Abstract
Although experience-dependent structural changes have been demonstrated in adult gray matter, there is little evidence for such changes in white matter. Using diffusion imaging, we detected a localised increase in fractional anisotropy, a measure of microstructure, in white matter underlying the intraparietal sulcus, following training of a complex visuo-motor skill. This provides the first evidence for training related changes in white matter structure in the healthy human adult brain.
The learning of a novel skill relies upon changes in brain function. This functional plasticity can be accompanied by structural changes in the gray matter of the human brain1. Such gross changes in gray matter structure could reflect underlying cellular events including synaptogenesis and dendritic arborisation2,3. By contrast, longitudinal experience-dependent white matter changes have not previously been reported in healthy humans. Yet evidence from animal studies suggests that white matter could alter with experience or training. For example, the amount of neuronal activity along an axon modulates its degree of myelination4,5 and dramatic cortico-cortical rewiring has been observed in response to training6 or rehabilitation7.
Diffusion Tensor Imaging (DTI) provides measures of white matter microstructure in the human brain. DTI is sensitive to the hindrance of water diffusion due to local tissue boundaries. Fractional anisotropy (FA), a DTI-derived quantitative measure of the directional dependence of water diffusion, reflects anatomical features of white matter, such as axon caliber, fiber density and myelination8.
Cross-sectional studies demonstrate that inter-individual variation in white matter microstructure, as indexed by FA, reflects behavioural variation9,10. Moreover, FA variation correlates with differences in experience, such as the amount of childhood piano practice11. Such cross-sectional studies, however, can never confirm a causal role of experience on white matter structure, because it is possible that common genetic factors influence both white matter structure and the propensity to train.
Here we used DTI to measure white matter changes, and voxel-based morphometry (VBM) to measure gray matter changes, in a longitudinal study of individuals learning a novel visuo-motor skill - juggling. 48 healthy adults gave informed consent to participate and were allocated to a training group (n=24) and an untrained control group (n=24). The training group were scanned before (scan 1) and after (scan 2) a six-week training period and following a subsequent four-week period without juggling (scan 3) (Supplementary Methods). After training, all subjects could perform at least two continuous cycles of the classic ‘3-ball cascade’ (Supplementary Fig. 1).
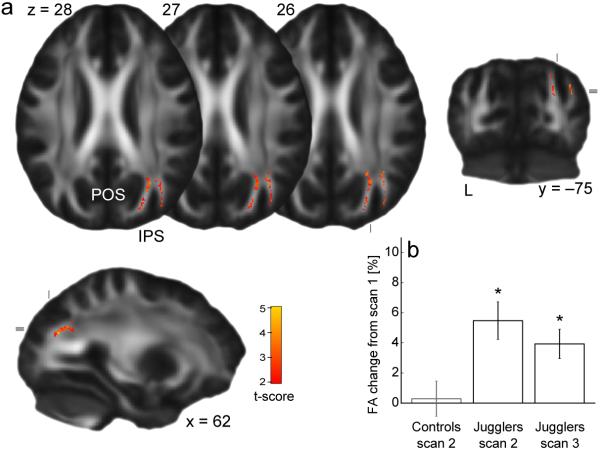
We fitted a diffusion tensor model to DTI data to create whole brain maps of FA which we compared between time points using Tract Based Spatial Statistics (TBSS) (Supplementary Methods). Comparisons between scan 2 and scan 1 in the trained group revealed significant training-related increases in FA within white matter underlying the right posterior intraparietal sulcus (IPS) (p<0.05, corrected, tmax=4.57, x=31, y=-59, z=31) (Fig. 1). We carried out a series of post hoc tests to probe this difference further, demonstrating that it was specific to the trained group and remained elevated relative to baseline after a four week period without juggling (Supplementary Results).
Figure 1.
FA increases after juggling training. (a) Colored voxels represent clusters (corrected p<0.05) of significant FA increase from scan 1 to scan 2, superimposed on the mean FA map. (b) Mean FA change from scan 1 from within the cluster shown in (a). Error bars represent standard errors. (*significant relative to baseline at p<0.05; IPS=intraparietal sulcus, POS=parieto-occipital sulcus.)
