Abstract
We analysed the influence of mesial temporal lobe epilepsy on the thickness of the corpus callosum (CC) in a large sample of well-characterized patients (n = 96) and healthy controls (n = 28). In particular, we investigated whether callosal structures are differentially affected depending on the affected hemisphere and age of epilepsy onset. Overall, we observed that epilepsy is associated with a decreased thickness in posterior callosal regions. Patients with an early onset, especially patients with left onset, additionally exhibited a smaller callosal thickness in more anterior and midbody regions. These findings may reflect non-specific as well as specific effects of temporal lobe epilepsy on CC development and interhemispheric connectivity.
Keywords: corpus callosum, temporal lobe epilepsy, MRI
Introduction
Temporal lobe epilepsy (TLE) is the most common form of symptomatic epilepsy. Usually the disease starts in early childhood and becomes pharmacologically intractable during the course of time (Engel, 2001). Although several studies have investigated the effect of epilepsy on cognitive functions as well as on cortical structures and development (Hermann et al., 2003b; Bernasconi et al., 2004; Elger et al., 2004; Oyegbile et al., 2004; Bernasconi et al., 2005; Gross et al., 2006; Mueller et al., 2006), only a few studies focused on the corpus callosum (CC) in association with TLE. Moreover, the studies performed differed in results and methods (Conlon and Trimble, 1988; Okusky et al., 1988; Atkinson et al., 1996; Hermann et al., 2003a). Therefore, we aimed to investigate the influence of TLE on the structure of the CC in a large sample of well-characterized patients with left or right TLE. Based on a previous study (Hermann et al., 2003a), we expected a smaller CC in patients with epilepsy as compared to the healthy control group. In addition, we set out to investigate whether the CC is differentially affected depending on the hemisphere of seizure onset (e.g. left versus right-hemisphere lateralization).
In contrast to previous studies, the current method allows a more subtle examination of callosal morphology by creating high-resolution spatial maps of deficits and does not require a priori specification of regions of interest.
Methods
A large number of TLE-patients from our presurgical evaluation program (n = 96) and control subjects (n = 28) were included in our study (for demographics and clinical data see Table 1). The patients were included consecutively when entering the presurgical workup, i.e. being pharmacoresistant and having evidence for a mTLE by MRI and electrophysiological diagnostic (for an overview of the pathologies and post-operative outcome see Supplementary Material). The controls were recruited from within the social environment of the patients and by means of newspaper ads. The control-subjects and the patient groups did not differ in regard to age (ANOVA: F = 1.718, P = 0.123) or gender distribution (χ2 = 8.615; P = 0.196). A T1-weighted 3D gradient echo sequence with 1 mm3 (isotropic) voxels was acquired for each subject (1.5 T: FOV 256 mm, matrix 256 × 256, sagittal orientation, no. of slices 140, slice thickness 1 mm, TR 15.2 ms, TE 3.6 ms, flip angle 30°).
Table 1.
Subjects demographics and clinical characteristics
| Left TLE (n = 48) |
Right TLE (n = 48) |
Controls (n = 28) | |||||
|---|---|---|---|---|---|---|---|
| Early N = 21 | Intermediate N = 8 | Late N = 19 | Early N = 9 | Intermediate N = 13 | Late N = 26 | ||
| Age (years) (Mean/SD) | 40.2 ± 11.4 | 33.5 ± 14.6 | 42.0 ± 11.2 | 31.6 ± 10.3 | 37.3 ± 11.5 | 40.5 ± 14.3 | 34.0 ± 11.8 |
| Gender | 14 (66.7%) female |
5 (62.5%) female |
11 (47.9%) female |
5 (55.6%) female |
3 (23.1%) female |
10 (38.5%) female |
13 (46.4%) female |
| Age at onset (years) | 3.0 ± 2.2 | 9.6 ± 2.5 | 25.8 ± 9.5 | 2.9 ± 1.6 | 10.6 ± 2.5 | 29.1 ± 11.7 | – |
| Duration of epilepsy (years) | 37.2 ± 10.6 | 23.9 ± 14.3 | 16.2 ± 10.8 | 28.7 ± 9.4 | 26.7 ± 11.8 | 11.4 ± 11.2 | – |
| Total brain volume (mm3) | 1449 ± 122* | 1475 ± 132 | 1515 ± 158 | 1442 ± 153 | 1585 ± 137 | 1582 ± 163 | 1602 ± 151 |
| Total grey matter volume | 686 ± 79** | 724 ± 67 | 708 ± 69 | 707 ± 75 | 750 ± 47 | 727 ± 90 | 777 ± 83 |
| Total white matter volume | 407 ± 47* | 413 ± 54 | 421 ± 54 | 403 ± 56 | 445 ± 38 | 460 ± 72 | 462 ± 52 |
P < 0.05 in comparison to controls.
