Abstract
F2-Isoprostanes (F2-IsoPs), regio- and stereoisomers of prostaglandin F2α (PGF2α), and urinary F2-IsoP metabolites including 2,3-dinor-5,6-dihydro-8-iso-PGF2α (2,3-dinor-8-iso-PGF1α (2,3-dinor-F1)) and 2,3 dinor-8-iso-PGF2α (2,3-dinor-F2), have all been used as biomarkers of oxidative stress. A novel method was developed to measure these biomarkers using a single solid phase extraction (SPE) cartridge, separation by HPLC, and detection by negative mode selected reaction monitoring (SRM) mass spectrometry (MS), using authentic standards of PGF2α; 8-iso-PGF2α; 2,3-dinor-F1 and 2,3-dinor-F2 to identify specific chromatographic peaks. The method was validated in a population of healthy, college-aged nonsmokers (n = 6M/8F) and smokers (n = 6M/5F). Urinary F2-IsoP concentrations were ~0.2–1.5 µg/g creatinine, 2,3-dinor-F1 was ~1–3 µg/g and 2,3-dinor-F2 was ~3–5 µg/g. Additional F2-IsoPs metabolites were identified using SRM. The sum of all urinary F2-IsoP metabolites was 50–100 µg/g creatinine indicating their greater abundance than F2-IsoPs. Women had higher F2-IsoP metabolite concentrations than did men (MANOVA, main effect p = 0.003); cigarette smokers had higher concentrations than did nonsmokers (main effect p = 0.036). For men or women, respectively, smokers had higher metabolite concentrations than did nonsmokers (p < 0.05). Thus, our method simultaneously allows measurement of urinary F2-IsoPs and their metabolites for the determination of oxidative stress.
Keywords: oxidative stress; LC-MS; F2-Isoprostanes; F2-Isoprostane metabolites; prostaglandin F2α (PGF2α); cigarette smoking; gender difference; 2,3-dinor-5,6-dihydro-8-iso-PGF2α; 2,3 dinor-8-iso-PGF2α
INTRODUCTION
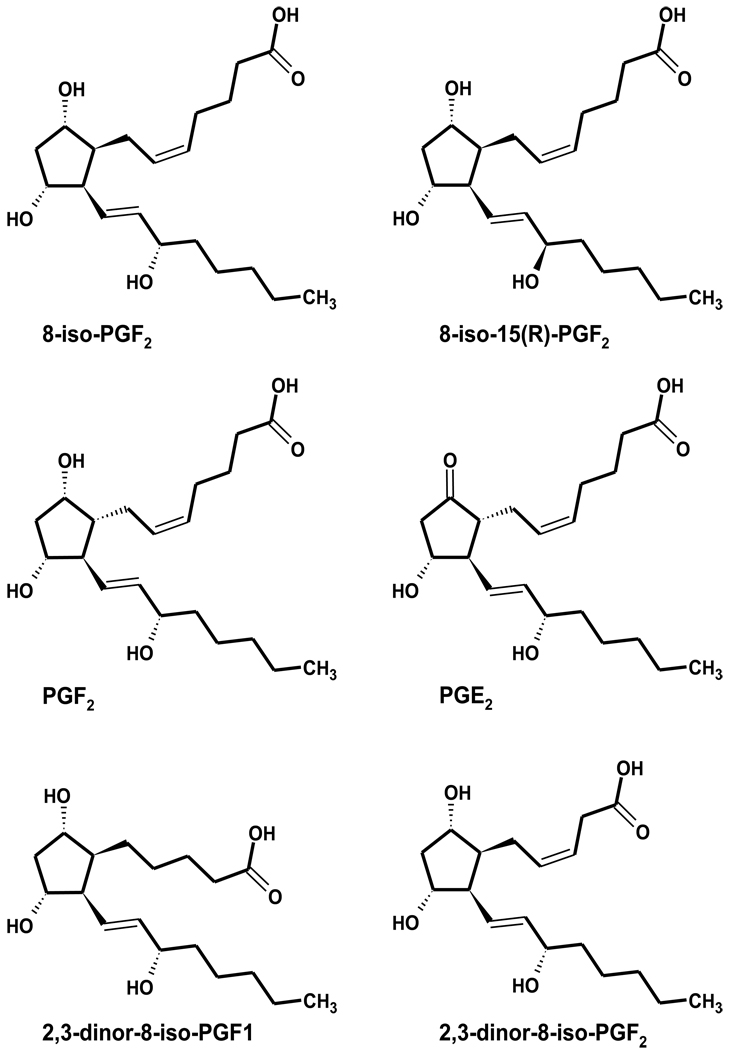
F2-Isoprostanes (F2-IsoPs, Figure 1) are regio- and stereoisomers of prostaglandin F2α (PGF2α) formed by the free radical-mediated oxidation of arachidonic acid (1). Thus, F2-IsoPs, particularly the 15-series regioisomer, 8-iso-PGF2α, are often used as biomarkers of oxidative stress (2, 3), and have been measured using well-established GC-MS and ELISA methods (4). Recent advances in LC-MS methods have allowed faster and simpler sample preparation (4, 5).
Figure 1. Structures of F2-Isoprostanes (F2-isoPs) and metabolites.
Plasma or tissue F2-IsoPs concentrations provide a “snap-shot” assessment of oxidative stress. However, concerns have arisen due to the potential for artifacts arising from in vitro lipid oxidation changes during sample preparation (6). Thus, measurement of urinary F2-IsoPs was proposed to be more reliable and less prone to artifactual increases in measured products thereby allowing for the more reliable assessment of oxidative stress in vivo. (6). Urine also contains appreciable levels of F2-IsoP metabolites (4, 6, 7), which could be measured simultaneously to bolster urinary F2-IsoP results. Additionally, urine can be collected non-invasively from humans. Thus, urinary F2-IsoPs may provide a better assessment of oxidative stress than samples from other biological materials, such as plasma.
Potentially, urinary F2-IsoP metabolites may be better biomarkers of oxidative stress (4, 6, 7). Roberts et al (6) determined that 2,3-dinor-5,6-dihydro-8-iso-PGF2α (2,3-dinor-F1, Figure 1) was the major metabolite of 8-iso-PGF2α. Chiabrando et al (4) also identified 2,3-dinor-8-iso-PGF2α (2,3-dinor-F2) as an additional urinary 8-iso-PGF2α metabolite. Interestingly, both 2,3-dinor-F1 and -F2 were present in similar urinary concentrations. Although both groups developed GC-MS procedures for the identification of these compounds (4, 8), the GC-MS methodology described by Nourooz-Zadeh et al (7) facilitated the simultaneous measurement of these metabolites along with the parent compound, 8-iso-PGF2α. Importantly, there is consensus among these investigators that the F2-IsoPs metabolites, 2,3-dinor-F1 and -F2, are present in significantly greater urinary concentrations than is 8-iso-PGF2α, suggesting that the metabolites may be more easily measured biomarkers. Moreover, F2-IsoP metabolism may be important in decreasing adverse consequences of oxidative stress and increasing removal of oxidatively damaged lipids (9).
LC-MS methodologies for the determination of these metabolites have been developed (5, 10). These methods stressed high sample throughput by using short HPLC columns (e.g. rocket method) and reported a single value for the F2-IsoP metabolitess. However, the metabolites from the 64 possible isomers of F2-IsoPs are likely to be numerous. Moreover, it is unlikely that all species from a biological sample having the same molecular-ion-to-product-ion reaction are derived from a single parent compound. Thus, the evaluation of F2-IsoP metabolites resolved by prolonged HPLC separation as we described for plasma F2-IsoPs (11), may be more advantageous than existing rocket methods because quantitation of specific biomarkers could potentially provide information concerning metabolism of specific precursors.
To this end, we developed a method that uses one solid phase extraction (SPE) cartridge, followed by prolonged gradient HPLC-MS-MS, to identify and quantitate urinary F2-IsoPs and their metabolites, specifically including 2,3-dinor-F1 and -F2. Additionally, we have identified several other potential F2-IsoP metabolites using selected reaction monitoring (SRM). The procedure was validated by assessing urinary F2-IsoPs and their metabolites from a cohort of otherwise healthy nonsmokers and smokers, described previously (12). Free F2-IsoPs were measured previously in the plasma from these individuals (12), as were total (free + esterified) 15-series F2-IsoPs (11). While mean F2-IsoP concentrations were numerically higher among smokers compared to nonsmokers, the differences were not statistically significant for any measured analyte due to large within-group variation. Thus, the simultaneous quantitation of urinary F2-IsoPs and their metabolites from this same cohort will assess the utility of measuring F2-IsoPs in urine versus plasma as well as measuring F2-IsoP metabolites versus parent F2-IsoPs in urine.
