Summary
The bacterial non-homologous end-joining (NHEJ) apparatus is a two-component system that uses Ku and LigD to repair DNA double-strand breaks. Although the reaction mechanism has been extensively studied, much less is known about the physiological role of bacterial NHEJ. Recent studies suggest that NHEJ acts under conditions where DNA replication is reduced or absent (such as in a spore or stationary phase). Interestingly, genes encoding Ku and LigD have been identified in a wide range of bacteria that can chronically infect eukaryotic hosts. Strikingly, Sinohizobium meliloti, an intracellular symbiont of legume plants, carries four genes encoding Ku homologues (sku1 to sku4). Deletion analysis of the sku genes indicated that all Ku homologues are functional. One of these genes, sku2, is strongly expressed in free-living cells, as well as in bacteroid cells residing inside of the host plant. To visualize the NHEJ apparatus in vivo, SKu2 protein was fused to yellow fluorescent protein (YFP). Ionizing radiation (IR) induced focus formation of SKu2-YFP in free-living cells in a dosage-dependent manner. Moreover, SKu2-YFP foci formed in response to IR in non-dividing bacteroids, indicating that NHEJ system is functional even during the chronic infection phase of symbiosis.
Introduction
DNA double-strand breaks (DSBs) pose a major threat to the genome and must be repaired properly to preserve genomic integrity. In higher eukaryotes, DSBs are predominately repaired by non-homologous end-joining (NHEJ), in which the two ends of a DSB are directly rejoined with little or no requirement for sequence homology (Hefferin and Tomkinson, 2005). Recently, an evolutionary-related NHEJ pathway was identified in many bacterial species (Weller et al., 2002; Della et al., 2004; Bowater and Doherty, 2006; Pitcher et al., 2007a). In bacteria, NHEJ is mediated by the DNA end-binding Ku protein and LigD, a multifunctional DNA repair enzyme (Weller et al., 2002; Della et al., 2004). Ku forms a stable homo-dimer in solution and preferentially binds to the linear, double-stranded DNA in vitro (Weller et al., 2002). LigD proteins often contain an ATP-dependent DNA ligase catalytic domain, a DNA polymerase domain and an exonuclease domain (Weller et al., 2002; Pitcher et al., 2005; 2007b; Zhu et al., 2005; 2006; Zhu and Shuman, 2005a,b; 2006; Akey et al., 2006; Yakovleva and Shuman, 2006). LigD therefore possesses most of the activities required to remodel the damaged DNA ends (filling in gaps and removing non-complementary extensions) and ultimately closing the nicks. Ku binds to the polymerase domain of LigD and stimulates the polymerase, exonuclease and ligase activities in vitro (Weller et al., 2002; Pitcher et al., 2005). These in vitro results suggest that, in vivo, Ku and LigD form a two-component DSB repair complex in which the Ku homo-dimer recruits LigD to a DSB and modulates the end-processing activity of LigD. Nucleotide sequence analysis of the repaired junctions often shows mutations, which results from the LigD-dependent end processing (Gong et al., 2005; Stephanou et al., 2007). Thus, NHEJ is referred to as an error-prone repair pathway (Gong et al., 2005).
Under laboratory conditions, bacteria generally replicate DNA during most of their life cycle. Thus, sister chromosomes are available for homologous recombination (HR), an error-free pathway of DSB repair that relies on strand exchange between the broken DNA and its intact sister chromosome (Friedberg et al., 2006). In contrast, cells of higher eukaryotes are usually in the non-replicating states (G1 phase of the cell cycle) or terminally differentiated (G0 phase). Repair of DSBs depends solely on NHEJ in such non-replicating cells, as a homologous chromosome is not available for HR. Analogously, bacterial NHEJ likely functions during the cell’s slow- or non-replicating stage: In Bacillus subtilis, the expression of ykoU and ykoV (encoding the LigD and Ku proteins respectively) is under the control of σG, the forespore-specific sigma factor for RNApolymerase, and is induced during the forespore development (Wang et al., 2006). The GFP-tagged Ku (YkoV) protein localizes to the nucleoid in newly germinating spores, whereas it is distributed equally in the spore core before germination and disappears in the later stage of germination. As ATP is not available in the dormant spores, it has been suggested that the NHEJ apparatus is accumulated during sporulation, stored in spores, and functions specifically during spore germination. Disruptions of ykoU and/or ykoV significantly sensitize spores to DSB-inducing treatments, such as ionizing radiation (IR), UV, dry heat and extreme desiccation (Moeller et al., 2007). In Mycobacterium smegmatis, disruption of NHEJ genes sensitizes stationary-phase cultures, but not logarithmic cultures, to IR and desiccation (Pitcher et al., 2007c; Stephanou et al., 2007).
Interestingly, genes encoding the NHEJ apparatus have been identified in a wide range of pathogens (such as Agrobacterium tumefaciens, Bacillus anthracis, Mycobacterium tuberculosis and Pseudomonas aeruginosa) and symbionts (such as Sinohizobium meliloti and other rhizobia) that can chronically infect their eukaryotic hosts (Bowater and Doherty, 2006). Entry into the chronic phase of infection is generally accompanied by a marked reduction or absence of bacterial DNA replication (Brown and Smith, 2001; Muñoz-Elías et al., 2005; Furukawa et al., 2006; Mergaert et al., 2006). It therefore seems plausible that NHEJ may function during chronic infection in these bacteria.
Sinorhizobium meliloti, a member of Rhizobiales, represents a particularly interesting model organism in this respect, because the symbiotic relationship with the plant host is based on a chronic intracellular infection (Jones et al., 2007). S. meliloti induces the formation of a highly specialized organ, the nodule, on the root of the host plant, and intracellularly colonizes the tissue of the nodule (Broughton et al., 2000; Perret et al., 2000). In soil or liquid culture, S. meliloti is a rod-shaped cell of 1–2 μm, the so-called ‘free-living’ form. Upon infecting the cytoplasm of host cells, bacteria differentiate into bacteroids, the specialized symbiotic form which enzymatically reduce atmospheric nitrogen, thereby providing a source of fixed nitrogen for the host (Kaminski et al., 1998; Prell and Poole, 2006). During the bacteroid differentiation, DNA replication is repeated several times without cell division, resulting in formation of elongated (5–10 μm) cells containing up to 24 copies of their genome. Once fully differentiated, however, bacteroids lose their ability to resume growth and no further DNA replication occurs in these cells (Kobayashi et al., 2001; Mergaert et al., 2006). Thus, bacteroids are terminally differentiated, which is analogous to the G0 phase of eukaryotic cells.
Strikingly, multiple copies of Ku have been detected in S. meliloti and all other rhizobia to date (Rocha et al., 2005). The genome of S. meliloti strain Rm1021 carries four open reading frames (ORFs) encoding Ku homologues (see Fig. 1), whereas other bacteria possessing the NHEJ system typically encode a single Ku (Wilson et al., 2003; Rocha et al., 2005). Here, we report the in vivo analysis of the multiple NHEJ systems in S. meliloti strain Rm1021. Our results suggest that S. meliloti Ku proteins contribute to maintaining genomic stability in the free-living form and during chronic intracellular infection.
Fig. 1.
