Abstract
Aims
Iron deficiency (ID) and anaemia are common in patients with chronic heart failure (CHF). The presence of anaemia is associated with increased morbidity and mortality in CHF, and ID is a major reason for the development of anaemia. Preliminary studies using intravenous (i.v.) iron supplementation alone in patients with CHF and ID have shown improvements in symptom status. FAIR-HF (Clinical Trials.gov NCT00520780) was designed to determine the effect of i.v. iron repletion therapy using ferric carboxymaltose on self-reported patient global assessment (PGA) and New York Heart Association (NYHA) in patients with CHF and ID.
Methods and results
This is a multi-centre, randomized, double-blind, placebo-controlled study recruiting ambulatory patients with symptomatic CHF with LVEF ≤ 40% (NYHA II) or ≤45% (NYHA III), ID [ferritin <100 ng/mL or ferritin 100–300 ng/mL when transferrin saturation (TSAT) < 20%], and haemoglobin 9.5–13.5 g/dL. Patients were randomized in a 2:1 ratio to receive ferric carboxymaltose (Ferinject®) 200 mg iron i.v. or saline i.v. weekly until iron repletion (correction phase), then monthly until Week 24 (maintenance phase). Primary endpoints are (i) self-reported PGA at Week 24 and (ii) NYHA class at Week 24, adjusted for baseline NYHA class.
Conclusion
This study will provide evidence on the efficacy and safety of iron repletion with ferric carboxymaltose in CHF patients with ID with and without anaemia.
Keywords: Chronic heart failure, Iron deficiency, Anaemia, Treatment, Ferric carboxymaltose
Introduction
Chronic heart failure (CHF) results in poor life expectancy, impaired quality of life, repeated hospitalizations and is a considerable economic burden to society.1 Despite advances in its treatment, the prognosis of CHF remains ominous,2 and in developed countries, total expenditure on heart failure ranges between 1 and 2% of the total healthcare budget.3
Patients with CHF often suffer from greatly reduced exercise capacity, which manifests itself as excessive fatigue and breathlessness. These symptoms are poorly related to myocardial function and lead to a decrease in quality of life and high morbidity.4 Numerous mechanisms may underlie exercise limitation in CHF, and among them inadequate oxygen supply and impaired oxygen utilization by the exercising skeletal muscle.5,6 Therapeutic options to improve functional capacity are still very limited, but it is plausible that targeting abnormalities impeding oxygen transportation and/or utilization may confer functional benefits.
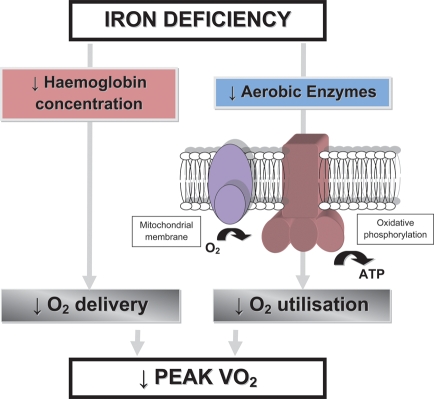
Iron plays a crucial role in oxygen uptake and transport [as part of the ferrous ring of haemoglobin (Hb)], oxygen storage (as a component of myoglobin), oxygen metabolism, and energy production (as a component of oxidative enzymes and respiratory chain proteins) and is involved in erythropoiesis.7,8 Therefore, iron deficiency (ID), with or without concomitant anaemia, may be associated with reduced functional capacity and is often accompanied by subjective complaints of poor physical condition with objective indices of exercise intolerance such as diminished oxygen consumption, and attenuated submaximal exercise performance (Figure 1).9,10 The correction of ID in anaemic and non-anaemic patients without heart failure has been shown to improve cognitive, symptomatic, and exercise performance.11,12 If clinical symptomatology of CHF resembles that of ID, one can simply imply impaired iron homeostasis as one of the mechanisms underlying exercise intolerance in CHF. Interestingly, ID as a problem in the CHF syndrome has only recently gained clinical interest, which coincided with the recognition of anaemia as a frequent co-morbidity in CHF.
Figure 1.