EXPERIMENTAL PROCEDURES
Materials
HPLC grade solvents and reagents were obtained from VWR (West Chester, PA). Purified water was obtained from a Millipore Milli-Q apparatus (Billerica, MA). Authentic samples of 8-iso-prostaglandin F2α (8-iso-PGF2α), PGF2α, PGE2, 2,3-dinor-8-iso-PGF1α, and 2,3-dinor-8-iso-PGF2α, and the deuterated internal standards 8-iso-PGF2α-d4 and PGE2-d4 were obtained commercially (Cayman Chemical; Ann Arbor, MI).
Study Design and Sample Collection
The study protocol for assessing vitamin E pharmacokinetics and oxidative stress markers in smokers and nonsmokers was reported previously (12). The protocol was approved by the Institutional Review Board for the protection of human subjects at Oregon State University. At the time of the study, participants consented to the use of archived specimens for the continued assessment of oxidative stress biomarkers.
In brief, healthy, 18–35 year old male and female nonsmokers (n = 6M/8F) and smokers (n = 6M/5F; 10–20 cigarettes/d) were recruited from the university area (12). Participants were not nutritional supplement users (>6-mo) and they maintained normal exercise patterns (<7 h/wk aerobic exercise). The study was designed as a randomized, double-blind, placebo-controlled vitamin C intervention study in which participants received vitamin C (500 mg; twice daily) or matching placebo for 17 d. On d 14, participants received an equimolar mixture of deuterium-labeled α- and γ-tocopherols (~50 mg each d6-α- and d2-γ-tocopheryl acetates) on a single occasion and blood was collected in regular intervals from 0 to 72 h following labeled-tocopherol administration. Urine was collected in 8 h intervals from 0 to 24 h, aliquoted, and subsequently stored at −20° C. The last 8 h collection (16–24 h) contained the first void on the second day of the trial. At the completion of the trial, all participants underwent a 3-month washout period, and subsequently returned to the study center to complete the alternate arm of the study as described. During each supplementation period, participants were instructed to follow a low ascorbic acid diet; plasma ascorbic acid was reported previously (12).
Plasma unesterified F2-IsoPs were measured previously using GC-MS (12). Plasma samples were analyzed previously for total (free + esterified) F2-IsoPs by HPLC-MS-MS (11). The data reported herein from 24 h urine collections are limited to those participants who completed the vitamin C intervention and respective placebo trial and provided complete urine samples from each 8 h collection period. Thus, the present investigation is limited to nonsmokers (n = 6M/8F) and smokers (n = 6M/5F). Smokers had lower body mass indexes (BMI) than nonsmokers and higher cotinine concentrations; women had higher high density lipoprotein (HDL) cholesterol concentrations (Table 1). Otherwise, there were no major differences between genders or smoking status groups.
Table 1.
Participant Characteristics1
| Serum Lipids | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gender | Group | Age (y) |
BMI* (kg/m2) |
TG (mg/dL) |
Chole- sterol (mg/dL) |
HDL- Chole- sterol (mg/dL) |
Cotinine (ng/mL) |
Cigarettes/d |
| Women | Non- smoker |
22.4 ± 0.7 | 24.3 ± 1.0a | 71 ± 12 | 164 ± 4 | 76 ± 5a | 28 ± 5b | 0c |
| Smoker | 20.2 ± 0.7 | 23.4 ± 2.2a,b |
92 ± 18 | 161 ± 13 | 72 ± 15a,b | 1473± 263a |
8 ± 1b | |
| Men | Non- smoker |
23.0 ± 0.7 | 26.6 ± 0.9a | 81 ± 17 | 160 ± 18 | 48 ± 5b | 23 ± 5b | 0c |
| Smoker | 22.5 ± 1.3 | 20.9 ± 0.4b | 84 ± 18 | 155 ± 9 | 64 ± 5a,b | 3037 ± 708a |
14 ± 2a | |
| Statistical Summary (MANOVA) | ||||||||
| Gender | NS | NS | NS | NS | 0.03 | NS | 0.022 | |
| Smoking | NS | 0.014 | NS | NS | NS | <0.0001 | <0.0001 | |
| Inter- action |
NS | NS | NS | NS | NS | NS | 0.022 | |
BMI = body mass index (body weight (kg)/height (m2)
Participant characteristics (mean ± SE) were analyzed using MANOVA and Tukey’s post hoc where appropriate for pair-wise comparisons. Main effects are listed in the table and pair-wise comparisons not sharing the same superscript are significantly different (p < 0.05).
Sample Preparation
Urine was thawed at room temperature, mixed by inversion, and centrifuged (200 × g, 5 min). The supernatant (600 µL) was mixed with 500 µL methanol, internal standards (IS, 12 ng 8-iso-PGF2α-d4 and 3 ng PGE2-d4 in 24 µL 1:1 methanol:water) added, followed by addition of buffer (2.0 mL 0.02 M bis-tris-HCl) with mixing at each step. The diluted sample pH was then adjusted using H3PO4 or KOH to pH 6.0 ± 0.05.
Strata X-AW cartridges (100 mg/3 mL, Phenomenex, Torrance, CA) were each pre-conditioned with 2 mL 98:2 methanol:formic acid (v:v) and subsequently equilibrated with 2 mL water. Diluted urine samples were loaded, the cartridges washed with 2 mL water, followed by 4 mL 25% methanol, 2 mL acetonitrile, and cartridges dried under a gentle vacuum (~5 mm Hg, 30 sec). Cartridges were then eluted 3-times with 1 mL methanol, and the eluants from each cartridge pooled and collected into glass tubes. Samples were dried under nitrogen gas, reconstituted in 200 µL methanol containing 0.1% formic acid (v:v), and injected onto the LC-MS-MS.
Chromatography and Mass Spectrometry
Separation was carried out using a Shimadzu HPLC system (Columbia, MD) consisting of two LC-10ADvp pumps, a DGU-14A degasser, a SIL-HTc autosampler-system controller maintained at 10° C, and a CTO-10Avp column oven (35° C) with a Synergi Hydro-RP column (250 × 2 mm i.d., 4 µm) equipped with a SecurityGuard C18 guard cartridge (4 × 2 mm; both from Phenomenex; Torrance, CA). Mobile phases A (water) and B (methanol) contained 0.01% acetic acid (v/v) and were delivered at a total flow of 200 µL/min using the following gradient scheme: 5 min equilibration at 30% B prior to injection, 30% B from 0–0.5 min, followed by a linear gradient over 1 min to 58% B, a linear gradient over 13.5 min to 66% B, a linear gradient over 7 min to 69% B, then to 100% B over 2 min which was held until the completion of the run at 32 min.
The HPLC system was coupled to a triple quadrupole mass spectrometer with TurboIon Spray source operated with both mass analyzers set at unit resolution in negative mode (Applied Biosystems/MDS Sciex API 3000, Foster City, CA). Nebulizer, curtain, and collision (CAD) gas parameters were set at 12, 10, and 7, respectively. Heater gas was supplied at 6 L/min at 425°C. All gases were high purity nitrogen supplied by a custom liquid nitrogen system (Polar Cryogenics, Portland, OR). The ionizing voltage was −4000 V, and the declustering, focusing, entrance, and exit potentials were −50, −250, −8, −35, and −11 V, respectively.
To identify chromatographic peaks, product ion spectra of authentic standards of 8-iso-PGF2α, PGF2α, 2,3-dinor-F1, and 2,3-dinor-F2 were acquired at collision energies (CE, see below) that produced a molecular ion intensity of 30–80% of the most abundant fragment ion. Then, each standard was analyzed by gradient HPLC and detected by single reaction monitoring (SRM) experimentation based on the major fragment ions in the corresponding product ion spectrum, thereby creating a multi-trace profile for each compound. Next, a human urine sample was analyzed similarly to identify corresponding chromatographic peaks eliciting identical SRM characteristics to each specific standard.
Analytes were detected and quantified using SRM of seven reactions:
15-series PGFs, m/z 353 to 193, CE −35 V
8-iso-PGF2α-d4 internal standard, m/z 357 to 197, CE −35 V
PGE2, m/z 351 to 189, CE −27 V; PGE2-d4, m/z 355 to 193, CE −27 V.