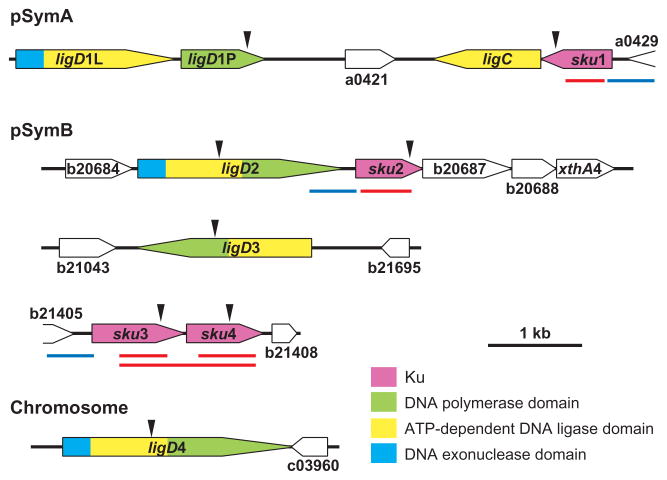
Genetic organization of loci encoding proteins of NHEJ apparatus. The genome of S. meliloti Rm1021 consists of three replicons: one chromosome, two megaplasmids (pSymA and pSymB) (Galibert et al., 2001). A blast database search detected four ORFs encoding close homologues of bacterial Ku in megaplasmids: SMa0426 (sku1) in pSymA; SMb20686, SMb21406 and SMb21407 (sku2, sku3 and sku4 respectively) in pSymB. Six ORFs encoding LigD homologues were also identified: SMa0414, SMa0417 and SMa0424 (ligD1L, ligD1P and ligC) in pSymA; SMb20685 and SMb21044 (ligD2 and ligD3) in pSymB; and SMC03959 (ligD4) in the chromosome. Genes are represented by arrows showing the direction of transcription and coloured depending on the predicted function of their encoding proteins: purple, bacterial Ku protein; green, DNA polymerase domain; yellow, ATP-dependent DNA ligase domain; blue, DNA exonuclease domain. The regions deleted in sku deletion mutants are marked with red lines. Regions subcloned in pMP220 are marked with blue lines. Arrowheads represent locations where pJH104 derivatives were inserted.
Results
S. meliloti Rm1021 possesses four functional homologues of Ku
Our examination of the sequenced S. meliloti Rm1021 genome revealed that it contained four ORFs encoding close homologues of bacterial Ku (sku1, sku2, sku3 and sku4; Fig. 1). Five ORFs encoding LigD homologues were also identified (ligD1L, ligD1P, ligD2, ligD3 and ligD4; Fig. 1). ligD2 and ligD4 encode proteins homologous to the P. aeruginosa LigD, in which the nuclease and polymerase domains are N-terminal and C-terminal extensions, respectively, of the ligase domain (Zhu and Shuman, 2005b). LigD3 lacks the nuclease domain, similar to the YkoU protein of B. subtilis (Bowater and Doherty, 2006). ligD1L encodes a ligase domain with an N-terminal nuclease domain, whereas ligD1P encodes only a polymerase domain. Additionally, the ligC ORF encodes only the ligase domain of LigD, which is similar to the M. smegmatis LigC (Gong et al., 2005). sku1 and sku2 are adjacent to ligC and ligD2 respectively, while sku3 and sku4 are arranged tandemly and likely form an operon (the intergenic region between two genes is 14 bp). Interestingly, only ligD4 is encoded in the chromosome. sku1, ligD1L, ligD1P and ligC are encoded on the symbiotic megaplasmid pSymA, while sku2, sku3, sku4, ligD2 and ligD3 are encoded on pSymB, a distribution that suggests a possible role for NHEJ during symbiosis.
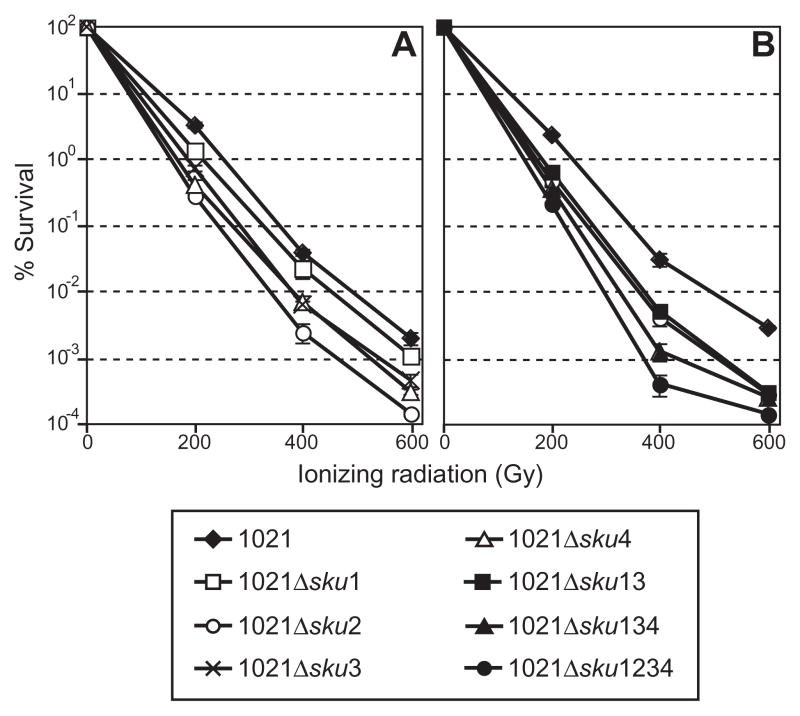
To examine the function of Ku homologues in strain Rm1021, non-polar deletion mutants of the sku genes (Rm1021Δsku1, Rm1021Δsku2, Rm1021Δsku3 and Rm1021Δsku4) were constructed. Saturated cultures of deletion mutants were irradiated with IR (0, 200, 400 and 600 Gy) and plated to measure viability. Irradiation of each sku deletion strain caused a significant decrease in viability compared with the wild type (Fig. 2A), indicating that all four sku genes encode functional orthologues of bacterial Ku protein. Interestingly, deletion of sku2 had a more profound effect on IR sensitivity than deletion of any other sku genes (Fig. 2A). The IR sensitivities of strains containing deletions of multiple sku genes (Rm1021Δsku13, Rm1021Δsku134 and Rm1021Δsku1234) were also determined (Fig. 2B). As expected, deletion of all four sku genes in the same background (Rm1021Δsku1234) resulted in an IR sensitivity greater than any of the single sku deletion strains (Fig. 2B). There was no significant difference between sku deletion mutants and the parental strain in the growth, viability or cell morphology under normal growth conditions (data not shown).
Fig. 2.
IR sensitivity of S. meliloti Ku (sku1, sku2, sku3 and sku4) deletion strains.
A. Stationary-phase cells of S. meliloti wild type (Rm1021), sku1, sku2, sku3, sku4 deletion mutant (Rm1021Δsku1, Rm1021Δsku2, Rm1021Δsku3 and Rm1021Δsku4) were irradiated with γ-rays. The percentage survival for each strain over the indicated range of doses was determined, and the mean from at least three independent experiments was plotted. Error bars denote standard error from the mean for each point.
B. IR sensitivity of the sku1sku3 double mutant (Rm1021Δsku13), sku1sku3sku4 triple mutant (Rm1021Δsku134) and sku1sku2sku3sku4 quadruple mutant (Rm1021Δsku1234). The wild type (Rm1021) and Rm1021Δsku2 were also treated as internal controls.
In vivo and in planta expression profiles of sku and other NHEJ genes
The existence of multiple NHEJ genes suggested that they might be differentially expressed under distinct physiological conditions, and thus might be specialized for different physiological roles. To address this model, we determined the expression of the S. meliloti sku genes. The regions inferred to contain promoters of sku1, sku2 and the sku3sku4 operon were cloned into pMP220, a broad-host range transcriptional-lacZ reporter system (Spaink et al., 1987; yielding plasmids pMP-sku1p, pMP-sku2p and pMP-sku3p respectively). The promoter region of recA, which encodes an essential protein for HR (Friedberg et al., 2006), was also examined as an example of a SOS-regulated gene whose expression is induced by DNA damage (plasmid pMP-recAp).