Effect of iron deficiency on erythropoiesis and oxygen metabolism. Adapted from Haas and Brownlie9 and Dallman.10
Estimates of the prevalence of anaemia in patients with CHF vary significantly, from 4 to 61%, and are related to clinical characteristics of the studied population. Anaemia is more common in the elderly, those with advanced CHF, concomitant renal dysfunction, diabetes mellitus, greater oedema, lower blood pressure, higher use of diuretics, and elevated levels of neurohormones and proinflammatory cytokines.13 Recent data from a cohort of 6159 consecutive outpatients with stable CHF revealed a prevalence of anaemia at baseline of 17%, with new-onset anaemia developing at 6-month follow-up in 16% of patients without prior anaemia.14 Data from clinical trials have shown that during the course of 1 year, new anaemia developed in 10–17% of CHF patients.15,16 In CHF patients, even mild anaemia is associated with worsening of symptoms, increased New York Heart Association (NYHA) class and impaired functional capacity, quality of life, and survival.17 The cost of hospitalization for anaemic CHF patients is higher than for non-anaemic CHF patients.18
Anaemia of chronic disease is characterized by a marked dysregulation of iron metabolism, in particular by a reduced level of iron available for erythropoiesis. Chronic heart failure is an inflammatory state with elevated levels of proinflammatory cytokines, which in turn block the intestinal absorption of iron and the release of iron, derived from phagocytised senescent red blood cells from the reticuloendothelial system, causing a ‘reticuloendothelial block’.19 Hepcidin, a small peptide produced by the liver in response to proinflammatory cytokines, has been shown to play a key role in the control of these processes.20 In this clinical situation, decreased iron absorption together with an accumulation within the reticuloendothelial stores leads to limited iron availability for erythropoiesis and subsequently iron-deficient anaemia.19 Since the main feature of this type of anaemia is an impaired mobilization of iron from cells, it may occur despite adequate iron stores in the body and, it is named ‘functional ID’ in contrast to absolute ID, when body iron stores are significantly depleted.
Ferric carboxymaltose, a new intravenous (i.v.) iron preparation, is designed to provide a high iron bioavailability without the disadvantageous characteristics associated with iron dextran, ferric gluconate, and iron sucrose therapy. Ferric carboxymaltose is dextran-free and thus does not react with dextran antibodies (therefore not requiring a test dose) and has a pH between 5.0 and 7.0. The pharmacokinetic properties of ferric carboxymaltose in humans lie between those of iron dextran and iron sucrose, i.e. the calculated terminal half-life is ∼16 h (in comparison to 3–4 days for iron dextran and 6 h for iron sucrose). The mechanism and the extent of utilization of iron are similar to that of iron sucrose.21
FAIR-HF (Ferinject® Assessment in patients with IRon deficiency and chronic Heart Failure) was designed to test whether correction of ID using i.v. iron (ferric carboxymaltose) confers benefits in symptomatic CHF patients with and without anaemia.
Methods
Study design
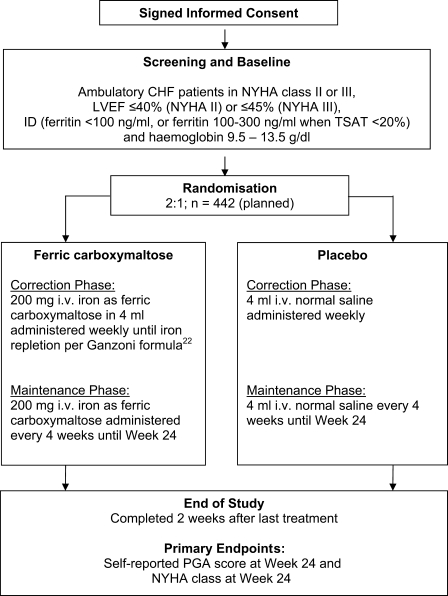
FAIR-HF is a multi-centre, double-blind, randomized, placebo-controlled parallel group study which is being conducted in 75 study sites mainly in Europe and Argentina. The study design is summarized in Figure 2.
Figure 2.
Study flow-chart. Abbreviations: CHF, chronic heart failure; ID, iron deficiency; i.v., intravenous; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PGA, patient global assessment; TSAT, transferrin saturation.
The study is conducted in accordance with the principles stated in the Declaration of Helsinki (1996), International Conference on Harmonisation Good Clinical Practice, and local and national regulations. Written informed consent has been provided by all patients prior to any study-related procedures.