P < 0.001 in comparison to controls.
The patient groups were divided in regard to the age of onset of the epilepsy into three different groups: early ≤ 7 years; intermediate 7–15 years; late ≥ 15 years). This division into age groups is based on previous studies showing the different susceptibility of the brain to developmental disturbances with the age of 6 years and under being very plastic in regard to language functions (Lenneberg, 1967) and own data showing that atypical language dominance — as a marker for brain plasticity — is very unlikely at ages above 14 years (Helmstaedter et al., 1997, 2004). This is confirmed also by studies of late plasticity (Hertz-Pannier et al., 2002; Loddenkemper et al., 2003).
A rigid-body-realignment was performed to place all images into the standard coordinate system of the ICBM-305 average brain, using the software package SPM2 (http://www.fil.ion.ucl.ac.uk/spm, FIL, London, UK). This procedure corrects for different head alignment between subjects and assures that callosal measurements are not influenced by different brain orientations.
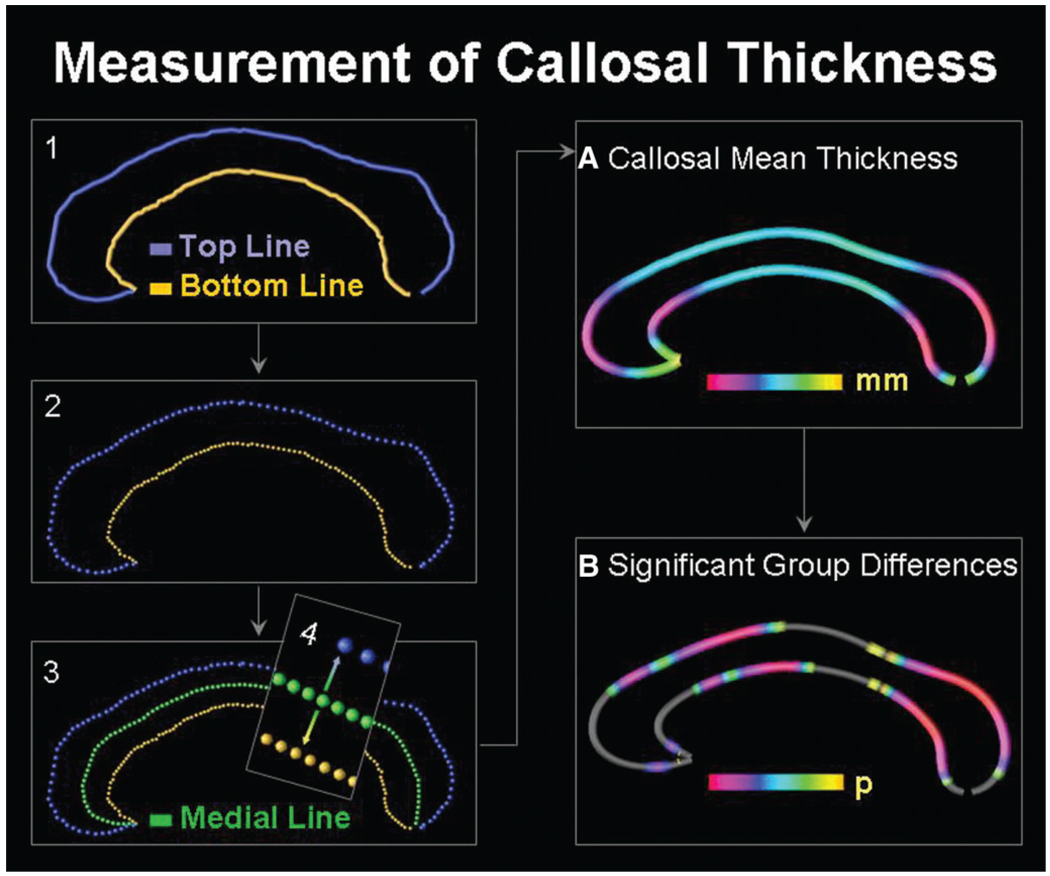
For a detailed description of the callosal measurements, see Luders et al., (2006). Briefly, one rater (JF) who was blind to group status identified the CC in midsagittal sections and delineated the upper and lower callosal boundaries (Fig. 1). Callosal top and bottom sections were redigitized, resulting in 100 equidistant points. Then a new callosal section was created by computing the spatial mean curve (also consisting of 100 equidistant points) from surface points representing the top and bottom sections. Finally, we calculated the point-wise distances (in mm) from the medial section to the callosal top and bottom sections. The resulting distance values in each subject were averaged within patients and controls in order to create colour-coded maps