For promoters of sku1 and the sku3sku4 operon, the regions upstream of sku1 and sku3 were examined (Fig. 1), because ORFs upstream of sku1 and the sku3 (SMa0429 and SMb21405) are distantly located (287 bp and 173 bp away from start codons of sku1 and sku3 respectively), and encode putative proteins that were not known to be involved in NHEJ. In the case of the sku2 promoter, sku2 is located 96 bp downstream of ligD2 in the same orientation, suggesting that these genes might form an operon, which is transcribed from a promoter upstream of ligD2. To determine the operon structure of lidD2 and sku2, the polar effect of the insertional disruption of ligD2 on the transcription of sku2 was examined. We replaced the genomic copy of sku2 by lacZ, resulting in the strain Rm1021sku2ΔlacZ. The strain showed significantly higher β-galactosidase activity (1393 ± 21 units) than the parental strain (2.2 ± 0.3 units) 24 h after subculture. A polar mutant of ligD2 was then created by inserting pJH104 (Ferguson et al., 2005), an integration vector carrying a promoter-less uidA followed by a transcriptional terminator, into ligD2 in the Rm1021sku2ΔlacZ background. More than 70% of the lacZ activity (1023 ± 27 units) was retained in the presence of the pJH104 insertion in ligD2. Thus, although the transcription of sku2 is affected by the readthrough from the ligD2 promoter, sku2 is mainly transcribed by the promoter located in the intergenic region between ligD2 and sku2. We therefore cloned the region directly upstream of sku2 as the sku2 promoter (Fig. 1).
Exponentially growing cells were inoculated into fresh culture media, and the β-galactosidase activities of Rm1021 strains harbouring these plasmids were monitored 1, 3, 6, 9 and 24 h after subculture (h) (Fig. 3A). Cells were in early- to mid-logarithmic growth phase (1cmOD600 of 0.3–0.6) from approximately 3 to 9 h and were in stationary phase (1cmOD600 of > 2.0) by 24 h (Fig. 2A).
Fig. 3.
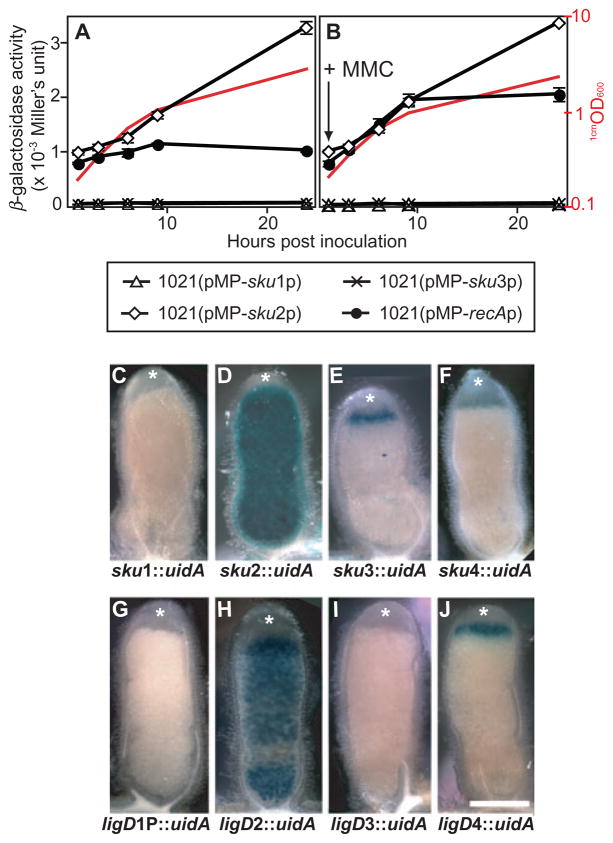
Expression of S. meliloti NHEJ genes in free-living cells and bacteroids. Panels A and B represent the levels of β-galactosidase activity (given in 103 Miller’s units) for the promoter region of sku1, sku2, sku3 and recA fused to lacZ respectively. Assays were performed at 1, 3, 6, 9 and 24 h after subculture. In B, 0.2 μg ml−1 of MMC was added to induce SOS response. The values reported represent the means of three independent experiments with standard errors (error bars). Growth curves of a representative strain (monitored by 1cmOD600) were also shown (red curves). Panels C–J represent histochemical localization of β-glucuronidase activity in nodule hand-sections. Nodules were harvested from alfalfa plants infected with Rm1021 strains carrying NHEJ gene–uidA fusions (Rm1021sku1::pJH104, Rm1021sku2::pJH104, Rm1021sku3::pJH104, Rm1021sku4::pJH104, Rm1021ligD1P::pJH104, Rm1021ligD2::pJH104, Rm1021ligD3::pJH104 and Rm1021ligD4::pJH104; C–J respectively). β-Glucuronidase activity was visualized as blue precipitates of the chromogenic substrate 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc). A total of 30–50 nodules from five plants were examined for each fusion. The meristematic zone of nodule is marked with white asterisks. Scale bar represents 0.5 mm.
In free-living conditions in rich medium, sku2 had the strongest promoter (3370 ± 15 units, 24 h), whereas significantly lower activities were recorded for the promoters of sku1 and the sku3sku4 operon (50 ± 3 and 30 ± 7 units, respectively) (Fig. 2A). Interestingly, the sku2 promoter activity was significantly enhanced at 24 h, by which time the cells were in stationary phase (a 3.3-fold higher activity than that of 1 h) (Fig. 3A). This observation suggested either that transcription of sku2 is enhanced during stationary phase or that it is under the control of one of S. meliloti’s quorum-sensing systems. To examine the possible involvement of quorum-sensing in sku2 regulation, the sku2 promoter activity was assessed in conditioned media derived from stationary culture of strain Rm1021 or Rm1021 (pMP-sku2p). Exposure of log-phase cells to conditioned media did not affect the transcription of sku2 (data not shown), indicating that quorum response is not involved in enhancing transcriptional activity of the sku2 promoter.
In S. meliloti (Tapias and Barbé, 1999), as in many other bacteria (Friedberg et al., 2006), expression of genes involved in HR pathway (such as recA) is induced by DNA damage in a process termed the SOS response, which is mediated by RecA and LexA proteins (Friedberg et al., 2006). To examine the effect of DNA damage on the transcription of sku genes, mitomycin C (MMC), a well-characterized inducer of the SOS response (Kenyon and Walker, 1980), was added to cultures at 1 h (Fig. 3B). Transcription from the recA promoter was induced twofold by the addition of MMC, consistent with a previous study of recA regulation in S. meliloti (Riera et al., 1994). In contrast, exposing cells to MMC did not affect the transcription of sku2 or other sku genes. As expected, a disruption of the chromosomal recA+ gene abolished the induction of the recA promoter by MMC; however, it did not alter the transcription of sku2 (data not shown). Thus, in the free-living cells of strain Rm1021, expression of sku2 is not controlled by the SOS response, but rather is growth phase-dependent.
To assess whether one or more of sku genes might be differentially expressed in nodules during symbiosis, the sku genes were transcriptionally fused to uidA by inserting pJH104 into sku1, sku2, sku3 and sku4. In the free-living stage, strong β-glucuronidase activity was only detected in the case of the sku2::uidA fusion on X-gluc indicator plates (data not shown), confirming the results we obtained with the sku promoter–lacZ fusions (Fig. 3A and B). Strains carrying sku::uidA fusions elicited functional pink nodules on alfalfa, indicating that bacteria successfully infected nodule tissues. The nodules were sectioned and stained for β-glucuronidase activity (Fig. 3C–F). S. meliloti induces formation of ‘indeterminate’-type nodules with a persistent meristem (which is marked with asterisks in Fig. 3C–J) on the roots of host plants. The sku2::uidA fusion exhibited strong β-glucuronidase expression throughout the nodule (Fig. 3D), whereas expression of sku3 and sku4 fusions occurred in a limited region close to the meristematic zone of the nodule. The region of the nodule where sku3 and sku4 are expressed corresponds to the invasion zone (Jones et al., 2007), where bacteria are being released into the plant cell and undergo the initial differentiation into bacteroids (Fig. 3E and F). The β-glucuronidase activity of the sku3 fusion was stronger than that of the sku4 fusion. As described above, sku3 and sku4 are likely in an operon which is transcribed from the promoter located upstream of sku3. The higher expression of the sku3 fusion compared with the sku4 fusion likely resulted from the pJH104 insertion being located closer to the promoter in Rm1021sku3::pJH104. We were unable to detect expression of the sku1 fusion in alfalfa nodules (Fig. 3C).