2,3-dinor-F1, m/z 327 to 283, CE −28 V
2,3-dinor-F2, m/z 325 to 237, CE −22 V
Standard curves were constructed at 8 concentrations for 8-iso-PGF2α, PGF2α, PGE2 (100–4000 pg/mL), 2,3-dinor-F1 (1250–50,000 pg/mL), and 2,3-dinor-F2 (2500–100,000 pg/mL). 8-iso-PGF2α-d4 was used as the IS for all analytes with the exception that PGE2-d4 was used for PGE2. Quantitation was performed using the Analyst 1.41 software (Applied Biosystems/MDS Sciex). The Analyst software calculated signal to noise (S/N) ratios for 2,3-dinor-F1 (m/z 327 to 283) ranging from 6 to 50 (15 – 20 as the mode values), when a representative, non-zero background region of a urine sample chromatogram (at 4 – 6 minutes) was chosen as the reference background.
Accuracy and Precision
Assay accuracy was evaluated by standard addition experiments conducted in urine and methanol. Known quantities of 8-iso-PGF2α, PGF2α, PGE2, 2,3-dinor-F1, and 2,3-dinor-F2 were spiked, in duplicate, into urine or methanol. Spiked urine was extracted as described above and spiked methanol was analyzed by LC-MS without prior extraction. Intra-day- and between-day precision was determined by analyzing five aliquots of urine on five separate occasions throughout a month.
Statistical Analysis
Data are expressed as mean ± SE. Repeated measures MANOVA was performed using JMP Statistical Discovery Software (SAS Institute, Cary, NC) to evaluate effects attributed to smoking status, gender, and with-in subject vitamin C treatment effects and time effects from urine samples collected in three 8 h intervals over 24 h (0–8, 8–16, and 16–24 h). For the analysis of repeated measures (multiple samples from the same subject), the natural logarithm of the dependent variables (urine concentration at the three time points in each study period, or vitamin C treatment) was used to minimize the correlation between the mean and variance of the data. Results were considered to be statistically significant at p < 0.05. Vitamin C had no effect on the oxidative stress parameters studied, as has been observed previously (13), so data from time points were averaged, as indicated. Similarly, when other parameters did not reach statistical significance, the values from each subject were averaged and the statistical analysis was repeated using matched pairs or MANOVA with repeated measures, as appropriate. It should be noted that as the numbers of data points decreased the statistical power decreased.
RESULTS
Precision and Accuracy of the SPE extraction and LC-MS analyses
The intra-day and inter-day precision of the SPE extraction and LC-MS analyses were evaluated from five aliquots of a single urine specimen analyzed over one month on five separate occasions. For the five analytes for which standards are available (8-iso-PGF2α; PGF22α; PGE2; 2,3-dinor-F1 and 2,3-dinor-F2), the intra-day coefficients of variation (CV) for each of the standards ranged from 6 to 9%, while the inter-day CVs for each ranged from 10 to 14%.
Assay accuracy was determined by comparing the ratio of the slopes of the line determined from measured amounts recovered from spiked human urine samples (standard addition) with the slope generated from spiked methanol. Standard addition experiments indicated that the accuracy of the assay was 101% for 8-iso-PGF2α, 104% for PGF2α, 102% for PGE2, 59% for 2,3-dinor-F1, and 86% for 2,3-dinor-F2. Because the measured concentrations of 2,3-dinor-F1 and F2 were within the quantitative range of the standard curves, the lower accuracy for these analytes was apparently the result of lower extraction recoveries. Therefore, the extraction protocol was optimized for maximum recovery of 2,3-dinor-F1 and -F2.
The standard addition regression estimates were linear in the range of 0, 20, 50, and 100% of standard mix added (equivalent to a urinary concentration of 8-iso-PGF2α, 1000 pg/mL; PGF2α and PGE2, 2000 pg/mL; 2,3-dinor-F1, 5000 pg/mL, and 2,3-dinor-F2 10,000 pg/mL at 100%). The standard curves used for quantitation were linear from 0 to 4000 pg/mL for 8-iso-PGF2α, PGF2α, and PGE2; 0 to 40 ng/mL for 2,3-dinor-F1; and 0 to 80 ng/mL for 2,3-dinor-F2.
Identification of Metabolites with SRM patterns similar to 2,3-dinor-F1 and 2,3-dinor-F2
Identification of biomarkers of lipid peroxidation is difficult due to the variety of lipid oxidation products, which potentially may be metabolized to an even greater variety of products. In addition, few authentic standards are available commercially. To circumvent these potential limitations, we used a prolonged HPLC gradient to separate multiple peaks from human urine samples and identified those peaks with product ion spectra matching those of available standards.
F2-IsoPs in human urine were identified tentatively as 8-iso-15(R)-PGF2α, 8-iso-PGF2α, and PGF2α because they had retention times identical to those of the standards and they had SRM profiles matching the authentic standards, 8-iso-PGF2α and PGF2α (data not shown).
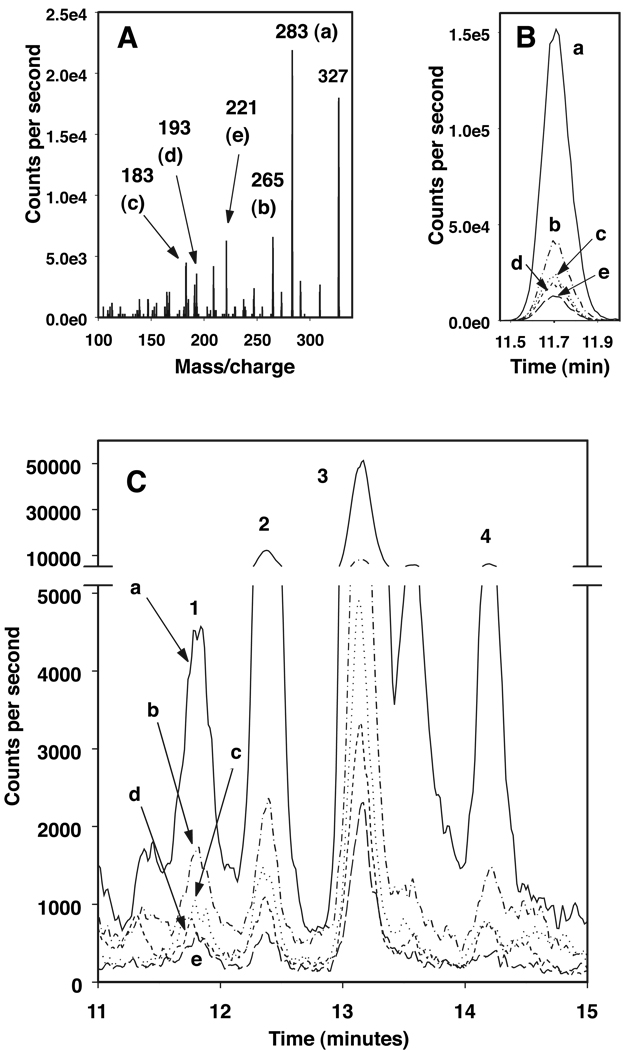
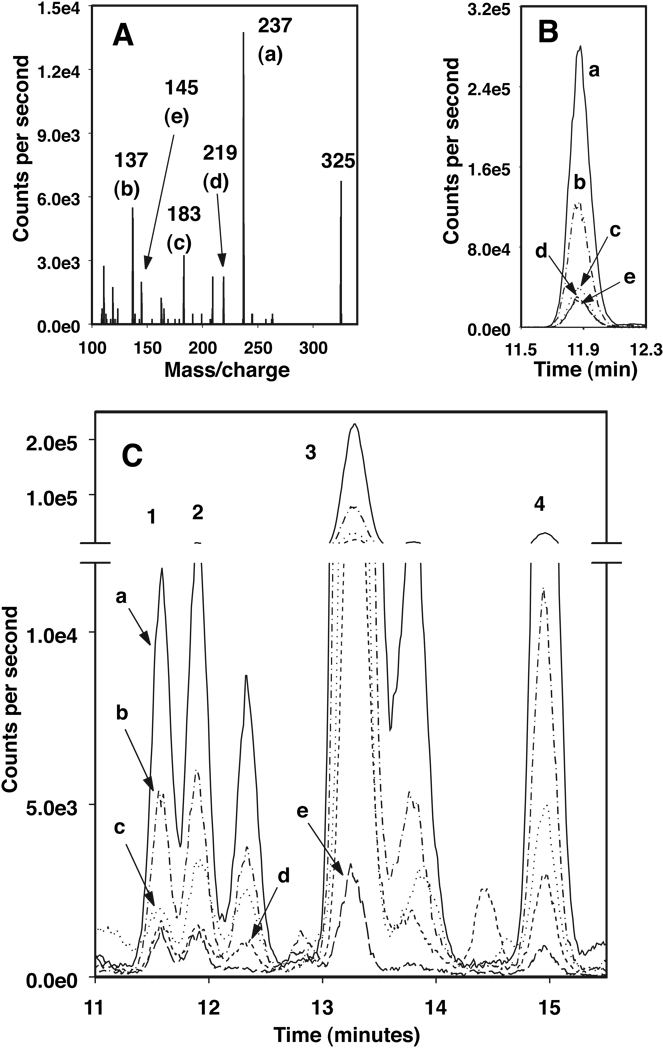
Product ion spectra of the 2,3-dinor-F1 and -F2 standards (Figure 2A and Figure 3A, respectively) were similar to those reported previously by others (5, 10). The retention times shown from the HPLC-SRM tracings of the 2,3-dinor-F1 and -F2 standards were 11.7 and 11.9 min, respectively (Figure 2B and Figure 3B, respectively). However, when urine sample extracts were analyzed, additional, sometimes larger, peaks had the same SRM profiles as these standards. Three peaks (identified as 2, 3 and 4 in Figure 2C), had SRM profiles that matched 2,3-dinor-F1 (Figure 2B, identified as 1 in 2C). Similarly, three peaks (identified as 1, 3 and 4 in Figure 3C) had SRM profiles that matched 2,3-dinor-F2 (Figure 3B, identified as 2 in 3C).