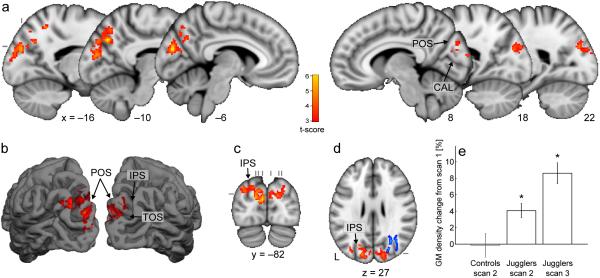
To explore the possibility that learning is associated with co-localized white matter/gray matter changes, we tested for gray matter density changes using VBM (Supplementary Methods). Following juggling training, gray matter density increased significantly in the medial occipital and parietal lobe in cortical regions overlying the white matter area of significant FA increase (Fig. 2). Again, we carried out a series of post hoc tests, demonstrating that this increase was specific to the trained group and continued after the four week period without juggling (Supplementary Results).
Figure 2.
Gray matter density increases after juggling training. (a-d) Red-yellow voxels represent clusters (p<0.05, corrected) of significant gray matter density increase from scan 1 to scan 2, superimposed on the MNI template. Sagittal (a), coronal (c) and axial (d) slices, and a surface rendering (b) are shown. (d) includes the white matter changes (blue, thickened for visibility) for comparison. (e) Mean gray matter density changes from scan 1 from within the clusters shown in (a-d). Error bars represent standard errors. (*significant at p<0.05 relative to scan 1; CAL=calcarine sulcus, IPS=intraparietal sulcus, POS=parieto-occipital sulcus, TOS=transverse occipital sulcus.)
Juggling is a complex motor skill that requires accurate bimanual arm movements, grasping and visual tracking in the periphery - precisely those functions in which the apparently structurally-altered brain regions specialize (Supplementary Discussion).
In general, structural changes did not correlate significantly with training progress or the performance level reached after the juggling period, consistent with previous reports12,13, although we did find a restricted effect at the peak voxel for our gray matter analysis (Supplementary Results). The absence of a strong and widespread relationship between performance and structural changes suggests that the majority of structural changes might be more closely related to amount of time spent training (which was kept constant in this study) than to training outcome.
Despite the close spatial proximity of gray matter and white matter regions showing training-related changes, we did not find any correlation between the magnitude of gray matter and white matter changes across subjects. This, along with the strikingly different time courses of gray matter and white matter change, suggests that relatively independent structural changes occur within these different tissue types. Future studies could use varying training regimes and longer periods of observation to more fully characterize the complex dynamics of gray matter and white matter change with learning.
Biological interpretation of changes in imaging measures is challenging (see Supplementary Discussion for interpretation of gray matter changes). FA in part reflects white matter properties such as axon caliber and myelination8. Changes in these properties might underlie behavioral improvements by altering conduction velocity and synchronisation of nervous signals14. Previous reports suggest that electrical activity within an axon could regulate its myelination over a time course of days to weeks4,5. Activity-dependent myelo-modulation, which would be expected to influence FA, is therefore a potential mechanism through which the functional properties of white matter are affected by experience. Changes in other structural features of the white matter, such as axon diameter (which could itself be regulated by myelin14), or packing density, could also underlie the results found here.
In summary, we provide the first evidence for experience dependent changes in white matter microstructure in healthy human adults. While neuroimaging techniques, such as DTI, provide opportunities for whole brain studies in living human subjects, the measures derived from MRI are indirect and their interpretation is complex. Therefore, future studies using cellular and biochemical techniques are required to determine the biological basis of the observed changes.
Supplementary Material
Acknowledgements
We are grateful for financial support from the Wellcome Trust (H.J.-B., J.S.) and UK Medical Research Council (J.S., T.E.J.B.). We thank Jesper Anderson, Mark Jenkinson, Gwenaelle Douaud and Mark Woolrich for technical assistance, Matthew Rushworth for useful discussions on functional anatomy, Rogier Mars for providing additional control data and Matt Mangham for juggling support.
References
- 1.Draganski B, et al. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 2.Volkmar FR, Greenough WT. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- 3.Turner AM, Greenough WT. Brain Res. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- 4.Demerens C, et al. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishibashi T, et al. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hihara S, et al. Neuropsychologia. 2006;44:2636–2646. doi: 10.1016/j.neuropsychologia.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Dancause N, et al. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaulieu C. The biological basis of diffusion anisotropy. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI: From quantitative measurement to in-vivo neuroanatomy. Elsevier; London: 2009. [Google Scholar]
- 9.Tuch DS, et al. Proc Natl Acad Sci U S A. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Neuroimage. 2007;36(Suppl 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengtsson SL, et al. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 12.Driemeyer J, Boyke J, Gaser C, Buchel C, May A. PLoS One. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draganski B, et al. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields RD. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.