To examine whether the various ligD genes were also differentially regulated, we constructed uidA fusions to the four ligD genes that contain a polymerase domain (ligD1P, ligD2, ligD3 and ligD4). Free-living cells of the strain carrying the ligD2::uidA fusion exhibited β-glucuronidase activity on the X-gluc indicator plate at a level similar to the sku2::uidA fusion strain (data not shown). Moreover, the ligD2::uidA fusion and the sku2::uidA fusion shared virtually an identical expression profile in the nodule (Fig. 3D and H). The similar expression pattern of sku2 and ligD2, and the fact that the two genes are adjacent, suggest that SKu2 and LigD2 function together in NHEJ. Similarly, the ligD4::uidA and the sku3::uidA fusions shared a similar pattern of expression specific to the invasion zone (Fig. 3E and J). We could not detect expression of ligD1P and ligD3 fusions in alfalfa nodules (Fig. 3G and I).
Our observations indicate that each NHEJ gene is regulated in a temporally and spatially distinct manner. Among sku genes, sku2 is strongly transcribed in liquid culture and throughout the various zones of the nodule, implying that sku2 is expressed in free-living bacteria, in newly formed bacteroids and also in older and senescing bacteroids. In contrast, sku3 and sku4 seem to be preferentially expressed in the invasion zone.
IR irradiation induces foci formation of SKu2–YFP fusion protein in the free-living Rm1021
The particularly significant effect of a sku2 deletion on IR survival and the high expression levels of the sku2 gene (in both free-living cells and bacteroids) indicate that SKu2, probably acting together with LigD2, mediates the main NHEJ system in Rm1021. As many DNA repair and recombination proteins undergo subcellular localization to form foci after DNA damage (Smith et al., 2001; Renzette et al., 2005; Simmons et al., 2007), we fused yfpmut2 (encoding a monomeric derivative of YFP) to the genomic copy of sku2. The fusion gene was present in single copy at the sku2 native locus in pSymB and expressed from the native sku2 promoter.
To assess the response of the fusion protein to IR, we first re-examined the sensitivity of strains to lower doses of IR (≤ 200 Gy) (Fig. S1). Only 3% of the Rm1021 cells survived after treated with 200 Gy of IR (Fig. 2). Under such severely damaging condition, subcellular localization of the fusion protein observed would mostly be occurring in dead cells. When cells were irradiated with 50 and 100 Gy of IR, significant populations (53% and 18% respectively) of the wild-type cells remained viable. We chose to use those doses of IR to treat the strain, in an effort to ensure that SKu2-YFP was examined under conditions where the localization pattern would be most relevant to surviving cells. Rm1021sku2-yfpmut2 showed a wild-type level of survival, when cells were treated with 50 and 100 Gy of IR (Fig. S2). This observation indicates that the SKu2–YFP fusion protein retains the activity to mediate NHEJ in vivo.
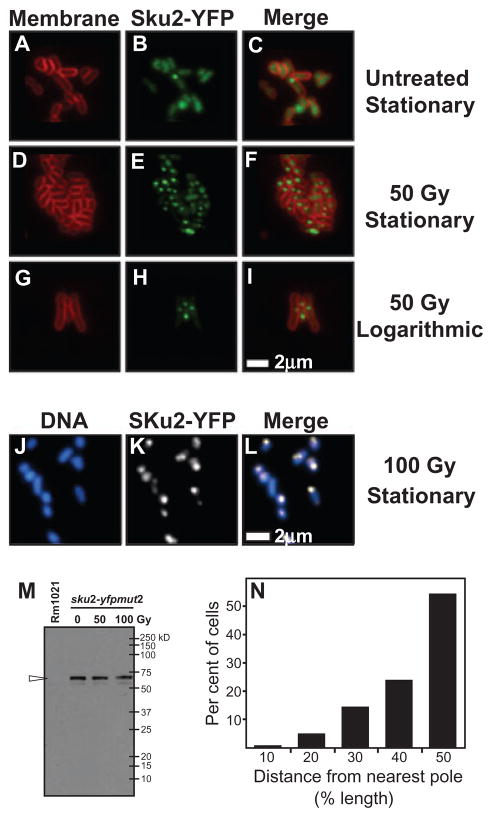
As expected, stationary-phase cells showed stronger SKu2-YFP fluorescence than cells in logarithmic phase, confirming our observation with the sku2–promoter fusion (data not shown). A strain lacking SKu2-YFP exhibited a lower level of background fluorescence and did not have foci (data not shown). In the stationary-phase culture without IR treatment, the SKu2-YFP fluorescence was distributed throughout the cell. Interestingly, in ~4% (n = 679) of cells, one focus of SKu2-YFP was visible above the background fluorescence (Fig. 4A–C; Table 1). Strikingly, after exposure to 50 Gy of IR, SKu2-YFP foci formed in ~60% (n = 726) of cells (Fig. 4D–F). When the IR dose was increased to 100 Gy, an even larger population of cells (~75%, n = 566) contained SKu2-YFP foci (Table 1). A significant fraction (~17%, n = 566) of cells contained more than two foci per cell. Thus, formation of SKu2-YFP foci is IR dosage-dependent. To confirm the localization of DNA, cells were stained with the fluorescent DNA dye 4′,6-diamidino-2-phenylindole (DAPI). As we expected, SKu2-YFP foci were 100% coincident with DNA (as represented in Fig. 4J–L). These observations suggested that SKu2 proteins accumulate on DSBs induced by IR irradiation in vivo. IR-inducible formation of SKu2-YFP foci was also observed in the Rm1021Δsku134 background (data not shown). This observation suggests that SKu2 is recruited independently of other SKu proteins and likely forms a homo-dimer in vivo.
Fig. 4.
Subcellular localization of SKu2 in free-living S. meliloti cells. Panels A–L represent subcellular localization of SKu2–YFP fusion proteins in free-living S. meliloti by epi-fluorescence microscopy. Rm1021sku2-yfpmut2 was grown in M9 medium supplemented with succinate. Cells of the stationary phase (A–E and J–L) or the logarithmic phase (G–I) were irradiated by 50 Gy of IR (D–I), 100 Gy of IR (J–L) or mock-treated (A–C). Localization of SKu2-YFP was visualized in living cells as represented in green (B, E and H) or white (K). The cell membrane was stained by FM4-64 as represented in red (A, D and G). Cellular DNA was stained by DAPI as represented in blue (J). Epi-fluorescence views of different filters were merged to analyse relative localization (C, F, I and L). No focus was observed in cells of the Rm1021 wild-type strain (data not shown). Scale bar represents 2 μm. Panel M represents immunoblot analysis of SKu2-YFP protein levels in Rm1021sku2-yfpmut2 after treatment with the indicated level of IR. Cells of stationary phase were treated with IR of 0, 50 and 100 Gy. Whole-cell extracts were blotted onto PVDF membrane and probed with anti-GFP (Abcam). One milligram of total protein from each cell extracts was loaded. Although few signals of endogenous proteins cross-reacting with anti-GFP were detected commonly in extracts from both Rm1021sku2-yfpmut2 (sku2-yfpmut2) and the wild-type control (Rm1021), strong signals of SKu2-YFP, a protein of expected molecular weight of 60 kDa, were only detected in extracts of Rm1021sku2-yfpmut2 (indicated with a open arrow head). Panel N represents the subcellular position of SKu2-YFP as a percentage of cell length for single-focus cells (n = 95). Cells of stationary phase were treated with IR of 50 Gy. The relative position of SKu2-YFP foci in each cell was determined as a ratio (%) of the distance between foci and the proximal pole to the longitudinal length of the cell.
Table 1.