Figure 2. LC-MS identification of 2,3-dinor-PGF1α.
(A) Fragmentation pattern of 2,3-dinor-PGF1α standard, m/z 327 to 283 (a), 265 (b), 183 (c), 193 (d) or 221 (e).
(B) LC-MS chromatogram of 2,3-dinor-PGF1α standard showing tracings of each of the fragments (a–e) identified in A.
(C) LC-MS chromatogram of a urine extract with tracings of peaks identified with SRM profiles showing each of the fragments (a–e, identified in A) that matched those of 2,3-dinor-PGF1α (2,3-dinor-F1, retention time same as the standard, labeled 1; F1-12, labeled 2; F1-13, labeled 3, and F1-14 labeled 4).
Figure 3. LC-MS identification of 2,3-dinor-PGF2〈.
(A) Fragmentation pattern of 2,3-dinor-PGF2α standard, m/z 325 to 237 (a), 137 (b), 183 (c), 219 (d) or 145 (e).
(B) LC-MS chromatogram of 2,3-dinor-PGF2α standard showing tracings of each of the fragments (a–e) identified in A.
(C) LC-MS chromatogram of a urine extract with tracings of peaks identified with SRM profiles showing each of the fragments (a–e, identified in A) that matched those of 2,3-dinor-PGF2α (2,3-dinor-F2, retention time same as the standard, labeled 2; F2-12, labeled 1; F2-13, labeled 3, and F2-15 labeled 4).
Peaks are named according to their metabolite class and retention time (e.g. F1-12 from a 2,3-dinor-F1 SRM match eluting at 12 minutes).
F2-IsoPs in Urine from Smokers and Nonsmokers
The method described herein allows the simultaneous measurement of both F2-IsoPs and F2-IsoP metabolites in a single extraction and LC/MS separation. Urinary F2-IsoPs (Figure 4), specifically 8-iso-PGF2α, 8-iso-15(R)-PGF2α and PGF2α, were measured in three separate urine specimens collected in 8 h intervals over 24 h from smokers and nonsmokers who completed placebo and vitamin C supplementation trials (12). Thus, six samples were analyzed for each subject. Remarkably, neither the time of day, nor vitamin C supplementation had any effect on the urinary excretion of any of the three F2-IsoPs measured (p > 0.05, MANOVA). The consistency of the methodology described herein is readily apparent in that the urinary concentrations for each of the subjects did not vary widely for any of the three F2-IsoPs measured (Figure 4).
Figure 4. Urinary F2-isoPs determined over 24 h in smokers and nonsmokers.
The urinary F2-isoPs: 8-iso-PGF2α, 8-iso-15(R)-PGF2α and PGF2α, were quantified both during placebo and vitamin C supplementation in smokers and nonsmokers from 3 separate urine collections (0 to 8 h, 8 to 16 h, and 16 to 24 h after taking deuterated α-tocopherol during a vitamin E kinetics study [12]). Neither the time of day, nor the supplementation with vitamin C had any effect on the urinary excretion of each of the three F2-isoPs measured (p > 0.05, MANOVA). 8-iso-PGF2α concentrations were significantly increased in smokers (p = 0.012, MANOVA, main effect of smoking). Women compared with men excreted significantly (MANOVA, main effect of gender) higher concentrations of 8-iso-PGF2α (p = 0.02), 8-iso-15(R)-PGF2α (p = 0.04), and PGF2α+ ent-PGF2α (p = 0.015).
In contrast to our expectation that cigarette smoking would increase all of the urinary F2-IsoPs identified, we found that only 8-iso-PGF2α concentrations were significantly increased in smokers (p = 0.012, MANOVA, main effect of smoking). However, women compared with men (MANOVA, main effect of gender) excreted significantly greater 8-iso-PGF2α (p = 0.02), 8-iso-15(R)-PGF2α (p = 0.04), and PGF2α (p = 0.015) concentrations.
Given the lack of statistical significance in the F2-IsoP concentrations between time points, or with vitamin C supplementation, the concentrations for each of the six samples were then averaged for each subject (three collections for each of the two supplements) and the statistical analyses repeated. Nonsmoking men had the lowest F2-IsoP concentrations, while women smokers had the highest concentrations (Table 2). In general, urinary PGF2α concentrations were approximately double those of either 8-iso-PGF2α or 8-iso-15(R)-PGF2α. Although PGF2α is the largest of the 15-series peaks in our samples, it is likely a mixture of PGF2α and ent-PGF2α that we cannot resolve, based on the findings by Yin et al (14), who used a chiral column and showed that the major portion of PGF2α in human urine is the enantiomer (ent) form of PGF2α. Thus, this peak is called PGF2α + ent-PGF2α.
Table 2.
Urinary F2-Isoprostanes in smokers and nonsmokers1
| Gender | Group | 8-iso-PGF2α (µg/g creatinine) |
8-iso-15(R)- PGF2α (µg/g creatinine) |
PGF2α+ ent-PGF2α (µg/g creatinine) |
|
|---|---|---|---|---|---|
| Women | Nonsmoker | 0.43 ± 0.06a | 0.61 ± 0.09a,b | 1.27 ± 0.16a | |
| Smoker | 0.52 ± 0.03a | 0.65 ± 0.09a | 1.24 ± 0.08a | ||
| Men | Nonsmoker | 0.28 ± 0.01b | 0.39 ± 0.03b | 0.74 ± 0.09b | |
| Smoker | 0.43 ± 0.06a | 0.54 ± 0.09a,b | 1.12 ± 0.15a | ||
| Statistical Summary (MANOVA) | |||||
| Gender | p = | 0.019 | 0.04 | 0.015 | |
| Smoking | p = | 0.012 | NS | NS | |
| Interaction | p = | NS | NS | NS | |
No significant differences were observed for vitamin C supplementation or for each 8 h collection interval; therefore, urinary F2-IsoPs were averaged within each participant over time and vitamin C supplementation. Urinary F2-IsoPs were analyzed using MANOVA and Tukey’s post hoc where appropriate for pair-wise comparisons. Main effects are listed in the table and pair-wise comparisons within a column not sharing the same superscript are significantly different (p < 0.05).