Eligibility
The main FAIR-HF study inclusion and exclusion criteria are listed in Table 1. Briefly, ambulatory CHF patients in NYHA class II or III, with a LVEF ≤ 40% (NYHA II) or ≤45% (NYHA III), with presence of ID (defined as ferritin <100 ng/mL or ferritin 100–300 ng/mL when TSAT <20%) and Hb between 9.5 and 13.5 g/dL (all at screening) were included. Patients with uncontrolled hypertension, other significant heart disease, inflammation, or significantly impaired liver or renal function were excluded. Pregnant women were excluded, and women with childbearing potential must not become pregnant during the study.
Table 1.
Summary of key inclusion and exclusion criteria at screening
| Key inclusion criteria | Key exclusion criteria |
|---|---|
| NYHA II–III functional class due to stable symptomatic CHF and all of the following | Known active infection, C-reactive protein>20 mg/L, clinically significant bleeding, active malignancy |
| Two weeks without cardiac hospitalization | ALT or AST >3× upper limit of normal |
| Patients in NYHA II: acute care admission or emergency room visit for worsening heart failure within 24 months prior to start of treatment | Anaemia due to reasons other than iron deficiency (e.g. haemoglobinopathy) |
| On optimal pharmacological treatment which includes a diuretic, a beta-blocker, and/or an ACE-inhibitor or ARB as determined by the investigator, unless contraindicated or not tolerated | Immunosuppressive therapy or renal dialysis |
| No dose changes of heart failure drugs during the last 2 weeks (exception: diuretics) | History of erythropoietin, i.v. or oral iron therapy, and blood transfusion in previous 12 weeks and/or such therapy planned within the next 6 months |
| No introduction of a new heart failure drug class during the last 4 weeks | Unstable angina pectoris, clinically significant uncorrected valvular disease or left ventricular outflow obstruction, obstructive cardiomyopathy |
| LVEF ≤40% for patients in NYHA II and LVEF ≤ 45% in NYHA III | Acute myocardial infarction or acute coronary syndrome, transient ischaemic attack or stroke within the last 3 months |
| Hb: 9.5–13.5 g/dL | Coronary-artery bypass graft, percutaneous intervention (e.g. cardiac, cerebrovascular, aortic; diagnostic catheters are allowed) or major surgery, including thoracic and cardiac surgery, within the last 3 months |
| Evidence of absolute or functional iron deficiency: screening ferritin <100 ng/mL or 100–300 ng/mL when TSAT < 20% | |
| Patient must be able to perform the 6 minute walk test according to investigator judgement |
ACE, angiotensin-converting enzyme; ALT, alanine transaminase; ARB, angiotensin II receptor blocker; AST, aspartate transaminase; CHF, chronic heart failure; Hb, haemoglobin; i.v., intravenous; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TSAT, transferrin saturation.
Screening and randomization
Randomization was achieved using a central Interactive Voice Response System to allocate patients to treatment groups and avoid any selection bias. Patients were stratified according to regions and randomized in a 2:1 ratio (ferric carboxymaltose:placebo).
Study treatment
Active treatment
Ferric carboxymaltose solution (Ferinject®, Vifor International) for parenteral application, 50 mg iron/mL iron. Medication is given as an i.v. bolus of 200 mg iron in 4 mL (can be 100 mg iron i.v. in 2 mL for last injection in correction phase).
Placebo
Normal saline (0.9% w/v NaCl) is administered in analogy to the active treatment.
For all patients, the total iron dose required for iron repletion is calculated at baseline, based on the mean of two Hb values obtained on site during the screening period. Calculations are done using the Ganzoni formula.22
†In patients with a body mass index >25, a normalized weight will be used to calculate the iron deficit. Normalized weight [kg] = 25 × height [m] × height [m].
††Factor 2.4 = 0.0034 (iron content Hb = 0.34%) × 0.07 (blood volume = 7% of body weight) × 10000 (conversion g/dL to mg/L).
†††Depot iron.
In both treatment groups, study drug is administered in doses of 4 mL once weekly up to calculated iron repletion. The calculated dose is rounded to the next 100 mg iron. After the correction phase, study drug is given monthly in doses of 4 mL from Week 8 (or week 12, depending on the number of infusions required during the correction phase) until Week 24 (maintenance phase).