The population of cells with SKu2-YFP foci increase in an IR dose-dependent manner.
| Percentage of cells with n foci |
||||||
|---|---|---|---|---|---|---|
| Ionizing radiation (Gy) | Growth phase | No. of cells | 0 | 1 | 2 | ≤ 3 |
| 0 | Stationary | 679 | 96 | 4 | < 1 | ND |
| 50 | 726 | 41 | 49 | 9 | < 1 | |
| 100 | 566 | 25 | 58 | 17 | < 1 | |
| 0 | Logarithmic | 474 | 74 | 23 | 3 | < 1 |
| 50 | 647 | 52 | 37 | 8 | 3 | |
| 100 | 550 | 27 | 49 | 20 | 4 | |
| 0 | Bacteroid | 915 | 83 | 9 | 5 | 3 |
| 50 | 919 | 59 | 21 | 11 | 9 | |
| 100 | 970 | 40 | 23 | 17 | 20 | |
Rm1021sku2-yfpmut2 was grown in M9 medium supplemented with succinate. Stationary-phase or the logarithmic phase cells were irradiated by 0, 50 and 100 Gy of IR. For bacteroids, alfalfa plants infected with Rm1021sku2-yfpmut2 were irradiated, and nodules were immediately crushed to observe bacteroids.
IR irradiation also induced formation of SKu2-YFP foci in log-phase cells, although these signals were faint compared with the foci intensity of cells in stationary phase (Fig. 4G–I). Interestingly, spontaneous formation (without IR treatment) of SKu2-YFP foci was observed in ~17% (n = 474) of cells during logarithmic phase, approximately fivefold more frequent than cells during stationary phase (Table 1). This could be caused by DSB formation as a result of replication stress in actively growing cells. Many studies have shown that replication fork collapse during DNA replication generates DSBs (Friedberg et al., 2006).
To examine whether formation of SKu2-YFP foci was due to an increased abundance of SKu2-YFP protein, we performed immunoblot analysis to monitor cellular SKu2-YFP protein levels (Fig. 4M). SKu2-YFP protein levels in cells irradiated with IR (50 and 100 Gy) were indistinguishable from the level of SKu2-YFP in untreated cells. We therefore conclude that SKu2 foci are assembled through a redistribution of existing protein. This observation is also consistent with our result from the analysis of the sku2 promoter activity (Fig. 3B), further indicating that expression of SKu2 is not regulated by DNA damage.
It has been reported that several proteins involved in HR localize to characteristic subcellular positions. For example, in E. coli and B. subtilis, RecA protein fused to GFP forms foci in response to DNA damage. RecA-GFP foci preferentially assemble at mid-cell, where DNA replication is expected to take place (Renzette et al., 2005; Simmons et al., 2007). To examine whether NHEJ apparatus localized to a specific subcellular location, we scored the position of SKu2-YFP foci related to the nearest cell pole in stationary-phase cells (Fig. 4N). Interestingly, more than 50% of SKu2-YFP foci were located near mid-cell, most likely because genomic DNA is more concentrated at mid-cell. Alternatively, the preferentially location of SKu2-YFP foci could result from DSB hot spots at mid-cell or the collapse of a replication fork.
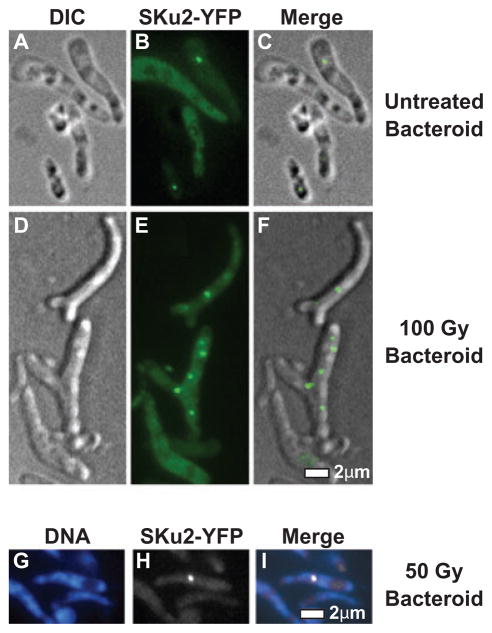
SKu2 can respond to IR irradiation in non-replicating bacteroids
To visualize the subcellular localization of the NHEJ apparatus during the chronic infection stage, alfalfa plants infected by Rm1021sku2-yfpmut2 were irradiated with IR. Nodules were crushed immediately after IR treatment and directly observed with the epi-fluorescence/DIC microscope. Fully developed bacteroids with the characteristic morphology (enlarged and branched cells) were readily distinguishable from undifferentiated bacteria or plant debris (Fig. 5A and D). In the non-IR treated control, SKu2-YFP foci were observed in ~17% (n = 915) of bacteroids (Fig. 5A–C). Given that bacteroids contain up to 24 copies of genomes (Mergaert et al., 2006), the frequency of spontaneous foci in bacteroids per genome is much less than that observed in free-living cells during logarithmic phase and probably less than in free-living stationary cells.
Fig. 5.
Subcellular localization of SKu2 in bacteroids. Panels A–I represent subcellular localization of SKu2–YFP fusion proteins in S. meliloti bacteroids by epi-fluorescence/DIC microscopy. Alfalfa plants infected with Rm1021sku2-yfpmut2 were irradiated by 100 Gy of IR (D–F), 50 Gy of IR (G–I) or mock-treated (A–C). Nodules were immediately harvested and crushed. Long, blanched bacteroid cells were visually distinguished from non-differentiated S. meliloti cells or plant-derived materials (A and D). In contrast to the free-living cells, the membrane of bacteroids could not be stained by FM4-64 (data not shown). Localization of SKu2-YFP was visualized in bacteroids as represented in green (B and E) or white (H). Cellular DNA was stained by DAPI as represented in blue (G). Epi-fluorescence views of different filters and DIC views were merged to analyse relative localization (C, F and I). No focus was observed in bacteroid cells of the Rm1021 wild-type strain (data not shown). Scale bar represents 2 μm.
IR irradiation significantly induced formation of SKu2-YFP foci in a dose-dependent manner (Table 1). Particularly, populations of bacteroids containing multiple foci (≤ 3) increased sevenfold after exposed to 100 Gy of IR (Fig. 5D–F; Table 1). Although multiple nucleoids appeared to be randomly organized in bacteroid cells, SKu2-YFP foci were always coincident with DAPI staining (as represented in Fig. 5G–I). SKu2-YFP was therefore still able to form foci in response to the IR-induced DSBs in non-replicating bacteroids.
NHEJ is not required for Rm1021 to establish a symbiosis with alfalfa
The preferential distribution of NHEJ genes on pSymA and pSymB and their differential expression in alfalfa nodules suggest that they act during symbiosis as well as in the free-living stage. To test whether NHEJ plays a crucial role in symbiosis, alfalfa seedlings were inoculated with all sku- and ligD-defective mutants. No significant difference was observed in nodule number or in plant growth in comparison with the parental strain. Coinoculation experiments in which two strains are forced to compete for colonization of a single niche can often reveal defects not observed during single-strain inoculations. To examine the competitiveness of the NHEJ-deficient mutant related to the wild type, we coinoculated alfalfa seedling with a Rm1021 derivative expressing β-glucuronidase (Rm1021bacA::pJH104, a strain with pJH104 insertion in the bacA promoter region without disrupting bacA gene) and Rm1021Δsku1234, a strain lacking all sku genes, or Rm1021, the wild-type parent. By chromogenically staining nodules with X-gluc, we observed that Rm1021Δsku1234 and Rm1021 competed with the uidA-tagged strain at a similar level, indicating that NHEJ is not required for nodulation competitiveness. The strong expression of sku2 in nodule and the active foci assembly of SKu2-YFP in bacteroids suggest that Ku proteins contribute to maintain the genomic integrity of S. meliloti in planta, but are not required to establish a functional symbiosis with alfalfa under laboratory conditions.