2,3-dinor-F1 and 2,3-dinor-F2 in Urine from Smokers and Nonsmokers
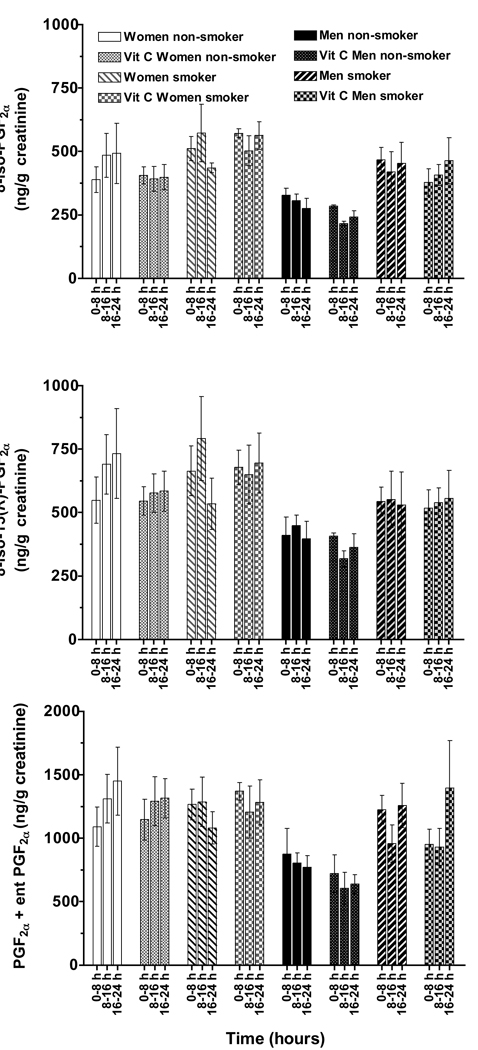
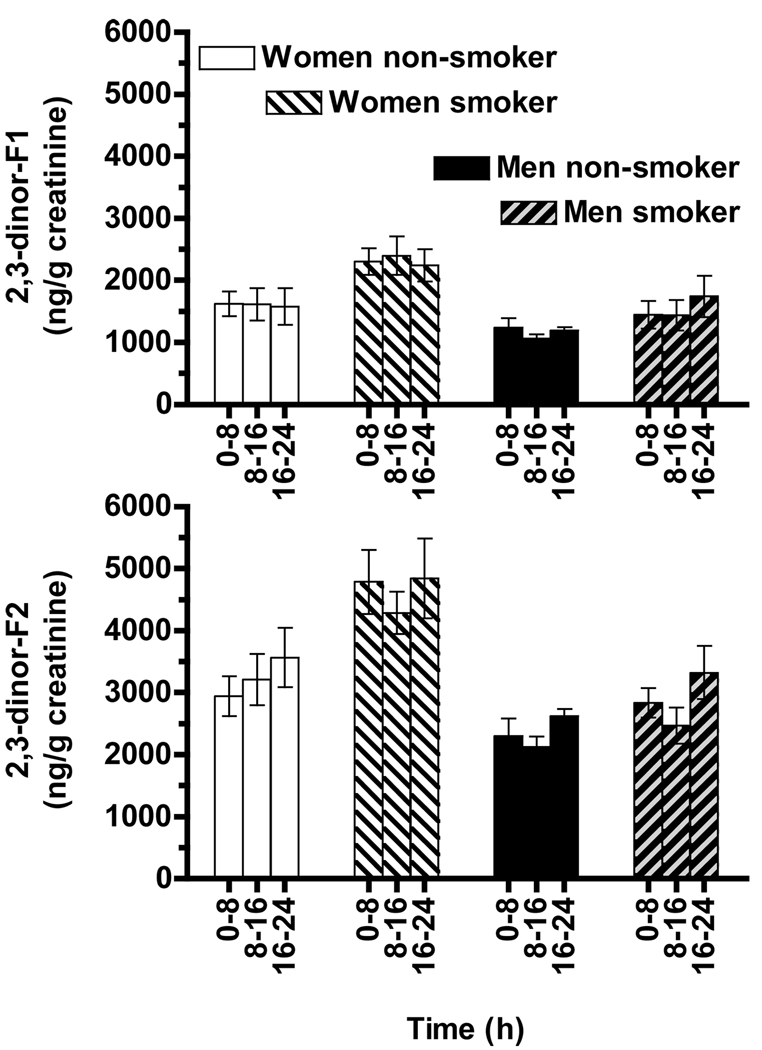
The urinary F2-IsoPs metabolites, 2,3-dinor-F1 and -F2, were measured in the same extract and during the same HPLC analyses as described above for the urinary F2-IsoPs. Similar to the F2-IsoPs data, no significant effects of vitamin C supplementation on urinary 2,3-dinor-F1 or -F2 concentrations were observed (data not shown). The consistency of the 2,3-dinor-F1 and 2,3-dinor-F2 measurements is readily apparent in the concentrations (averages for each time point obtained during the vitamin C and placebo trials) shown for the three time points for each group (Figure 5A and B).
Figure 5. Urinary isoprostane metabolites excreted by smokers and nonsmokers.
Urinary 2,3 dinor F1 (A) and 2,3 dinor F2 (B) concentrations, respectively, were averaged for the vitamin C and placebo supplementation trials at each of the three time points.
A. Women excreted higher 2,3-dinor-F1 concentrations than did men (MANOVA, main effect of gender p = 0.025); cigarette smokers excreted higher concentrations than did non-smokers (main effect of smoking p = 0.039).
B. Women excreted more 2,3-dinor-F2 than did men (MANOVA, main effect of gender p = 0.0012); cigarette smokers excreted higher concentrations than did non-smokers (main effects of smoking p = 0.013). 2,3-dinor-F2 concentrations also varied with time (main effect, p = 0.006).
Women excreted more 2,3-dinor-F1 than did men (MANOVA, main effect of gender, p = 0.025); cigarette smokers also excreted more 2,3-dinor-F1 than did nonsmokers (main effect of smoking P=0.039, Figure 5A). When 2,3-dinor-F1 concentrations were averaged over the six time points for each subject, women smokers had significantly higher concentrations (p < 0.05, 2.3 ± 0.3 µg/g creatinine) than did nonsmokers (women 1.6 ± 0.3, or men 1.2 ± 0.1), or male smokers (1.5 ± 0.3).
Women also excreted more 2,3-dinor-F2 than did men (MANOVA, main effect of gender p = 0.0012). Similarly, cigarette smokers excreted more 2,3-dinor-F2 than did nonsmokers (main effect of smoking p = 0.013, Figure 5B). However, 2,3-dinor-F2 concentrations varied with time (main effect, p = 0.006). When the 2,3-dinor-F2 data were analyzed separately for men and women, interesting differences were apparent. Women smokers excreted more 2,3-dinor-F2 (4.6 ± 0.5 µg/g creatinine) than did nonsmokers (3.2 ± 0.4 µg/g, p = 0.036), but concentrations did not vary significantly with the time of day. In contrast, cigarette smoking had no significant effect on the 2,3-dinor-F2 excretion in men. However, men excreted greater 2,3-dinor-F2 concentrations (3.0 ± 0.3 µg/g creatinine) during the overnight collection (16 to 24 h) compared with 8 to 16 h (2.3 ± 0.2, p = 0.001). The collections taken during the day 0 to 8 h (2.6 ± 0.3 µg/g creatinine) were also greater than during 8 to 16 h (p = 0.03). These latter data suggest that men may have increased F2-IsoPs metabolism and excretion overnight into morning.
Additional Urinary F2-IsoPs Metabolites in Smokers and Nonsmokers
In addition to 2,3-dinor-F1 and -F2, we identified using SRM several other potential urinary F2-IsoP metabolites (Figure 2 and Figure 3, respectively). Pair-wise correlations between all data (n = 144) showed that F1-12 and F1-13 concentrations were correlated with 2,3-dinor-F1 (r > 0.9, p < 0.0000); F2-12, F2-13 and F2-15 concentrations were correlated with 2,3-dinor-F2 (r = 0.7 to 0.9, p < 0.0000); while F1-14 had lower correlation coefficients with concentrations of either 2,3-dinor-F1 or -F2 (r < 0.4, Table 3). Of particular interest, F1-14 was not correlated (p > 0.05) with 8-iso-15(R)-PGF2α suggesting that it has a different precursor than do the other metabolites.
Table 3.
