al. 2006). As we have previously discussed, important methodological and biological issues for interpreting gray matter thickness measurements on MRI should be considered when evaluating developing populations (Sowell, Peterson, et al. 2003; Sowell et al. 2004). As with MRI, even in histological data, the boundary between gray and white matter is not “black-and-white” (Annese et al. 2004). Essentially, tissue that appears as cortical gray matter on MRI during development may actually be unmyelinated peripheral axonal fibers. Thus, the increased cortical thickness observed in FASD here with MRI could result from decreased myelination. Further support for this can be found in earlier reports of white matter volume decreases in parietal and temporal lobes (Archibald et al. 2001) and white matter concentration decreases in subjects with FASDs relative to controls in the same locations where we observed gray matter concentration increases (Sowell, Thompson, Mattson, et al. 2001). Animal models of FAS have shown delayed myelination and loss of myelination in optic axons following prenatal exposure to ethanol (reviewed in Stromland and Pinazo-Duran 2002). Other animal studies have shown that the brain may be highly vulnerable to ethanol-induced brain size reductions during the brain growth spurt at which time glial and myelin structures are developing rapidly (reviewed in Guerri 1998). Given these findings in humans and animals with prenatal alcohol exposure, increased gray matter thickness likely does not reflect an increase of the constituents that actually comprise healthy cortex (i.e., cell bodies, glia, synapses) and “more” is not likely “better” given the cognitive and behavioral problems characteristic of FASD.
Although we speculate that neuronal events that occur normally during childhood and adolescence go awry in children with FASD, this is not to imply that children with FASD eventually “catch-up” in the maturational trajectory and at some point are indistinguishable from their nonexposed counterparts. Ultimately, longitudinal studies will help determine whether brain dysmorphology is static or progressive during the years of development in children with heavy prenatal alcohol exposure. Some studies have show that neuropsychological impairments and brain dysmorpholgy are present even in adulthood (Bookstein et al. 2002), though the question of the long-term effects of alcohol-induced anomalies have not been adequately addressed in human or animal studies (West et al. 1994).
In this report, we measured relationships between cognitive functioning and cortical thickness and found these relationships altered as a function of prenatal alcohol exposure. On a measure of verbal recall, the FASD subjects but not the controls exhibited positive correlations between cortical thickness and test performance in dorsal frontal regions (i.e., thinner cortex, worse performance). The differences between groups for brain-behavior relationships in verbal recall were confirmed by significant interactions in the right dorsal frontal region. These are the same brain regions where functional activation increases have been observed in a different group of FASD subjects relative to controls during verbal learning and recall (Sowell et al. forthcoming). Combined, these results suggest that FASD subjects may rely more heavily on dorsal frontal structures than nonexposed individuals, perhaps to compensate for dysfunction in other brain regions responsible for verbal learning and recall.
On a measure of visuospatial functioning, cortical thickness in large areas of the frontal, parietal, and temporal lobes were negatively associated with test performance in the controls but not the FASD subjects. That is, in the controls, thinner cortex was associated with better performance. This finding is easily reconciled with findings in other typically developing samples, studied cross-sectionally and longitudinally, where thinner cortex was associated with improved performance on tests of verbal learning and verbal intellectual functioning (Sowell, Delis, et al. 2001; Sowell et al. 2004). This is possibly due to increased cortical efficiency as unnecessary or underutilized synaptic connections are pruned, as has been shown in animal models (Nordeen KW and Nordeen EJ 1997), and increasing myelination, which results in more efficient connections between complementary brain regions (reviewed in Paus 2005). Both of these processes result in what we observe as cortical thinning with MRI during normal development, and the dorsal cortices of the frontal and parietal lobes are regions where the most dramatic cortical thinning is occurring during the age range studied here (Sowell, Peterson, et al. 2003; Gogtay et al. 2004; Sowell et al. 2004; Shaw et al. 2006). Thus, thinner cortex cooccurring with better performance in the control subjects is to be expected. Why these negative correlations were not observed in the FASD subjects is not clear, but the group differences in cortical thickness reported here suggest that pruning and myelination processes may not be occurring normally in these children. Significant group by score interactions in the left occipital region suggest that the cortical thinning that occurs in controls is related to improved cognitive test performance, but this relationship does not hold for that region in the FASD subjects.
Taken together, these results suggest that heavy prenatal alcohol exposure impacts the organization of structures within the cortex, and that abnormalities in cortical thickness may result from cellular changes that occur during postnatal development. Further, the significant group-by-score interactions in predicting cortical thickness suggest that brain-behavior relationships do not develop normally in individuals with FASDs.
Limitations
The sample sizes studied here were relatively small, especially for the within-group neurocognitive test score cortical thickness analyses where only 15–18 subjects were evaluated for each correlation map. Nonetheless, significant neurocognitive-thickness effects were seen within groups when permutation methods were use to correct for multiple comparisons. Our predictions regarding the regional correlates were supported in that the visuospatial measure was predominantly correlated with right hemisphere thickness in the controls. Within the FASD group, verbal recall was significantly correlated with cortical thickness in frontal lobe structures, though, contrary to our predictions, the effect was more prominent in the right hemisphere. The effect sizes for brain-behavior relationships were quite small in some regions, perhaps due to the relatively small sample sizes. Replication in independent samples would increase confidence in the unpredicted effects. Not knowing how much and when the pregnant mother consumed alcohol, and having small sample sizes that do not permit a longitudinal analysis of changes, limits the power of human studies of prenatal alcohol exposure. Controlled animal studies, however, can provide insight into potential mechanisms of the teratogenicity of alcohol.