Adjustment algorithm
In case of elevated levels of ferritin >800 ng/mL or ferritin >500 ng/mL when TSAT >50% or Hb >16 g/dL at any stage, iron treatment is discontinued and placebo is given instead. In this case, ferritin, TSAT, and Hb are re-checked every 2 weeks (correction phase) or every 4 weeks (maintenance phase). Once ferritin has dropped to <400 ng/mL and TSAT to <45% and Hb to <16 g/dL, treatment with iron can be restarted. In case severe anaemia develops (i.e. Hb ≤ 9 g/dL), the patient is to discontinue treatment but remain in the study, and further management of anaemia is at the investigator's discretion.
Blinding
The ferric carboxymaltose solution is dark brown in appearance and is therefore easily distinguishable from the placebo (0.9% saline). Hence, unblinded study personnel (at least one physician) not involved in any study assessments are responsible for preparing and administering the study treatment injections in black syringes and using a curtain (or similar) to maintain patient blinding. Unblinded personnel make sure the patient is not able to observe the preparation of study treatment injections. To ensure the blinded investigator remains blinded, post-baseline iron values are sent by the central laboratory only to the unblinded personnel, who then are responsible for evaluating the presence of elevated iron levels or severe anaemia. In case of elevated iron parameters or severe anaemia, the unblinded physician amends the study treatment as described above.
Assessments
Planned clinical assessments for efficacy are completed for all patients who start investigational drug. In addition to screening and baseline visits, assessments are completed at Weeks 4, 12, and 24. A follow-up visit is completed at Week 26 (or within 2 weeks after last treatment). Primary efficacy assessments for NYHA functional class and self-reported patient global assessment (PGA) are performed at weeks 4, 12 and 24.
Study endpoints
The primary endpoints are self-reported PGA score at Week 24 and NYHA class at Week 24, adjusted for baseline NYHA class.
Key secondary endpoints are:
PGA score at Weeks 4 and 12
NYHA class at Weeks 4 and 12, adjusted for baseline NYHA class
Six minute walk test (6MWT) distance at Weeks 4, 12, and 24, adjusted for baseline distance
Kansas City Cardiomyopathy Questionnaire scores (overall summary score and all sub-scores) at Weeks 4, 12, and 24, adjusted for baseline
European Quality of Life-5 Dimensions (EQ-5D) questionnaire score (visual assessment score and all sub-scores) at Weeks 4, 12, and 24, adjusted for baseline
Health resource utilization at each assessment time point
Days alive and out of hospital
Hospitalization (total, cardiovascular, due to worsening of CHF)
Mortality (total, cardiovascular, due to worsening of CHF)
Change in estimated glomerular filtration rate (e-GFR)
Standard safety assessments (adverse events, vital signs, physical examinations, 12-lead electrocardiograms, clinical laboratory panels).
Statistical considerations
Sample size
Sample size calculations are based on the PGA score and NYHA class 24 weeks after start of iron repletion. A two-group t-test with a 0.025 two-sided significance level has 90% power to detect a difference in PGA score means of 0.900, assuming that the common standard deviation is 2.407, when the sample sizes in the two groups are 134 and 268, respectively (a total sample size of 402). A two-group t-test with a 0.025 two-sided significance level has 90% power to detect a difference in NYHA class means of 0.500, assuming that the common standard deviation is 1.337, when the sample sizes in the two groups are 134 and 268, respectively (a total sample size of 402). With an estimated rate of 10% of patients without a Week 24 assessment, we aimed to recruit 442 patients.
Statistical analysis
Two analysis populations will be distinguished—full analysis set (FAS) and per-protocol (PP) analysis population. The analysis will be performed according to the intention-to-treat principle, by assigned study treatment and ignoring changes to study treatment during follow-up. The FAS will comprise all patients for whom study treatment administration was started. The PP analysis population will comprise all patients for whom study treatment administration was started and for whom no major baseline protocol violations were present. Analyses will be available for the FAS and PP analysis populations. The primary efficacy analysis will be based on the FAS. For the primary efficacy analysis, the 24 week changes from baseline for the two co-primary outcomes (i.e. NYHA class, adjusted for the baseline value, and the PGA score) will be compared using standard, two-sided significance tests. Alpha adjustment will be made using the method of Benjamini and Hochberg.23 The P-values for the two tests (P-value of the treatment covariate in the ordered polytomous regression for the PGA and NYHA values, respectively) will be ordered in size (P1≤P2). P2 will be compared with a significance level of 5%. If P2 ≤ 5%, both alternative hypotheses will be proven. If P2>5% and P1 ≤ 2.5%, the corresponding alternative hypothesis of the smaller P-value (H1) will be proven. Time-to-event data will be analysed by Kaplan–Meier plots and appropriate event rates using person-time ‘at risk’ denominators will be given. Secondary and subgroup analyses for efficacy and safety analyses will also be performed. Descriptive statistics will include means, standard deviations, medians, and ranges for continuous variables (including changes), and frequency distributions for discrete variables. The Statistical Analysis Plan will clearly describe the analytic methods that will be used to analyse the data.