Pair-wise Correlations between F2-IsoPs and their Putative Metabolites
| 8-iso- PGF2α |
8-iso- 15(R)- PGF2α |
PGF2α+ ent- PGF2α |
2,3- dinor- F1 |
F1-12 | F1-13 | F1-14 | 2,3- dinor- F2 |
F2-12 | F2-13 | F2-15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8-iso-PGF2α | 1.0000 | 0.9066 | 0.7938 | 0.6008 | 0.5641 | 0.5797 | 0.2407 | 0.6317 | 0.6360 | 0.5143 | 0.4448 |
|
8-iso-15(R)- PGF2α |
1.0000 | 0.7263 | 0.4304 | 0.3834 | 0.4474 | 0.1090 | 0.4629 | 0.4678 | 0.4286 | 0.3674 | |
|
PGF2α+ ent- PGF2α |
1.0000 | 0.3280 | 0.3307 | 0.3458 | 0.3046 | 0.4434 | 0.4372 | 0.4294 | 0.4987 | ||
|
2,3-dinor- F1 |
1.0000 | 0.9292 | 0.9399 | 0.4010 | 0.8555 | 0.8552 | 0.6641 | 0.4483 | |||
| F1-12 | 1.0000 | 0.9367 | 0.5028 | 0.8336 | 0.8192 | 0.6879 | 0.4841 | ||||
| F1-13 | 1.0000 | 0.3794 | 0.8628 | 0.8729 | 0.7539 | 0.4982 | |||||
| F1-14 | 1.0000 | 0.2894 | 0.2636 | 0.2343 | 0.3642 | ||||||
|
2,3-dinor- F2 |
1.0000 | 0.9700 | 0.8790 | 0.7298 | |||||||
| F2-12 | 1.0000 | 0.8763 | 0.7129 | ||||||||
| F2-13 | 1.0000 | 0.8767 | |||||||||
| F2-15 | 1.0000 |
All pair-wise correlations are statistically significant (P < 0.0001) with the exception of the correlation of F1-14 with 8-iso-PGF2α (P = 0.0037), 8-iso-15(R)-PGF2α (not significant), PGF2α+ ent-PGF2α (P = 0.0002), 2,3-dinor-F2 (P = 0.0004), F2-12 (P = 0.0014), and F2-13 (P = 0.0047).
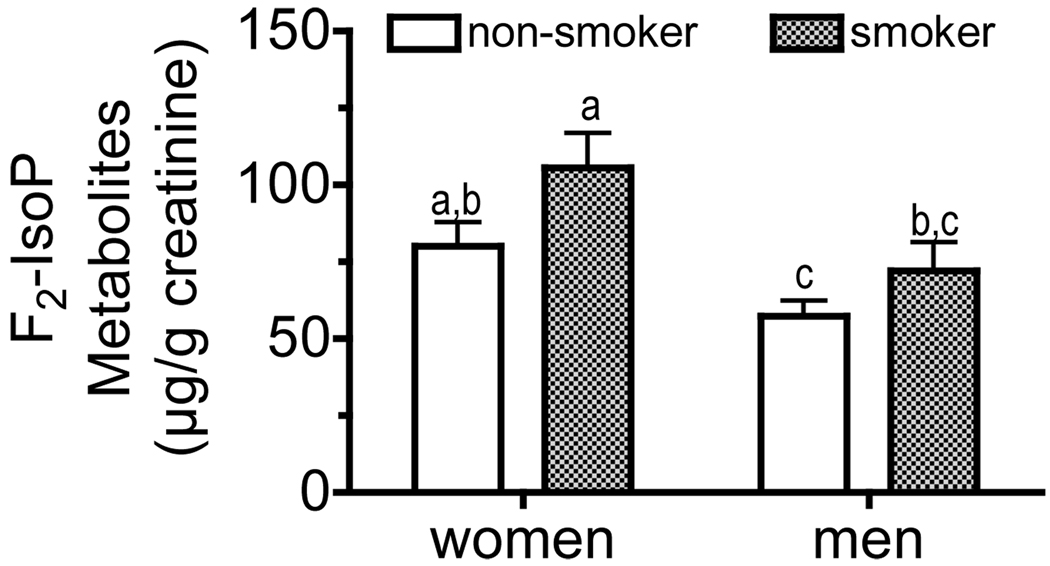
Given that all of the “identified” urinary F2-IsoPs metabolite concentrations (except F1-14) were correlated, the concentrations calculated for each metabolite were summed for each subject at each time point and then averaged for the six time points within each subject. Women had higher F2-IsoPs metabolite concentrations than did men (MANOVA, main effect p = 0.0033), while cigarette smokers had higher concentrations than did nonsmokers (main effect p = 0.036, Figure 6). Women smokers had higher metabolite concentrations than did men (p < 0.05); nonsmoking men had lower concentrations than did women (P<0.05).
Figure 6. Urinary total isoprostane metabolites excreted by men and women smokers and nonsmokers.
Urinary F2-isoP metabolite concentrations (identified as shown in Figure 2 & Figure 3, except F1-14) were individually calculated, summed for each subject at each time point and then averaged for the six time points for each subject. Women had higher F2-isoP metabolite concentrations than did men (MANOVA, main effect p = 0.0033), while cigarette smokers had higher concentrations than did nonsmokers (main effect p = 0.036). Bars not sharing the same letter are significantly different by Tukey’s pair-wise comparisons (p < 0.05).
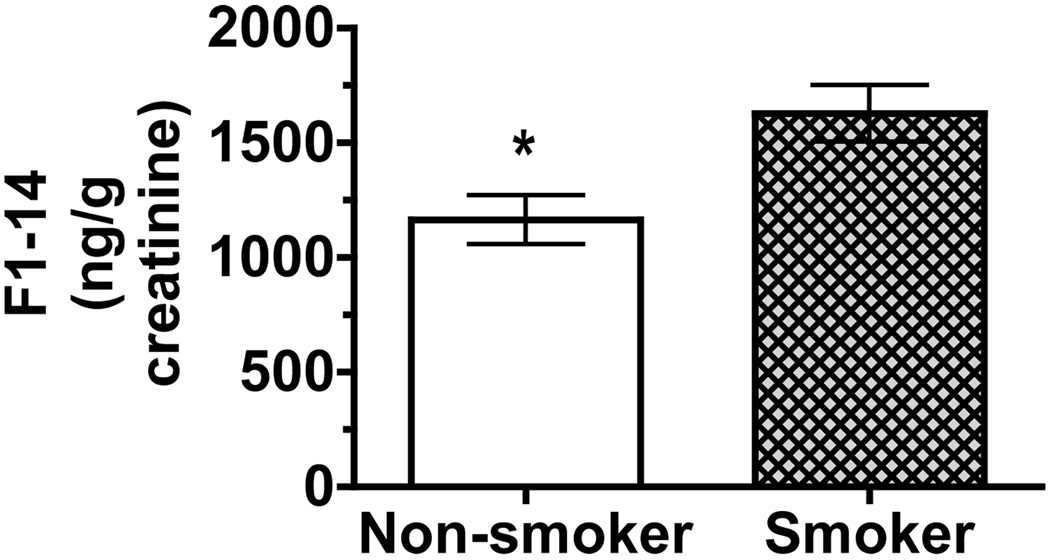
Although F1-14 concentrations were not used in the above estimation of F2-IsoPs metabolites, the SRM characteristics of the peaks were consistent with the F2-IsoPs metabolite standards, 2,3-dinor-F1 and -F2. F1-14 concentrations (averaged over the six time points for each subject) showed that smokers had higher F1-14 concentrations than did nonsmokers (p = 0.025, Figure 7). Unlike all of the other biomarkers of lipid peroxidation that were evaluated and reported herein, there was no statistically significant effect of gender on F1-14 concentrations even when all six data points per subject (rather than averages) were evaluated.
Figure 7. Urinary F1-14 excreted by smokers and nonsmokers.
F1-14 (identified in Figure 2C) concentrations were averaged over the six time points for each subject. Smokers had higher F1-14 concentrations than did nonsmokers (p = 0.025). There were no significant effects of gender on F1-14 concentrations.
DISCUSSION
The strategy used in the current study was to identify multiple biomarkers of oxidative stress in human urine. Rather than using a rapid passage through the HPLC column to obtain a single peak containing various F2-IsoPs or their metabolites, the separation was prolonged to resolve individual peaks of interest. Our hypothesis was that some biomarkers may be more sensitive to oxidative stress than others. Our strategy has been effective in that significant differences in urinary F2-IsoPs were detected between women and men, nonsmokers and smokers (~10 cigarettes/day); differences that our plasma F2-IsoP assay was unable to detect in these same subjects (11). Perhaps more importantly, we have identified a series of metabolites that have mass spectral characteristics of the relatively low abundance 2,3-dinor-F1 and -F2, but when summed together, these apparent metabolites are excreted in quantities 50 to 100 times greater than the two metabolites for which we have commercially available standards. The urinary F2-IsoPs were found at concentrations of about 0.2 to 1.5 µg/g creatinine (Figure 4), while urinary 2,3-dinor-F1 was about 1 to 3 µg/g and 2,3-dinor-F2 was about 3 to 5 µg/g (Figure 5). In contrast, the sum of the various F2-IsoP metabolites was in the range of 50 to 100 µg/g (Figure 6). Thus, these urinary F2-IsoPs metabolite concentrations are supportive of rapid F2-IsoP metabolism (15) and the excretion of the metabolites as potentially important in ameliorating the adverse effects of oxidative stress.