of callosal mean thickness. Finally, utilizing independent sample Student’s t-tests, we tested for group differences in callosal thickness and generated colour-coded maps illustrating statistically significant regions where epilepsy patients differ from controls. Given that independent sample Student’s t-tests were made at many callosal surface points and adjacent data points are highly correlated, permutation testing was employed to control for multiple comparisons. For this purpose, we first determined the spatial extent (supra-threshold count) of the effect in the real experiment as shown in the uncorrected statistical maps in Fig. 3. That is, we calculated the number of significant callosal points at P < 0.05. Subsequently, callosal sections were randomly assigned to either patient or control groups 100 000 times (while keeping the number of subjects in each group the same), and a new statistical test was performed at each callosal point for each random assignment. The number of supra-threshold counts from these randomizations was then compared to the number of significant results in the true assignment (real experiment) to produce a corrected overall significance value for the uncorrected statistical maps (Thompson et al., 2003, 2004).
Fig. 1.
Illustration of callosal thickness measurements. Left panel: (1) After delineating upper callosal boundaries (top line) and lower callosal boundaries (bottom line), we (2) redigitized both callosal sections and (3) computed the spatial mean (medial line) between them. The (4) point-wise distances from the medial line (green) to the top line (blue) and the bottom line (yellow) were calculated. Right panel: Point-wise distance values were averaged within groups to create (A) maps of callosal mean thickness within groups and (B) maps of significant differences between groups.
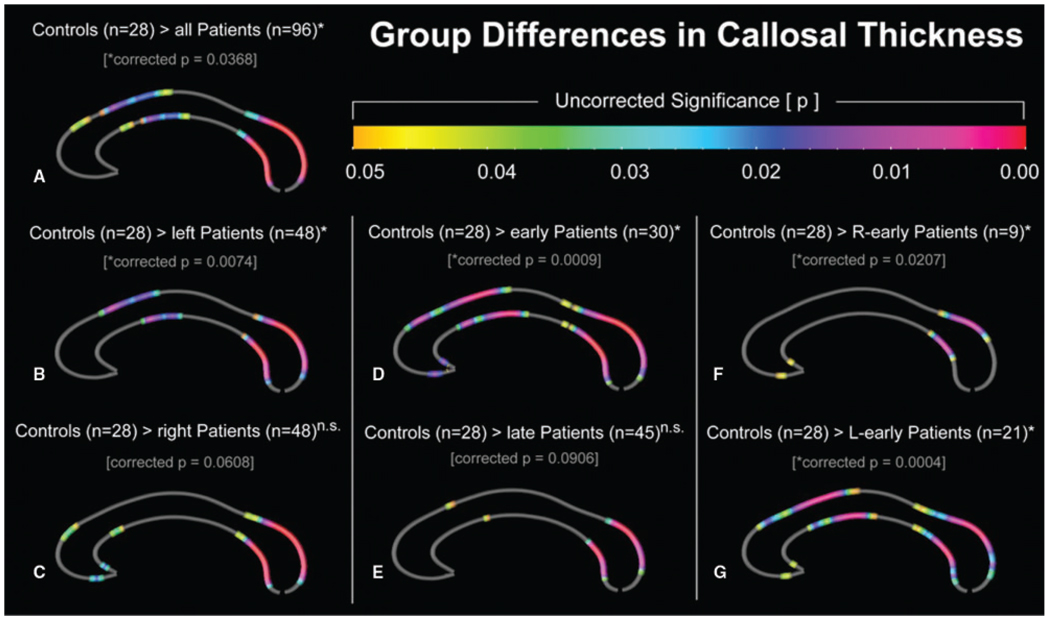
Fig. 3.