Discussion
Bacterial NHEJ is a two-component DSB repair system that consists of a bacterial Ku protein and a multifunctional DNA repair enzyme LigD (or, to some extent, LigC) (Gong et al., 2005). By tagging one of S. meliloti Ku proteins (SKu2) with YFP, we discovered that this component of NHEJ can undergo subcellular localization. Strikingly, SKu2–YFP fusion protein formed foci in response to IR irradiation (Fig. 4). The formation of SKu2-YFP foci increased in an IR dosage-dependent manner (Table 1). This observation, the first demonstration of DNA damage-dependent subcellular localization of NHEJ components in bacteria, clearly suggests that the Ku proteins accumulate at DSBs induced by IR. The significant intensity of foci above the background fluorescence suggests that foci are the result of increasing the local concentration of SKu2-YFP proteins at specific locations. Previous in vitro studies showed that multiple molecules of purified Ku (either bacterial or eukaryotic) could tightly bind to a single DNA substrate yielding multimeric Ku–DNA complexes (de Vries et al., 1989; Yaneva et al., 1997; Weller et al., 2002). This is likely due to the unique structure of a Ku dimer, an open ring-like molecule encircling the DNA that can freely slide along the DNA (Paillard and Strauss, 1991; Walker et al., 2001). Recently, localization of eukaryotic Ku was visualized in hamster cells by using a physiologically functional eGFP–Ku80 fusion protein (Mari et al., 2006). In agreement with our results, eGFP-Ku80, as a hetero-dimer with Ku70, accumulated at DSB induced by a focused laser beam. The FRAP (Fluorescence Recovery After Photobleaching; Houtsmuller and Vermeulen, 2001) assay revealed that accumulated eGFP-Ku80 could exchange with the soluble fraction, showing that Ku on DNA ends is in dynamic equilibrium with protein in solution.
Surprisingly, IR challenge induced SKu2-YFP foci during logarithmic phase (Fig. 4G–I), indicating that NHEJ also operates in replicating cells. Our experiments did not detect significant IR sensitization by sku deletions in logarithmically growing cultures (data not shown) as previously reported in M. smegmatis (Pitcher et al., 2007c; Stephanou et al., 2007). As DSBs are exclusively repaired through HR during logarithmic phase, HR might compensate for the loss of the NHEJ pathway under such conditions. Furthermore, we confirmed that Ku could still actively respond to DSBs in mature bacteroids, representing a non-replicating and terminally differentiated state (Fig. 5 and Table 1). Recently, it has been shown in B. subtilis that ongoing DNA replication was required to assemble RecA at a DSB and for functional HR (Simmons et al., 2007). Our result presents the first indication that NHEJ is a DSB repair pathway functional in bacteria under conditions of chronic infection, where DNA replication is slow or absent. Although Rm1021 does not require NHEJ to establish symbiosis with alfalfa, it is yet possible that, in certain pathogen–host interactions, NHEJ allows repair of DSBs induced by the genotoxic defence of host cells, and may also be able to promote mutagenesis inside of host tissues, as DSB repair by bacterial NHEJ is highly error-prone (Gong et al., 2005).
Rhizobia are unique among bacteria possessing NHEJ, as multiple Ku homologues are conserved in all genomes of rhizobia sequenced to date. Four homologues of Ku are detected in Bradhyrhizobium japonicum USDA110, Rhizobium etli CFN42, Rhizobium leguminosarum bv. viciae 3841, Mesorhizobium loti MAFF303099 and S. meliloti Rm1021. A. tumefaciens C58, a plant pathogen closely related to S. meliloti, also possesses five Ku homologues. By deleting each sku gene, we showed that all four Ku proteins of Rm1021 were functional in vivo (Fig. 2A). Very recently, LigD and LigC proteins of A. tumefaciens C58 (AtuLigD1, D2, AtuLigC1, C2 and C3) were biochemically characterized (Zhu and Shuman, 2007). Purified P. aeruginosa Ku protein significantly enhanced the activity of AtuLigD2, C2 and C3 in vitro, indicating that those three proteins are capable of mediating NHEJ. Thus, those bacteria possess multiple sets of functional NHEJ components. It remains to be determined how each Ku protein interacts with various LigDs (or other Ku proteins) to mediate NHEJ.
Our identification of the involvement of multiple SKus in NHEJ in strain Rm1021 raised an important question: how are these sku genes regulated? Our analysis revealed that the transcriptional level of the sku2 gene in free-living cells was significantly higher than that of sku1 and the sku3sku4 operon (Fig. 3A). Strong expression of sku2 was also observed in all zones of alfalfa nodules, whereas sku1 appeared silent in the nodule (Fig. 3C and D). Interestingly, expression of the sku3sku4 operon was limited to the infection zone (Fig. 3E and F), suggesting that transcription of those genes is induced under a specific condition. Indeed, a microarray analysis of the response of S. meliloti to osmotic shock revealed that transcription of sku3 was induced by 0.4 M of NaCl (Domínguez-Ferreras et al., 2006). The induction of ligD3 transcription was detected in nodules of Medicago truncatula, another host of Rm1021, by transcriptome analysis (Barnett et al., 2004). By the histochemical staining of β-glucuronidase activity, we also observed that the expression of the ligD3::uidA fusion, which was not detected in alfalfa nodule (Fig. 3I), was induced in mature bacteroids in nodules elicited on M. truncatula (data not shown). This suggests that some of NHEJ genes are regulated in a host-specific manner. It is interesting to note that MMC, a typical inducer of the SOS response, does not affect the expression of sku genes.
The physiological importance of NHEJ in the symbiotic system of S. meliloti Rm1021 and host plants remain unclear. Rm1021Δsku1234, a strain lacking all sku genes, still can nodulate and establish a functional symbiosis with alfalfa under laboratory conditions. Nevertheless, by sealing DSBs in bacteroid DNA, NHEJ might be able to delay senescence and thus prolong nitrogen fixation capability, especially under stressful conditions. Given that mature bacteroids of Rm1021 cannot resume growth outside of the nodule (Mergaert et al., 2006), it has been proposed that, upon the senescence of nodules, the undifferentiated population of bacteria in the nodule (invaded the tissue but remained not to be released into the host cytoplasm) are released into the environment to repopulate. As undifferentiated bacteria are thought to persist in lumens of the nodule tissue with little or no growth (Gage, 2004), it is possible that NHEJ is important for survival of those persisting bacteria as well. It is also possible that multiple NHEJ genes are evolved for fitness or survival under various stressful conditions found in the natural environment or for the symbiosis between S. meliloti and its alternative hosts. Expression patterns of sku genes suggest that each Ku protein of Rm1021 has a distinct role under different conditions. SKu2 probably mediates the main NHEJ pathway in Rm1021, given that SKu2 is highly expressed in both the free-living cells and bacteroids. This model is consistent with our observation that a deletion of sku2 sensitized Rm1021 to IR at a higher extent than other sku single deletions (Fig. 2A). On the other hand, other NHEJ proteins could be auxiliary NHEJ components induced under specific environmental circumstances (such as under high salt stress or in nodules of M. truncatula). To this end, it would be interesting to examine the biochemical properties of each NHEJ component under stressful conditions.
Experimental procedures
Microbiological techniques
Strains and plasmids used in this study were listed in Table 2. E. coli recombinants were grown at 37°C on Luria–Bertani (LB) media/medium (Sambrook et al., 1989). S. meliloti strain Rm1021 and its derivatives were raised at 30°C in/on M9 succinate medium with biotin or LBMC (LB supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2). Gentamycin (Gm), neomycin (Nm), streptomycin (Sm) and tetracycline (Tet) were added at concentrations of 50, 200, 500 and 15 μg ml−1 respectively. Plasmids were mobilized into Rm1021 and derivative strains by tri-parental matings using pRK600 as the helper plasmid (Ditta et al., 1980). Pfu turbo (Stratagene) was used in all PCR reaction followed by cloning. The amplified products were cloned into pCR-Blunt II-TOPO (Invitrogen) and verified by sequencing the inserts.
Table 2.