To identify F2-IsoPs and F2-IsoPs metabolites, we first acquired product ion spectra of the individual standards. We then used these results to design an SRM program for each class of analyte. For example, authentic 2,3-dinor-F1 had a molecular ion of m/z 327 (negative mode), and major products ions, in order of abundance, of m/z 283, 265, 209, 183, 193, and 221 (Figure 2). The SRM program detected these ions as m/z 327 products during an HPLC separation of authentic 2,3-dinor-F1 and also during analysis of a human urine extract. The peaks in the urine extract chromatogram that had SRM profiles resembling those of authentic 2,3-dinor-F1, were likely to have a similar chemical structure, but perhaps with stereochemical differences. Once specific peaks were identified based on SRM profiles as likely members of a particular analyte type, we integrated only those peaks. Note that that Figure 2C and Figure 3C also show peaks in the urine extract that did not have the appropriate SRM profile, thus, were not included in our analyses.
Previous methods have utilized mass spectrometry to identify and quantify components with the same single molecular ion-to-product ion reaction as that of available standards with minimal HPLC separation. We found that human urine chromatograms contained a peak, F1-14, with a product ion spectrum similar to that of 2,3-dinor-F1; however, the amounts were not correlated with F2-IsoP concentrations (Table 3). These findings suggest that peak F1-14 is not derived from the F2-IsoPs that we quantitated. Moreover, analyses based solely on tandem MS detection with minimal or no HPLC separation may not provide accurate results. Importantly, unlike every other F2-IsoP and metabolite we detected, there was no gender effect, just increased F1-14 concentrations in smokers (Figure 7). Without the prolonged HPLC separation we used, this very interesting biomarker would have escaped discovery. F1-14 likely has a molecular structure similar to that of 2,3-dinor-F1, but with perhaps different stereochemistry. Clearly, further investigation is needed to characterize this molecule(s).
The SPE method described is a new innovation compared with previously published methods by others. We sought to extend our LC-MS-MS assay for plasma 15-series F2-IsoPs (11) for use with urine samples and to simultaneously measure urinary F2-IsoPs metabolites. However, our initial efforts using liquid-liquid extraction or published SPE protocols (5, 10) yielded poor recoveries (~25–50% of spiked internal standard) from urine samples. A generic anion exchange SPE protocol (including a methanol wash, then elution with acidified methanol) using mixed-mode weak anion exchange-reverse phase cartridges gave satisfactory recovery of the internal standard, 8-iso-PGF2α-d4, when added to water, but not when added to urine. Moreover, when the internal standard was added to an extracted urine sample, only half the expected increase in peak area was observed, suggesting a matrix effect, possibly ion suppression in the mass spectrometer. In the course of developing a new F2-IsoP extraction method, we observed that when urine samples were loaded on mixed-mode reverse phase-weak anion exchange cartridges at ~1 pH unit above the typical carboxylic acid pKa (e. g., n-butyric, pH 4.81), the protic solvent, methanol (but not acetonitrile) was capable of eluting F2-IsoPs and F2-IsoP metabolites, indicating ionic forces still contributed to F2-IsoP binding to the cartridge at the lower pH. Acetonitrile could then be used to wash any neutral, nonpolar compounds from the cartridge, as well as any residual water. Good recoveries of F2-IsoPs and F2-IsoP metabolites were achieved with neutral methanol elution, while agents that caused ion suppression in the MS were eluted with acidified solvents. These findings explain the low recoveries of urinary F2-IsoPs and F2-IsoP metabolites when we used conventional anion exchange methods, in which samples are usually loaded at greater than or equal to 2 pH units higher than the pKa of the analyte of interest, which in turn, is eluted with acidified solvents.
Intra- and inter-day precision of the final assay compare satisfactorily with previously published methods, and the accuracy of measurements of the F2-IsoPs was nearly 100%. However, the accuracies of 2,3-dinor-F1 and -F2 were lower. We believe the lower accuracy is due to lower rates of recovery of these analytes from urine. The SPE procedure was more robust for variations in the loading pH and elution volumes when determining recoveries of 8-iso-PGF2α, PGF2α, and PGE2 than for 2,3-dinor-F1 and -F2. As a consequence, attention was focused on achieving as good recovery as possible of the latter without compromising the recovery of the F2-IsoPs. Our accuracy of 59% for 2,3-dinor-F1 and 86% for 2,3-dinor-F2 is not as high as the 80% reported for the former (5) or the 98 – 99% reported for the latter (10). Nevertheless, the recoveries were reproducible and were linear over a large range, allowing the use of our protocol in analyzing a set of samples collected from college-age subjects, some of them light smokers and others nonsmokers. Bohnstedt et al. (16) previously reported that reverse phase SPE was unsatisfactory for urine sample preparation; they then developed a liquid-liquid extraction method for F2-IsoPs. Herein we report a SPE procedure using a different stationary phase chemistry that was satisfactory for both F2-IsoPs and their metabolites. Bohnstadt et al. (16) reported urinary concentrations of iPF2a from control subjects (216 pg/mg creatinine) that were similar to those we observed of 8-iso-PGF2a (the same compound) from nonsmoking male subjects (280 pg/mg creatinine), while we report higher concentrations in female smokers (530 pg/mg creatinine). Welsh et al. (17) also report satisfactory recoveries of F2-IsoPs and their metabolites using SPE technology.
In the present study, we observed that smokers had greater urinary 8-iso-PGF2α concentrations than did nonsmokers, but cigarette smoking did not significantly increase urinary 8-iso-15(R)-PGF2α or PGF2α concentrations. We previously reported plasma F2-IsoPs measured from a similar cohort of otherwise healthy college-aged nonsmokers and smokers. Utilizing GC-MS (12) or LC-MS (11) approaches, smokers had numerically higher plasma F2-IsoPs but without statistical significance between the groups. In a separate study, plasma F2-IsoPs were significantly higher among smokers, but their smoking history and frequency (>30 cigarettes/d) were substantially greater than observed in the participants from the present study (18, 19). Also, proposed biomarkers of oxidative stress are often tested with a strong stress such as CCl4 poisoning (20). Thus, significantly greater urinary 8-iso-PGF2α in smokers supports the well-established literature indicating that smoking enhances oxidative stress (21, 22) and supports the hypothesis of the present work that specific F2-IsoPs may be more sensitive biomarkers of oxidative stress in vivo. The latter is important for studies conducted in populations that are otherwise healthy, young, and with a limited history (<5 years) and frequency (~10 cigarettes/d) of smoking, that is, subjects with a relatively low magnitude of oxidative stress, where decreased variability in the methodology would allow increased sensitivity to detect differences between subjects.
It should be noted that our method also allows quantitation of PGE2. In general, PGE2 is formed enzymatically (23), but there is evidence that non-enzymatic generation can also occur (24). Given our interest in oxidative stress markers, and the non-specific origin of PGE2, this molecule was not quantitated for smokers and non-smokers.
Of particular interest is that plasma F2-IsoP turnover is rapid (15); therefore, blood levels may vary widely over time with transient oxidative stress. Thus, urinary F2-IsoPs and their metabolite concentration would be expected to provide a more reliable index of systemic oxidative stress with less variability than that of circulating concentrations. Indeed, our measurements confirmed this notion (Table 2) and demonstrated the significance of measuring these biomarkers in urine.
Consistent with the notion of enhanced oxidative stress in smokers, we observed significantly higher urinary 2,3-dinor-F1 and -F2 concentrations in smokers compared with nonsmokers (Figure 5), as were urinary total F2-IsoP metabolites (Figure 6). However, to our surprise, we observed that women compared with men had higher urinary concentrations of all F2-IsoPs measured (Table 2 and Figure 4), as well as urinary F2-IsoP metabolites (Figure 5–Figure 6) despite men and women having similar characteristics (Table 1). Gender effects on lipid peroxidation or oxidative stress biomarkers have been addressed in clinical studies only to a limited extent and the results have been equivocal. Men had higher urinary F2-IsoPs and plasma thiobarbituric acid-reactive substances (TBARS) than did women (25), as well as higher plasma malondialdehyde (MDA) (26). However, in a study with nearly 300 men and women, Block et al (27) demonstrated that women had significantly higher plasma MDA and F2-IsoPs than did men. Moreover, the gender effect was maintained despite efforts to adjust for BMI which would potentially control for differences in adiposity. In the present study, F2-IsoPs and F2-IsoP metabolite concentrations were not correlated with any parameter shown in Table 1, including BMI (data not shown). Coudray et al (28) also observed significantly greater evidence of lipid peroxidation in women compared with men in a cohort of nearly 1400 participants. Clearly, additional study is needed to further characterize gender differences on oxidative stress as well as to potentially unravel the mechanism leading to such effect.