Increased callosal thickness in healthy controls compared to epilepsy patients (Controls > Patients): (A) all patients; (B) patients with left temporal lobe epilepsy (left patients); (C) patients with right temporal lobe epilepsy (right patients); (D) patients with early onset temporal lobe epilepsy (early); (E) patients with late onset temporal lobe epilepsy (late); (F) patients with early onset right temporal lobe epilepsy (R-early), and (G) patients with early onset left temporal lobe epilepsy (L-early). The colour bar encodes the uncorrected P-value associated with the t-test performed at each distance value from upper and lower callosal boundaries. The asterisks indicate results that were confirmed by permutation testing (n.s. is non-significant).
The extraction of total brain volume as well as of grey and white matter volumes were performed with the VBM-Toolbox for SPM by Christian Gaser (http://dbm.neuro.uni-jena.de/vbm2_v1.06.zip). The volumes were then entered into separate ANOVAs for total brain volume, grey and white matter volumes to test for group differences.
Results
Volumetric differences
While the early onset left-TLE group differed significantly from the control group in regard to total brain volume (P = 0.021), gray matter volume (P = 0.001) and white matter volume (P = 0.016), no significant differences were detected between any other groups. Notwithstanding, smaller sample sizes and/or slightly higher standard deviations (SD) in, for example, the early onset right-TLE group (in contrast to the early onset left-TLE group) appear to be the reason for the observed non-significant findings. For instance, one might argue that the volumes are comparably decreased for both left and right early onset groups, considering the percent reductions compared to controls (−11.9 and −12.8% for white matter; −11.7 and −9% for gray matter, and −9.6 and −9.98% for total brain volume.
Callosal measurements
All patients
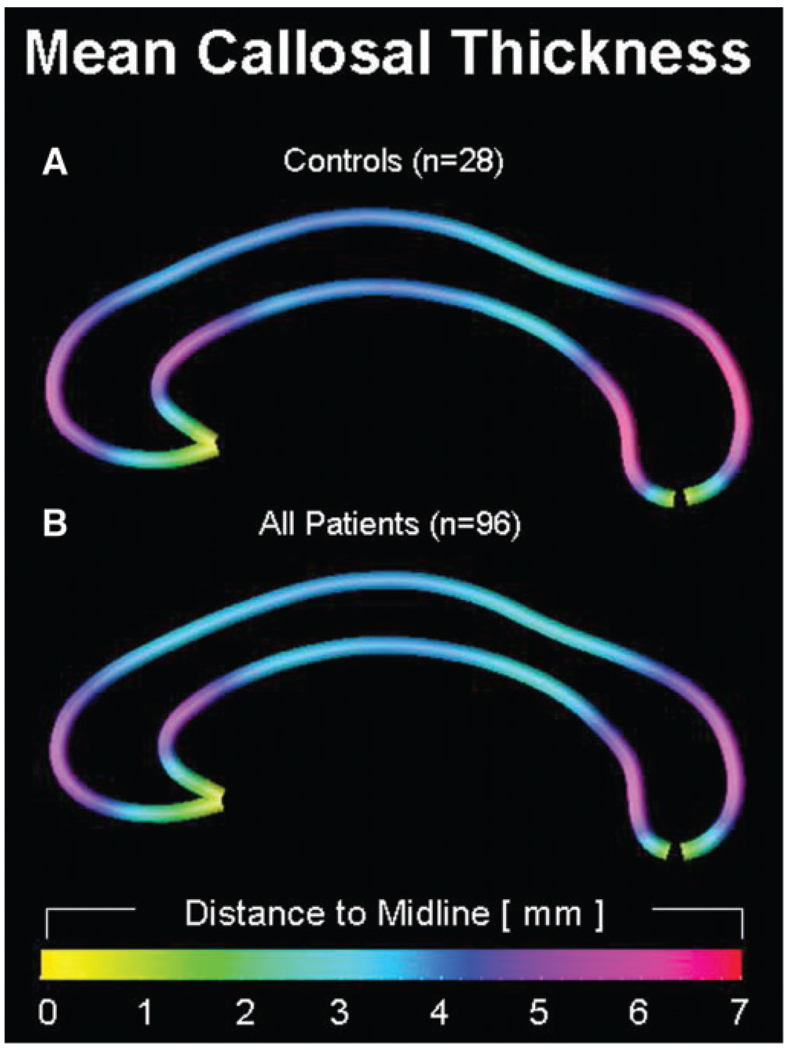
As demonstrated in Fig. 2, the regional patterns of callosal thickness are similar in (a) healthy controls and (b) epilepsy patients. In both groups, callosal thickness is largest where the callosal body bends near its anterior and posterior end. The averaged distances from the upper and lower callosal boundaries to midline attain values as high as 6.4 mm in healthy controls (observed in the splenium), indicating a maximum callosal thickness of 12.8 mm. In contrast, the maximum averaged distance value of the same location in the patient group is only 5.6 (11.2 mm thickness). In both groups, the CC was thinnest at its anterior tip, where upper and lower callosal boundaries merge into each other.