Strains and plasmids used in this study.
| Relevant characteristics | Reference | |
|---|---|---|
| Sinorhizobium meliloti strains | ||
| Rm1021 | SmR derivative of SU47 | Meade et al. (1982) |
| Rm1021Δsku1 | Deletion mutant of Rm1021 where the 0.3 kb SalI-XhoI fragment was removed from sku1 | This study |
| Rm1021Δsku2 | Deletion mutant of Rm1021 where the 0.5 kb fragment was removed from sku2 | This study |
| Rm1021Δsku3 | Deletion mutant of Rm1021 where the 0.5 kb XhoI fragment was removed from sku3 | This study |
| Rm1021Δsku4 | Deletion mutant of Rm1021 where the 0.6 kb SalI–XhoI fragment was removed from sku4 | This study |
| Rm1021Δsku13 | Rm1021Δsku1 strain whose sku3 was also deleted as in Rm1021Δsku3 | This study |
| Rm1021Δsku134 | Rm1021Δsku1 strain where the 1445 bp XhoI fragment was removed from sku3-sku4 region | This study |
| Rm1021Δsku1234 | Rm1021Δsku134 whose sku2 was also deleted as in Rm1021Δsku2 | This study |
| Rm1021sku2ΔlacZ | Derivative of Rm1021 whose sku2 and 0.6 kb of the downstream ORF was replaced with lacZ | This study |
| Rm1021sku1::pJH104 | Derivative of Rm1021 with a pJH104 insertion in sku1 at 628 bp downstream from the start codon | This study |
| Rm1021sku2::pJH104 | Derivative of Rm1021 with a pJH104 insertion in sku2 at 561 bp downstream from the start codon | This study |
| Rm1021sku3::pJH104 | Derivative of Rm1021 with a pJH104 insertion in sku3 at 649 bp downstream from the start codon | This study |
| Rm1021sku4::pJH104 | Derivative of Rm1021 with a pJH104 insertion in sku4 at 478 bp downstream from the start codon | This study |
| Rm1021ligD1P::pJH104 | Derivative of Rm1021 with a pJH104 insertion in ligD1P at 703 bp downstream from the start codon | This study |
| Rm1021ligD2::pJH104 | Derivative of Rm1021 with a pJH104 insertion in ligD2 at 1003 bp downstream from the start codon | This study |
| Rm1021ligD3::pJH104 | Derivative of Rm1021 with a pJH104 insertion in ligD3 at 1099 bp downstream from the start codon | This study |
| Rm1021ligD4::pJH104 | Derivative of Rm1021 with a pJH104 insertion in ligD4 at 962 bp downstream from the start codon | This study |
| Rm1021sku2-yfpmut2 | Rm1021 derivative where sku2-yfpmut2 replaced the native sku2 | This study |
| Rm1021bacA::pJH104 | Derivative of Rm1021 with a pJH104 insertion in the bacA promoter region without disrupting bacA | G.P. Ferguson (unpublished) |
| Plasmids | ||
| pJH104 | Suicide vector containing a promoterless uidA, Km/NmR | Ferguson et al. (2005) |
| pMP220 | IncP expression vector containing a promoterless lacZ gene, TcR | Spaink et al. (1987) |
| pMP-sku1p | The sku1 promoter region cloned in pMP220 as a 0.5 kb XbaI–PstI fragment | This study |
| pMP-sku2p | The sku2 promoter region cloned in pMP220 as a 0.5 kb XbaI–PstI fragment | This study |
| pMP-sku3p | The sku3 promoter region cloned in pMP220 as a 0.5 kb XbaI–PstI fragment | This study |
| pMP-recAp | The recA promoter region cloned in pMP220 as a 0.5 kb XbaI–PstI fragment | This study |
Construction of sku non-polar deletion mutants
To construct the deletion of sku genes, we amplified each full-length ORF with flanking regions (for sku1 and sku3) or the 5′ region and the 3′ region with flanking regions separately (for sku2 and sku4) by PCR using Rm1021 genomic DNA and the following primer pairs: Δsku1 (5′-GGATCCATGGCCCTGCGTCCCTATTGGAAAGGCT-3′/5′-GGATCCTCATTTGGTCGCCCGTTTCTCAGCCGCG-3′); Δsku2-up (5′-TCTAGAAGAAATTCCATCAGGCGACGAGCG-3′/5′-CTGCAGATATAGCCCTTCCAACTTGCCCTG-3′); Δsku2-down (5′-GGATCCATGGCACCCAGGGCAAGTTGGAAGGGCT-3′/5′-GGATCCAGGCTTTCTTCTTGGCCGCGGGTTTAGC-3′); Δsku3 (5′-TTGGATCCCCTGGATATGCAGGGAGTTAGCCATGG-3′/5′-GCGGATCCAATTTCTCGATGTCGATCGTCCTCGC-3′); Δsku4-up (5′-AGTTCATTGACAGCGTCTCTGGCAAGCCG-3′/5′-TTTCACCGCGCTCGTAACCTTTCACCTCG-3′); Δsku4-down (5′-TTGGATCCCCTGGATATGCAGGGAGTTAGCCATGG-3′/5′-CCGGATCCACTTCTACTTCCAGCCCGTCATGCAG-3′). Underlined nucleotides correspond to mismatches introduced to create selected restriction sites. To delete both sku3 and sku4 at the same time, the region containing sku3 and sku4 was also amplified with the primer set used for Δsku3 and Δsku4-down. Most parts of sku coding sequences were deleted by restriction digestion of internal sites (for sku1, sku3 and sku3sku4), or by combining two PCR fragments of 5′ and 3′ regions of genes (for sku2 and sku4). DNA fragments containing in-frame sku deletions were subcloned into pJQ200-SK (Quandt and Hynes, 1993), and the resulting plasmids, pJQ-Δsku1, Δsku2, Δsku3, Δsku4 and Δsku34 were mobilized into Rm1021 or its derivatives by tri-parental mating. Transconjugants were first selected on LBMC plates containing Sm and Gm, then on LBMC plates containing Sm and 5% (w/v) of sucrose to select for double cross-overs. Replacement of wild-type sku genes with its deleted loci was confirmed by PCR. The position of deleted sku protein-coding regions on Rm1021 genome are shown in Fig. 1.
IR survival assay
Cultures of each strain were grown to saturation (1cmOD600 of > 1.5) in M9 succinate medium, washed in 0.85% NaCl, and diluted to 1cmOD600 of 0.1 in 0.85% NaCl. Suspensions were aliquoted into 1.5 ml tubes and irradiated in a 60Co gamma irradiator. Following IR irradiation of 0, 200, 400 and 600 Gy or 0, 10, 50, 100 and 200 Gy, cells were diluted in 0.85% NaCl, plated on LBMC, and incubated for 3 days before colony-forming units (cfu) were examined. Survival at each dose was determined as the percentage (%) of cfu formed by irradiated samples to cfu of the untreated sample. The results reported represent the means of at least three independent experiments.
Construction of reporter gene fusions
To construct promoter–lacZ fusions, predicted promoter regions of sku1, sku2 and the sku3sku4 putative operon were amplified by PCR using the following primer pairs: the sku1 promoter (5′-TCTAGATCTGACACCGTCCGGAGATG GTC-3′/5′-CTGCAGGGCCATGGCGACCTCCGATCAA-3′); the sku2 promoter (5′-TCTAGAAGAAATTCCATCAGGCGACGAGCG-3′/5′-CTGCAGATATAGCCCTTCCAACTTGCCCTG-3′); the sku3 promoter (5′-TCTAGACCTGGCAGGATGAGAAGGTCGTG-3′/5′-CTGCAGTGGCTAACTCCCTGCATATCCAG-3′); the recA promoter (5′-TCTAGATTCTGCGACTTCACCTGTGCTTGC-3′/5′-CTGCAGCGATTTGTCCTCTACGAGCCGCAA-3′). To construct transcriptional–uidA fusions, internal regions of sku1, sku2, sku3, sku4, ligD1P, ligD2, ligD3 and ligD4 were amplified by PCR using the following primer pairs: sku1 (5′-AGGATCCGGTGAAGGGCTATCAGCGA-3′/5′-TCGGGATCCAGTCCTTGGTCTGCTTT-3′); sku2 (5′-CGGATCCACATGAAGCCCGTCGATC-3′/5′-TCGGCAAACCGGATCCCGAGATGCT-3′); sku3 (5′-CCGGATCCCCTTCCACACGGTCAAC-3′/5′-AGGGATCCTCGAATTCTTCGGCCGAG-3′); sku4 (5′-CGGATCCACACGCTCAACCGCAAGT-3′/5′-GGATCCATACGACGATGCCTTTGCC-3′); ligD1P (5′-GAGGATCCCGCCCGCTACCATGAAG-3′/5′-CTGGATCCAGTTGAGCGGCATCGAG-3′); ligD2 (5′-ATGGATCCTGGCCCGTCTGAAGCGT-3′/5′-AAGGATCCAGCCGTTGAGATGCAGG-3′); ligD3 (5′-CTGGATCCGACGACGGAATTTGAGG-3′/5′-TCGGATCCAGGCAGGTGCAGTAGTC-3′); ligD4 (5′-CAGGATCCGGTTCCTGGTTCAGAAGC-3′/5′-GTGGATCCGCCTTCCGAAAGCGCCA-3′). The cloned fragments were subcloned into the broad-host-range reporter vector pMP220 (for promoter–lacZ fusions) or the suicide uidA reporter vector pJH104 (for transcriptional–uidA fusions). Resulting plasmids were mobilized into Rm1021 or its derivatives by tri-parental mating. Transcon-jugants were selected on LBMC plates containing Sm and Tet (for pMP220 derivatives) or Sm and Nm (for pJH104). It must be noted that derivatives of pJH104 were inserted into native gene loci by single cross-over recombination, resulting in polar disruptions of corresponding genes. Insertions of pJH104 were confirmed by PCR. The position of the cloned sku promoters and pJH104 insertions in relation to their predicted protein-coding regions on Rm1021 are shown in Fig. 1. To replace the genomic copy of sku2 with lacZ, the XbaI–SacI fragment of pMP-sku2p containing the sku2 ptomoter–lacZ fusion was ligated with the XbaI–SacI fragment of pJQ-Δsku2. The resulting plasmid was integrated into Rm1021 by double cross-over as described above, to give rise of Rm1021sku2ΔlacZ.