Study management
A Data Safety Monitoring Board (DSMB) independently monitors the safety of the study participants and suggests amendments to the protocol if deemed necessary for reasons of patient safety or feasibility of study procedures. The DSMB also includes an independent statistician. The independent statistician performs analyses on safety data in support of the DSMB during the study. To minimize potential bias, DSMB members do not have direct contact with the study site personnel or with study patients.
Study status
The study protocol and amendments have been approved by all relevant regulatory authorities and participating centre's Ethics Committees. The first patient was randomized on 13 July 2007, and the last patient was randomized on 30 December 2008.
Discussion
Studies on the epidemiology and pathophysiology of ID in CHF are rare, and there is no gold standard to diagnose ID in CHF. Ezekowitz et al.24 have shown that, according to hospital discharge records, 17% of CHF patients had anaemia and, of the latter, 21% also show a diagnosis of ID. Case records as source of epidemiological information, however, require physicians to remember this issue and to perform appropriate diagnostic tests. Hence this may be an underestimate of the true frequency of ID in CHF. Opasich et al.25 studied 148 patients with CHF and low Hb level. They found that the majority of the patients (57%) presented with anaemia of chronic disease, and in this group nearly all (92%) demonstrated defective iron supply for erythropoiesis and/or blunted endogenous erythropoietin production. Nanas et al.26 used bone marrow biopsies to investigate the aetiology of anaemia in advanced CHF in 37 patients admitted to hospital (NYHA IV, mean LVEF 22%). The authors found that 73% of them suffered from ID anaemia, as confirmed by the depletion of iron stores in the bone marrow aspirates. Interestingly, iron-deficient patients had fairly normal levels of iron metabolism blood biomarkers (i.e. ferritin, transferrin saturation (TSAT), iron). Grzeslo et al.27 reported a high prevalence of ID among an unselected cohort of CHF patients. The authors studied 165 consecutive, symptomatic, stable CHF patients under optimal pharmacological therapy and used the following laboratory criteria of ID: ferritin <100 ng/mL or if ferritin 100–300 ng/mL, TSAT < 20%. Thirty-eight percent of patients fulfilled laboratory criteria for ID, which was related to disease severity and status of low-grade inflammation. They also reported that ID was associated with poor outcome independently of standard prognosticators and Hb level.27
In disease areas other than HF, ID has been linked to the presence of anaemia, and several studies have investigated the effects of a combination of treatment based on erythropoiesis stimulating agents with oral or i.v. iron. Many of these studies demonstrated meaningful benefits of such therapy, not only to correct anaemia or ID, but also to improve clinical indices.28–30 A study investigating the effects of darbepoietin alpha given together with i.v. iron to CHF patients is not available.
Patients with CHF suffer from excessive fatigue, breathlessness, and a reduced exercise capacity that all lead to decreased quality of life. Iron deficiency, either with or without anaemia, can worsen all these symptoms. If correction of ID in anaemic and non-anaemic patients with CHF using i.v. iron could be linked with clinical benefits, this would ultimately prove the pathophysiological importance of ID in CHF. To date, four studies have been reported (Table 2). Bolger et al.31 in a short study, showed that iron given intravenously as iron sucrose over 5–17 days improved Hb, reduced symptoms and improved exercise capacity over a 3-month follow-up period in anaemic patients with CHF. Toblli et al.32 further demonstrated that 5 weeks of i.v. iron sucrose treatment in anaemic CHF patients (baseline NYHA class III or IV and LVEF < 35%) improved quality of life, 6MWT distance, and LVEF and reduced the number of hospitalizations compared with controls. Plasma levels of N-terminal pro-brain natriuretic peptide and C-reactive protein also decreased.
Table 2.