Fig. 2.
Group effects on callosal morphology. Callosal thickness profiles are displayed as colour-coded maps, illustrating the averaged point-wise distances (mm) from the medial line to the top and bottom line, within healthy controls (n = 28) and epilepsy patients (n = 96). Smaller distances correspond to a decreased callosal thickness, and larger distances correspond to an increased callosal thickness.
As demonstrated in Fig. 3A, the control group exhibits significantly thicker CCs than the patient group as a whole in a number of callosal regions, especially posteriorly, but also in regions of the medial and anterior CC (permutation corrected P = 0.0368). The opposite contrast revealed no significant differences.
Lateralization of epilepsy
As further shown in Fig. 3B and C, separate comparisons in left and right-TLE patients reveal smaller posterior CC regions in both patient groups compared to healthy controls. With respect to callosal regions located toward the front of the CC, patients with left TLE exhibit effects of decreased callosal thickness in the medial CC (Fig. 3B; permutation corrected P = 0.0074), while patients with right-lateralized TLE are affected in the anterior CC (Fig. 3C; permutation corrected P = 0.0608). Overall disease effects in anterior callosal regions appear to be more pronounced in patients with left TLE. The control group did not show any decreased callosal area in comparison to left and right-TLE patients.
Onset of epilepsy
As demonstrated in Fig. 3D and E, both early and late onset epilepsy appear to affect posterior callosal regions. Early onset epilepsy, however, tends to additionally affect regions corresponding to the anterior body and also anterior third (Fig. 3D; permutation corrected P = 0.0009; Fig. 3E; permutation corrected = 0.0906). The control group did not show any decreased area in comparison to the early or late onset patient groups.
Interaction of side and onset
As illustrated in Fig. 3F and G, the analysis of left and right early onset TLE patients revealed a decreased CC thickness in posterior regions (Fig. 3F; permutation corrected P = 0.0207), with additional midbody and anterior CC regions affected only in the left early onset group (Fig. 3G; permutation corrected P = 0.0004). The opposite contrasts revealed no significant differences.