Acknowledgments
Support was provided by the National Institutes of Health (NIDA R21 DA15878 and RO1 DA017831, NIAAA U24AA014808, U01AA014834, R01 AA010820, R01 AA010437, and NCRR U54 RR021813) and the March of Dimes (5FY03-12). PMT is also supported by AG016570, LM05639, EB01651, RR019771, and HD050735.
Footnotes
Conflict of Interest: None declared.
References
- Anderson MJ, Legendre P. An empirical comparison of permutation methods for tests of partial regression coefficients in a linear model. J Stat Comput Simul. 1999;62:271–303. [Google Scholar]
- Anderson MJ, Ter Braak CJ. Permutation tests for multi-factorial analysis of variance. J Stat Comput Simul. 2003;73:85–113. [Google Scholar]
- Annese J, Pitiot A, Dinov ID, Toga AW. A myelo-architectonic method for the structural classification of cortical areas. Neuroimage. 2004;21:15–26. doi: 10.1016/j.neuroimage.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd LL, Webber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep. 2005;54:1–14. [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 2001;64:4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer J, Kaplan E, Ober BA. California Verbal Learning Test for Children. New York: Psychological Corporation; 1994. [Google Scholar]
- Evans AC, Collins DL, Holmes CJ. Automatic 3D regional MRI segmentation and statistical probabilistic anatomical maps. New York: Academic Press; 1996. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D, Lane D. A nonstochastic interpretation of reported significance levels. J Bus Econ Stat. 1983;1:292–298. [Google Scholar]
- Gogtay N, Giedd J, Lusk L, Hayashi KM, Greenstein DK, Vaituzis AC, Nugent TF, Herman D, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW, II, Sato Y, Andreasen NC. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genet. 1996;61:329–339. doi: 10.1002/(SICI)1096-8628(19960202)61:4<329::AID-AJMG6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jones KL. The fetal alcohol syndrome. Addict Dis. 1975;2:79–88. [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. The fetal alcohol syndrome. Teratology. 1975;12:1–10. doi: 10.1002/tera.1420120102. [DOI] [PubMed] [Google Scholar]
- Jones SE, Buchbinder BR, Aharon I. Three-dimensional mapping of cortical thickness using Laplace’s equation. Hum Brain Mapp. 2000;11:12–32. doi: 10.1002/1097-0193(200009)11:1<12::AID-HBM20>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13:375–380. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Avis D, Evans A. Multiple surface identification and matching in magnetic resonance images. Proc Vis Biomed Comput. 1994;2359:160–169. [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc. 1999;5:462–471. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J The International Consortium for Brain Mapping (ICBM) A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Miller MI, Massie AB, Ratnanather JT, Botteron KN, Csernansky JG. Bayesian construction of geometrically based cortical thickness metrics. Neuroimage. 2000;12:676–687. doi: 10.1006/nimg.2000.0666. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- National Center on Birth Defects and Developmental Disabilities Centers for Disease Control and Prevention Department of Health and Human Services. [Accessed April 6, 2007];Fetal Alcohol Syndrome Guidelines for Referral and Diagnosis: National Task Force on Fetal Alcohol Syndrome and Fetal Alcohol Effects [Internet] 2004 Available from http://www.cdc.gov/ncbddd/fas/documents/FAS_guidelines_accessible.pdf.
- Nordeen KW, Nordeen EJ. Anatomical and synaptic substrates for avian song learning. J Neurobiol. 1997;33:532–548. doi: 10.1002/(sici)1097-4695(19971105)33:5<532::aid-neu4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe. Arch Psychol. 1944;30:206–356. [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Sapiro G. Geometric partial differential equations and image analysis. New York: Cambridge University Press; 2001. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I–V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt S, Mattson SN, O’Connor MJ, Bookheimer SY. Medial temporal and frontal lobe activation abnormalities during verbal learning in children with fetal alcohol spectrum disorders. NeuroReport. Forthcoming. [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex Differences in Cortical Thickness Mapped in 176 Healthy Individuals between 7 and 87 Years of Age. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl066. August 31, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002c;17:1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, Toga AW. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 2002;12:17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Stern RA, Singer EA, Duke LM, Singer NG. The Boston qualitative scoring system for the Rey-Osterrieth Complex Figure: description and interrater reliability. Clin Neuropsychol. 1994;8:309–322. [Google Scholar]
- Stromland K, Pinazo-Duran MD. Ophthalmic involvement in the fetal alcohol syndrome: clinical and animal model studies. Alcohol Alcohol. 2002;37:2–8. doi: 10.1093/alcalc/37.1.2. [DOI] [PubMed] [Google Scholar]
- Swayze VW, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, et al. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23 Suppl 1:S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Von Economo CV. The cytoarchitectonics of the human cerebral cortex. London: Oxford Medical Publications; 1929. [Google Scholar]
- West JR, Chen WJ, Pantazis NJ. Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis. 1994;9:291–322. doi: 10.1007/BF02098878. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li TK. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Brain Res Dev Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]