Summary of previous studies using intravenous iron in patients with chronic heart failure
| Authors | n | Design | Inclusion (Hb, ferritin) | Regimen and total iron dose per protocol | Follow-up time | Key results |
|---|---|---|---|---|---|---|
| Bolger et al.31 | 16 | Open, no control | Hb ≤ 12 g/dL and Ferritin ≤ 400 ng/mL | Iron sucrose, maximum 1000 mg iron i.v. over a 17-day period (200 mg iron on Days 1, 3, 5, plus Days 15 and 17, if ferritin <400 ng/mL on day 12) | 3 months | ↑Hb |
| ↑QoL | ||||||
| ↑Exercise capacity (6MWD) | ||||||
| Toblli et al.32 | 40 | Double-blind, randomized, placebo-controlled | Hb < 12.5 g/dL for men; <11.5 g/dL for women | Iron sucrose, 200 mg iron i.v. weekly for 5 weeks (total 1000 mg iron) | 6 months | ↑Hb |
| Ferritin <100 ng/mL and/or TSAT ≤ 20% | ↑QoL | |||||
| ↑Exercise capacity (6MWD) | ||||||
| ↑LVEF | ||||||
| ↓Hospitalizations | ||||||
| ↓NYHA | ||||||
| ↑Renal function | ||||||
| ↓NT-proBNP level | ||||||
| Okonko et al.33 | 35 | Single blind, randomized, controlled | Hb < 12.5 g/dL (anaemic group) | Iron sucrose, 200 mg iron i.v. weekly until ferritin ≥500 ng/mL (correction phase), then 200 mg iron every 4 weeks (maintenance phase) to Week 16 | 4 months | ↓HF symptoms (PGA) |
| 12.5–14.5 g/dl (non-anaemic group) | Required iron dose calculated using Ganzoni formula: body weight (kg) × 2.4 × [15—patient's Hb (g/dl)] + 500 mg (for stores) | ↑Exercise tolerance (peak VO2) | ||||
| Ferritin <100 ng/mL or 100–300 ng/mL with TSAT < 20% | ↓NYHA | |||||
| ↓Fatigue score | ||||||
| Usmanov et al.34 | 32 | Open, no control | Hb < 11 g/dL | Iron sucrose, 100 mg iron i.v. three times weekly for 3 weeks, then once weekly for 23 weeks (total 3200 mg iron) | 6 months | ↑Hb |
| Ferritin not specified | ↓NYHA (in NYHA class III patients) | |||||
| Echocardiographic indices: ↓PWT, ↓ST (in NYHA class III), ↓LVESD, ↓LVESV, ↓LVEDD (in NYHA class III), ↓LVEDV, ↓LVMI, ↑LVEF (in NYHA class III) |
6MWT, 6 min walking distance; Hb, haemoglobin; HF, heart failure; i.v., intravenous; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; LVMI, left ventricular mass index; NT-proBNP, NT-pro-brain natriuretic peptide; NYHA, New York Heart Association; PGA, Patient's Global Assessment; PWT, posterior wall thickness; QoL, quality of life; ST, septal thickness; TSAT, transferrin saturation; VO2, oxygen consumption.
Another study by Okonko et al.33 confirmed benefits of i.v. iron loading with iron sucrose in CHF patients. The authors randomized 35 (18 anaemic and 17 non-anaemic) patients with symptomatic CHF and abnormal iron metabolism to 16 weeks of i.v. iron sucrose or no treatment. Iron therapy was well tolerated and improved exercise capacity and symptoms. Benefits were more evident in anaemic patients. Increments in peak oxygen consumption related only to increments in TSAT, a putative marker of circulating iron status, but not to changes in Hb levels. Finally, in patients with severe CHF, anaemia and chronic kidney insufficiency, Usmanov et al.34 demonstrated that correction of anaemia using i.v. iron sucrose over a prolonged period (26 weeks) resulted in beneficial effects on electrocardiographic indices of cardiac remodelling, including improvement in cardiac hypertrophy, cardiac dilation, and ejection fraction. An improvement in NYHA status was observed in 47% of NYHA class III patients, but no improvement was seen in NYHA class IV patients.
In patients with chronic CHF and ID, treatment with oral iron has not been assessed in a double blind study. Oral iron has gastrointestinal side effects and limited absorption, which may limit its use in chronic illness.