Discussion
The CC plays a pivotal role in cognitive functions and seems especially prone to disturbances throughout cortical development. The callosal measurements applied in the present study differ from other approaches, in that no arbitrary sections of the CC needed to be defined. That is, the current method allows the investigation of more subtle differences between groups with a high spatial resolution and without an a priori bias (Luders et al., 2006).
To our knowledge, only one study focused on callosal alterations in dependence of epilepsy onset and the lateralization of the seizure origin (Hermann et al., 2003a). This previous study indicated an effect of early onset TLE on callosal volume, especially in posterior regions. In agreement with these results (Hermann et al., 2003a), we revealed major decreased callosal thickness in posterior regions in TLE patients. Additionally we observed clusters of decreased thickness in further anterior regions. Moreover, differences between left-TLE patients and controls appeared slightly more pronounced than differences between right-TLE patients and controls. Furthermore, the spatial locations of the affected regions were slightly different between both groups in the anterior parts of the CC. These new observations could be related to the more sensitive callosal measurements but also to the larger sample size and thus the increased statistical power in the current study. Finally, the spatial location and magnitude of the disease effect were considerably different, depending on whether early onset or late onset patients were compared to the control group. Hence, although both early and late onset epilepsy appear to affect posterior callosal regions, early onset epilepsy tends to additionally affect regions located further anterior. This holds true especially for early onset left-TLE patients who exhibit, in contrast to early onset right-TLE patients, more pronounced anterior and midbody CC effects. However, given that the sample size is considerably smaller in the early onset right-TLE group (n = 9) compared to early onset left-TLE group (n = 21), limited statistical power may have caused the lower significance and more spatially restricted abnormality clusters in early onset right TLE. Future studies will have to establish whether callosal morphology is similarly affected in early onset TLE, regardless of laterality.
The areas found to be mostly affected by TLE in general are located in the isthmus and splenium of the CC. These areas connect the inferior temporal, occipital as well as superior temporal and parietal regions of each hemisphere. These regions seem to be affected regardless of the lateralization or the age at onset of the epilepsy. The result so far appears to reflect a general non-specific effect of temporal lobe epilepsy on the CC, as it has also been described for whole brain cerebrospinal fluid or gray- and white-matter volumes (Oyegbile et al., 2006). The non-specific effects on brain development correspond well with the neuropsychological finding of a generally impaired intelligence apart from specific temporal-lobe-related memory problems (Helmstaedter and Kockelmann, 2006).
More anterior regions of the CC seem to be affected only in left early onset TLE, indicating that early onset left TLE has a differential impact on anterior brain regions and interhemispheric connection. It could be hypothesized that these additional callosal lesions correspond to distant extra-temporal effects of mesial TLE. These effects could be more pronounced if the dominant hemisphere, which is usually the left hemisphere, is affected. Corresponding distant effects of mesial TLE have been described for frontal lobe metabolism and frontal lobe functions (Jokeit et al., 1997; Nelissen et al., 2006). In parallel to our findings these effects are also pronounced in left lateralized TLE. Metabolic disruption to frontostriatal neural network systems has been discussed as a possible reason of such impairments secondary to temporal lobe pathology (Martin et al., 2000). To further investigate differential impact of early left onset TLE on frontal lobe structure, function and connectivity, studies including larger sample sizes with thorough neuropsychological examinations are needed.
In conclusion, the present study shows a clear influence of TLE on the structure of the CC. Most pronounced effects were present in patients with early onset TLE. Further studies in larger and well-matched patient samples will have to establish whether the stronger effect in early onset left TLE (in contrast to early onset right TLE) is merely a by-product of larger statistical power, or possibly reflect additional functional disturbances associated specifically with left TLE. Finally, future studies examining the relationship between anatomical and neuropsychological measures will elucidate the link between epilepsy, neuropsychology and structural disturbances.