ACKNOWLEDGEMENTS
The study authors would like to thank Katie Payne for excellent technical assistance, Dr. Clifford Pereira and Cody Olsen for statistical consultations, and Phenomenex for the donation of SPE cartridges used during method development. The project described was supported by grants to MGT (R01DK059576 and R01DK067930 from the National Institute of Diabetes and Digestive and Kidney Diseases and the Office of Dietary Supplements) and to the Environmental Health Sciences Center at Oregon State University from the National Institute of Environmental Health Sciences (NIH P30 ES00210). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
LIST OF ABBREVIATIONS
- CE
collision energies
- F2-IsoPs
F2-Isoprostanes
- GC-MS
gas chromatography-mass spectrometry
- LC-MS
liquid chromatography-mass spectrometry
- SPE
solid phase extraction
- SRM
selected reaction monitoring
- 2,3-dinor-F1
2,3-dinor-5,6-dihydro-8-iso-PGF2α, or 2,3-dinor-8-iso-PGF1α
- 2,3-dinor-F2
2,3-dinor-8-iso-PGF2α
REFERENCES
- 1.Morrow JD, Harris TM, Roberts LJ., 2nd Noncyclooxygenase Oxidative Formation of a Series of Novel Prostaglandins: Analytical Ramifications for Measurement of Eicosanoids. Anal Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 2.Lawson JA, Rokach J, FitzGerald GA. Isoprostanes: Formation, Analysis and Use as Indices of Lipid Peroxidation in Vivo. J Biol Chem. 1999;274:24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LJ, II, Morrow JD. Measurement of F2-Isoprostanes as an Index of Oxidative Stress in Vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 4.Chiabrando C, Valagussa A, Rivalta C, Durand T, Guy A, Zuccato E, Villa P, Rossi J-C, Fanelli R. Identification and Measurement of Endogenous B-Oxidation Metabolites of 8-Epi-Prostaglandin F2a. J Biol Chem. 1999;274:1313–1319. doi: 10.1074/jbc.274.3.1313. [DOI] [PubMed] [Google Scholar]
- 5.Davies SS, Zackert W, Luo Y, Cunningham CC, Frisard M, Roberts LJ., 2nd Quantification of Dinor,Dihydro Metabolites of F(2)-Isoprostanes in Urine by Liquid Chromatography/Tandem Mass Spectrometry. Anal Biochem. 2006;348:185–191. doi: 10.1016/j.ab.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Roberts LJ, II, Moore KP, Zackert WE, Oates JA, Morrow JD. Identification of the Major Metabolite of the F2-Isoprostane 8-Iso-Prostaglandin F2alpha in Humans. J Biol Chem. 1996;271:20617–20620. doi: 10.1074/jbc.271.34.20617. [DOI] [PubMed] [Google Scholar]
- 7.Nourooz-Zadeh J, Cooper MB, Ziegler D, Betteridge DJ. Urinary 8-Epi-Pgf2a and Its Endogenous B-Oxidation Products (2,3-Dinor and 2,3-Dinor-5,6-Dihydro) as Biomarkers of Total Body Oxidative Stress. Biochem Biophys Res Comm. 2005;330:731–736. doi: 10.1016/j.bbrc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Morrow JD, Zackert WE, Yang JP, Kurhts EH, Callewaert, Oates JA, Roberts LJ., II Quantification of the Major Urinary Metabolite of 15-F2t-Isoprostane (8-Iso-Pgf2a) by a Stable Isotope Dilution Mass Spectrometric Assay. Anal Biochem. 1999;269:326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg JB, Frei B. Why Clinical Trials of Vitamin E and Cardiovascular Diseases May Be Fatally Flawed. Commentary On "The Relationship between Dose of Vitamin E and Suppression of Oxidative Stress in Humans". Free Radic Biol Med. 2007;43:1374–1376. doi: 10.1016/j.freeradbiomed.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Wei P, Duke RW, Reaven PD, Harman SM, Cutler RG, Heward CB. Quantification of 8-Iso-Prostaglandin-F2alpha and 2,3-Dinor-8-Iso-Prostaglandin-F2alpha in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. Free Radic Biol Med. 2003;34:409–418. doi: 10.1016/s0891-5849(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AW, Bruno RS, Frei B, Traber MG. Benefits of Prolonged Gradient Separation for High-Performance Liquid Chromatography-Tandem Mass Spectrometry Quantitation of Plasma Total 15-Series F2-Isoprostanes. Anal Biochem. 2006;350:41–51. doi: 10.1016/j.ab.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Bruno RS, Leonard SW, Atkinson JK, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster Vitamin E Disappearance in Smokers Is Normalized by Vitamin C Supplementation. Free Radic Biol Med. 2006;40:689–697. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 13.Levine M, Wang Y, Padayatty S, Morrow J. A New Recommended Dietary Allowance of Vitamin C for Healthy Young Women. Proc Natl Acad Sci USA. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin H, Gao L, Tai H-H, Murphey LJ, Porter NA, Morrow JD. Urinary Prostaglandin F2a Is Generated from the Isoprostane Pathway and Not the Cyclooxygenase in Humans. J Biol Chem. 2007;282:329–336. doi: 10.1074/jbc.M608975200. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Helmersson J. Factors Regulating Isoprostane Formation in Vivo. Antioxid Redox Signal. 2005;7:221–235. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- 16.Bohnstedt KC, Karlberg B, Wahlund LO, Jonhagen ME, Basun H, Schmidt S. Determination of Isoprostanes in Urine Samples from Alzheimer Patients Using Porous Graphitic Carbon Liquid Chromatography-Tandem Mass Spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:11–19. doi: 10.1016/s1570-0232(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 17.Welsh TN, Hubbard S, Mitchell CM, Mesiano S, Zarzycki PK, Zakar T. Optimization of a Solid Phase Extraction Procedure for Prostaglandin E2, F2 Alpha and Their Tissue Metabolites. Prostaglandins Other Lipid Mediat. 2007;83:304–310. doi: 10.1016/j.prostaglandins.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., II Increase in Circulating Products of Lipid Peroxidation (F2-Isoprostanes) in Smokers. Smoking as a Cause of Oxidative Damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 19.Reilly NDM N, Delanty JA, Lawson GA, FitzGerald Modulation of Oxidant Stress in Vivo in Chronic Cigarette Smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJ, 2nd, Burk RF. Formation of Novel Non-Cyclooxygenase-Derived Prostanoids (F2-Isoprostanes) in Carbon Tetrachloride Hepatotoxicity. An Animal Model of Lipid Peroxidation. J Clin Invest. 1992;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruno RS, Traber MG. Cigarette Smoke Alters Human Vitamin E Requirements. J Nutr. 2005;135:671–674. doi: 10.1093/jn/135.4.671. [DOI] [PubMed] [Google Scholar]
- 22.Bruno RS, Traber MG. Vitamin E Biokinetics, Oxidative Stress and Cigarette Smoking. Pathophysiology. 2006;13:143–149. doi: 10.1016/j.pathophys.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Hao CM, Breyer MD. Physiological Regulation of Prostaglandins in the Kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Zackert WE, Hasford JJ, Danekis ME, Milne GL, Remmert C, Reese J, Yin H, Tai HH, Dey SK, et al. Formation of Prostaglandins E2 and D2 Via the Isoprostane Pathway: A Mechanism for the Generation of Bioactive Prostaglandins Independent of Cyclooxygenase. J Biol Chem. 2003;278:28479–28489. doi: 10.1074/jbc.M303984200. [DOI] [PubMed] [Google Scholar]
- 25.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater Oxidative Stress in Healthy Young Men Compared with Premenopausal Women. Arterioscler Thromb Vasc Biol. 2002;22:438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma Malondialdehyde as Biomarker for Oxidative Stress: Reference Interval and Effects of Life-Style Factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- 27.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors Associated with Oxidative Stress in Human Populations. Am J Epidemiol. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 28.Coudray C, Roussel AM, Mainard F, Arnaud J, Favier A. Lipid Peroxidation Level and Antioxidant Micronutrient Status in a Pre-Aging Population; Correlation with Chronic Disease Prevalence in a French Epidemiological Study (Nantes, France) J Am Coll Nutr. 1997;16:584–591. [PubMed] [Google Scholar]