Furthermore, a pilot, randomized, double-blind, controlled phase II study35 was previously conducted to compare the efficacy and safety of ferric carboxymaltose and iron sucrose vs. standard therapy in 72 patients with CHF (NYHA II–IV class), renal failure (GFR < 60 mL/min) and ID (Hb at screening: 10–14.5 g/dL; ferritin <100 ng/mL or 100–300 ng/mL with TSAT < 20%, or hypochromic red cells >10%). Iron was dosed weekly (i.v. injections of 200 mg iron or 100 mg iron for the last dose, if necessary) until the calculated iron dose was reached, then one injection (200 mg iron) every 4 weeks until Week 12.35 From this 12-week pilot study, an improvement in PGA score was observed in 80% of patients in the ferric carboxymaltose group and in 74% of patients in the iron sucrose group, and an improvement in NYHA class by one step, from baseline to last observation, was observed in 23 and 33% of patients, respectively. Also, a lesser proportion of patients in the placebo group showed an improvement in PGA score or improvement in NYHA class (47 and 13%, respectively). However, none of the between group differences in PGA or change in NYHA between last observation and baseline was statistically significant (Data on file, Vifor Pharma Ltd). The dosing schedule used in this study in patients with CHF did not raise any clinically relevant safety concerns; the safety profile of ferric carboxymaltose was similar to that of placebo.35 Although these data are encouraging, they require confirmation in a larger placebo controlled study.
FAIR-HF is an international, multi-centre, randomized, placebo controlled study and it will provide important efficacy and safety information on the impact of i.v. iron supplementation in CHF. FAIR-HF will enable us to make statements about the health economic impact that i.v. iron therapy may have in CHF patients.
Study Committees
Executive Committee Members: S.D.A. (Chair), P.P. (Co-Chair), J.C.C., G.F., R.W., K.D., H.D. (deceased), T.F.L., P.A.P.-W. (deceased).
DSMB Members: Jacobus Lubsen, SOCAR Research SA, 18, Chemin Chantemerle, Nyon 2, Switzerland. Rudolf P. Wüthrich, Klinik für Nephrologie, Universitätsspital, Rämistrasse 100, Zürich, Switzerland.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
The study is sponsored by Vifor Pharma Ltd. Funding to pay the Open Access publication charges for this article was provided by Vifor Pharma Ltd.
Acknowledgements
We acknowledge the editorial assistance in preparing this manuscript of Alison Dev, PhD, PAREXEL International Ltd, based on input and guidance provided by the authors.
Conflict of interest: S.D.A., P.P., J.C.C., G.F., R.W., K.D., H.D., and T.L. are members of the FAIR-HF steering committee. S.D.A. and R.W. are consultant and has received honoraria for speaking from Vifor Pharma Ltd and Amgen Inc. S.D.A. has received honoraria for speaking from Roche Pharma and Teva. G.F., T.L., and H.D. have received honoraria for speaking from Vifor Pharma Ltd. P.P. is a consultant and has received honoraria for speaking from Vifor Pharma Ltd. C.M. and B.E.R. are employees of Vifor Pharma Ltd, Switzerland and hold stock in Galenica Ltd. SP has no conflicts to declare.
References
- 1.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. J Am Med Assoc. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Berry C, Murdoch DR, McMurray JJ. Economics of chronic heart failure. Eur J Heart Fail. 2001;3:283–291. doi: 10.1016/s1388-9842(01)00123-4. [DOI] [PubMed] [Google Scholar]
- 4.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ American Heart Association Committee on exercise, rehabilitation, prevention. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 5.Clark AL, Poole-Wilson PA, Coats AJS. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 6.Harrington D, Anker SD, Chua TP, Webb-Peploe K, Ponikowski P, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 7.Fairbanks V, Beutler E. Iron deficiency. In: Beutler E, editor. Williams Hematology. 6th ed. New York: McGraw-Hill; 2001. pp. 295–304. and p.447–440. [Google Scholar]
- 8.Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Haas JD, Brownlie T., IV Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–688S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- 10.Dallman PR. Iron deficiency: does it matter? J Intern Med. 1989;226:367–372. doi: 10.1111/j.1365-2796.1989.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 11.Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol. 1982;242:E418–E427. doi: 10.1152/ajpendo.1982.242.6.E418. [DOI] [PubMed] [Google Scholar]