Acknowledgements
Carlos M. Quesada and Bernd Weber were supported by the BMBF Grant 01GW0511. Paul M. Thompson was supported by the National Institute for Biomedical Imaging and Bioengineering, the National Center for Research Resources, the National Institute for Neurological Disorders and Stroke, National Institute on Aging and the National Institute for Child Health and Development (EB01651, RR019771, AG016570, NS049194 and HD050735). Moreover, this work was supported by the National Institutes of Health through the NIH Roadmap for Medical Research, grant U54 RR021813 entitled Center for Computational Biology (CCB). Information on the National Centers for Biomedical Computing can be obtained from <http://nihroadmap.nih.gov/bioinformatics>. Additional support was provided by the NIH/NCRR resource grant P41 RR013642.
Abbreviations
- CC
corpus callosum
- TLE
temporal lobe epilepsy
Footnotes
Supplementary material
Supplementary material is available at Brain online.
References
- Atkinson DS, Aboukhalil B, Charles PD, Welch L. Midsagittal corpus callosum area, intelligence, and language dominance in epilepsy. J Neuroimaging. 1996;6:235–239. doi: 10.1111/jon199664235. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray alter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717–723. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy - Differential atrophy in mesial temporal structures. Neurology. 2005;65:223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- Conlon P, Trimble MR. A study of the corpus-callosum in epilepsy using magnetic-resonance imaging. Epilepsy Res. 1988;2:122–126. doi: 10.1016/0920-1211(88)90029-0. [DOI] [PubMed] [Google Scholar]
- Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Engel J. Mesial temporal lobe epilepsy: what have we learned? Neuroscientist. 2001;7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Brosch T, Kurthen M, Elger CE. The impact of sex and language dominance on material-specific memory before and after left temporal lobe surgery. Brain. 2004;127:1518–1525. doi: 10.1093/brain/awh174. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47:96–98. doi: 10.1111/j.1528-1167.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain Cogn. 1997;33:135–150. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- Hermann B, Hansen R, Seidenberg M, Magnotta V, O’Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003a;18:284–292. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, et al. Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. J Int Neuropsych Soc. 2003b;9:353–362. doi: 10.1017/S1355617703930013. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaque I, et al. Late plasticity for language in a child’s non-dominant hemisphere - a pre- and post-surgery fMRI study. Brain. 2002;125:361–372. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Seitz RJ, Markowitsch HJ, Neumann N, Witte OW, Ebner A. Prefrontal asymmetric interictal glucose hypometabolism and cognitive impairment in patients with temporal lobe epilepsy. Brain. 1997;120:2283–2294. doi: 10.1093/brain/120.12.2283. [DOI] [PubMed] [Google Scholar]
- Lenneberg EH. Biological foundations of language. New York: John Wiley & Sons; 1967. [Google Scholar]
- Loddenkemper T, Wyllie E, Lardizabal D, Stanford LD, Bingaman W. Late language transfer in patients with Rasmussen encephalitis. Epilepsia. 2003;44:870–871. doi: 10.1046/j.1528-1157.2003.66402.x. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Martin RC, Sawrie SM, Gilliam FG, et al. Wisconsin card sorting performance in patients with temporal lobe epilepsy: clinical and neuroanatomical correlates. Epilepsia. 2000;41:1626–1632. doi: 10.1111/j.1499-1654.2000.001626.x. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006;47:900–907. doi: 10.1111/j.1528-1167.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen N, Van Paesschen W, Baete K, et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2006;32:684–695. doi: 10.1016/j.neuroimage.2006.04.185. [DOI] [PubMed] [Google Scholar]
- Okusky J, Strauss E, Kosaka B, et al. The corpus-callosum is larger with right-hemisphere cerebral speech dominance. Ann Neurol. 1988;24:379–383. doi: 10.1002/ana.410240305. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Bhattacharya A, Seidenberg M, Hermann BP. Quantitative MRI biomarkers of cognitive morbidity in temporal lobe epilepsy. Epilepsia. 2006;47:143–152. doi: 10.1111/j.1528-1167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, et al. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23:S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]