- 12.Satija P, Ondo WG. Restless legs syndrome: pathophysiology, diagnosis and treatment. CNS Drugs. 2008;22:497–518. doi: 10.2165/00023210-200822060-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lainscak M, von Haehling S, Anker SD. Natriuretic peptides and other biomarkers in chronic heart failure: from BNP, NT-proBNP, and MR-proANP to routine biochemical markers. Int J Cardiol. 2009;132:303–311. doi: 10.1016/j.ijcard.2008.11.149. [DOI] [PubMed] [Google Scholar]
- 14.Tang WH, Tong W, Jain A, Francis GS, Harris CM, Young JB. Evaluation and long-term prognosis of new-onset, transient, and persistent anemia in ambulatory patients with chronic heart failure. J Am Coll Cardiol. 2008;51:569–576. doi: 10.1016/j.jacc.2007.07.094. [DOI] [PubMed] [Google Scholar]
- 15.Anand IS, Kuskowski MA, Rector TS, Florea VG, Glazer RD, Hester A, Chiang YT, Aknay N, Maggioni AP, Opasich C, Latini R, Cohn JN. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation. 2005;112:1121–1127. doi: 10.1161/CIRCULATIONAHA.104.512988. [DOI] [PubMed] [Google Scholar]
- 16.Komajda M, Anker SD, Charlesworth A, Okonko D, Metra M, Di Lenarda A, Remme W, Moullet C, Swedberg K, Cleland JG, Poole-Wilson PA. The impact of new onset anaemia on morbidity and mortality in chronic heart failure: results from COMET. Eur Heart J. 2006;27:1440–1446. doi: 10.1093/eurheartj/ehl012. [DOI] [PubMed] [Google Scholar]
- 17.Szachniewicz J, Petruk-Kowalczyk J, Majda J, Kaczmarek A, Reczuch K, Kalra PR, Piepoli MF, Anker SD, Banasiak W, Ponikowski P. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol. 2003;90:303–308. doi: 10.1016/s0167-5273(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 18.Nordyke RJ, Kim JJ, Goldberg GA, Vendiola R, Batra D, McCamish M, Thomasson JW. Impact of anemia on hospitalization time, charges, and mortality in patients with heart failure. Value Health. 2004;7:464–471. doi: 10.1111/j.1524-4733.2004.74009.x. [DOI] [PubMed] [Google Scholar]
- 19.Weiss G, Goodnough LT. Anemia of chronic disease. N Eng J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 20.Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93:90–97. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 21.Crichton RR, Danielson BG, Geisser P. Iron Therapy with Special Emphasis on Intravenous Administration. 4th ed. London, Boston: International Medical Publishers; 2008. [Google Scholar]
- 22.Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. 1970;100:301–303. [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc. 1995;B57:289–300. [Google Scholar]
- 24.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12,065 patients with new-onset heart failure. Circulation. 2003;107:223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 25.Opasich C, Cazzola M, Scelsi L, De Feo S, Bosimini E, Lagioia R, Febo O, Ferrari R, Fucili A, Moratti R, Tramarin R, Tavazzi L. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J. 2005;26:2232–2237. doi: 10.1093/eurheartj/ehi388. [DOI] [PubMed] [Google Scholar]
- 26.Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou-Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48:2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Grzeslo A, Jankowska EA, Witkowski T, Okonko DO, Majda J, Anker SD, Poole-Wilson PA, Banasiak W, Ponikowski P. Iron deficiency: a frequent and morbid condition in patients with chronic heart failure. Eur Heart J. 2007;28(Abstract Suppl):773. [Google Scholar]
- 28.Silverberg DS, Wexler D, Sheps D, Blum M, Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I, Laniado S, Iaina A. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 29.Mancini DM, Katz SD, Lang CC, Lamanca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 30.Comin-Colet J, Ruiz S, Cladellas M, Rizzo M, Torres AG, Serrat R, Bruguera J. A pilot evaluation of the long-term effect of combined therapy with intravenous iron sucrose and erythropoietin in elderly patients with advanced chronic heart failure and cardio-renal anemia syndrome. J Cardiac Fail. doi: 10.1016/j.cardfail.2009.05.010. doi:10.1016/j.cardfail.2009.05.010. Published online ahead of print 29 June 2009. [DOI] [PubMed] [Google Scholar]
- 31.Bolger AP, Bartlett FR, Penston HS, O'Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:1225–1227. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 34.Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol. 2008;21:236–242. [PubMed] [Google Scholar]
- 35.Arutyunov GP, Bylova NA, Ivleva AY, Kobalava ZD. The safety of intravenous (i.v.) ferric carboxymaltose versus i.v. iron sucrose in patients with chronic heart failure (CHF) and chronic kidney disease (CKD) with iron deficiency (ID) Eur J Heart Fail. 2009;8(Suppl. 2):ii71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.