β-Galactosidase assay in free-living Rm1021 strains
The β-galactosidase activity of Rm1021 transconjugants was assayed according to Miller (1972). Overnight exponentially growing cultures (1cmOD600 of 0.4–0.6) were initially adjusted to 1cmOD600 of 0.1 in LBMC (0 h after subculture). β-Galactosidase activities were monitored 1, 3, 6, 9 and 24 h after subculture. To induce SOS response, 0.2 μg ml−1 of MMC was added 1 h after subculture.
β-Glucuronidase assay in nodules
Seedlings of alfalfa (Medicago sativa cv. Iroquois) were inoculated with Rm1021 derivatives on Petri dishes containing Jensen agar as described previously (Leigh et al., 1985; Pellock et al., 2000). Plants inoculated with Rm1021 derivatives containing pJH104 insertions showed a wild-type phenotype for nodulation. Four-week-old nodules were excised, sectioned and incubated in X-gluc staining buffer [1 mM 5-bromo-4-chloro-3-indoyl-β-d-glucuronide, 0.1 M Na-phosphate buffer (pH 7), 10 mM EDTA, 0.5 mM K-Ferricyanide, 0.5 mM K-Ferrocyanide, 0.1% Triton X-100] at 37°C for overnight.
Construction of Rm1021sku2-yfpmut2
The 3′ region of sku2 coding sequence and the region downstream of sku2 (starting from the stop codon of sku2) were amplified by PCR using the following primer pairs: sku2-yfpmut2-up (5′-GGGCCCTATCAGACTGAGTCTCGTGAGCTGTCC-3′/5′-CTCGAGGGCTTTCTTCTTGGCCGCGGGTTTAGCAG-3′); sku2-yfpmut2-down (5′-CTGCAGATGGCGCTTGCCGGTCGGACATTCGGTATC-3′/5′-TCTAGATAACGCATCCACCAGCGGCGGGCTTCGGC-3′). Two fragments were subcloned in pJQ200SK and combind with the XhoI–PstI fragment containing yfpmut2, encoding the A206K missense mutation generating monomeric YFP, of pKL183 (Lemon and Grossman, 2000). The resulting pJQ200SK derivative carried sku2-yfpmut2 followed by the downstream sequence of native sku2 locus. Replacement of wild-type sku2 locus with sku2-yfpmut2 was achieved by a similar procedure as in construction of sku deletion strains.
Live cell microscopy
Aliquots of cells grown in M9 medium supplemented with succinate were stained with the vital membrane dye FM4-64 (Molecular Probes) and the DNA stain DAPI. The following Chroma filter sets were used: 41029 for YFP, 41002C for FM4-64 and 31000 for DAPI. Exposure time for SKu2–YFP fusion protein was 3 s. Images were acquired, colourized and merged using OpenLab software (Improvision). To assess the effect of IR irradiation in free-living cells, aliquots of M9 cultures of stationary phase (1cmOD600 of > 1.5) or log-phase (1cmOD600 of 0.4–0.5) were irradiated with IR of 0, 50 and 100 Gy and observed under the microscope.
For bacteroids, whole alfalfa plants on Petri dishes containing Jensen agar were irradiated in a 60Co gamma irradiator. Nodules were immediately harvested, crushed, and bacteroids were observed under the microscope. Positions of foci were scored after colorization and merging of microscopic images. All data presented here are cumulative from at least two independent experiments, each of which gave nearly identical results.
Immunoblot analysis of SKu2-YFP protein
Rm1021sku2-yfpmut2 and Rm1021 were grown in M9 medium supplemented with succinate. Cells of the stationary phase were irradiated by IR of 50 and 100 Gy, then lysed by boiling in the presence of 1% SDS. Total proteins were acetone precipitated and quantified by Bradford assay (Bio-Rad). One milligram of protein from each extract was separated on SDS-polyacrylamide (4% to 20% w/v) gels. For immunodetection, separated proteins were transferred on PVDF Immobilon-P membranes (Millipore Corporation) and probed with 1/4000 working dilutions of the anti-GFP (Abcam). Horseradish peroxidase-labelled goat anti-rabbit immunoglobulin antibodies of the ECL kit (Amersham Pharmacia Biotech) were used as secondary antibodies. Reactions were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech).
Supplementary Material
Acknowledgments
We thank Gail P. Ferguson (University of Edinburgh) for providing us with her unpublished strain, Rm1021bacA::pJH104, used in our studies. We are indebted to Mary Lou Pardue (Massachusetts Institute of Technology) and Alan D. Grossman (Massachusetts Institute of Technology) for the use of their microscopes. We wish to thank Judith Carlin, Marianne White and Dora Gerber for their help. The authors thank Katherine E. Gibson for insightful discussions and kind suggestions; Melanie Barker Berkmen for help with the microscopy; Mary Ellen Wiltrout for critical reading of the manuscript. This work was supported by National Institutes of Health Grant GM31010 (to G.C.W.), National Cancer Institute (NCI) Grant CA21615-27 (to G.C.W.), the Fonds National Suisse de la Recherche Scientifique (Projects 31-63893.00 and 3100AO-104097/1; to W.J.B.), University of Geneva, MIT Center for Environmental Health Sciences NIEHS P30 ES002109, a postdoctoral fellowship from the NCI (to L.A.S.) and JSPS Postdoctoral Fellowships for Research Abroad (to H.K.). G.C.W. is an American Cancer Society Research Professor.
Footnotes
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2958.2007.06036.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Akey D, Martins A, Aniukwu J, Glickman MS, Shuman S, Berger JM. Crystal structure and nonhomologous end-joining function of the ligase component of Mycobacterium DNA ligase D. J Biol Chem. 2006;281:13412–13423. doi: 10.1074/jbc.M513550200. [DOI] [PubMed] [Google Scholar]
- Barnett MJ, Toman CJ, Fisher RF, Long SR. A dual-genome Symbiosis Chip for coordinate study of signal exchange and development in a prokaryote–host interaction. Proc Natl Acad Sci USA. 2004;101:16636–16641. doi: 10.1073/pnas.0407269101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowater R, Doherty AJ. Making ends meet: repairing breaks in bacterial DNA by non-homologous end-joining. Plos Genet. 2006;2:e8, 93–99. doi: 10.1371/journal.pgen.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton WJ, Jabbouri S, Perret X. Keys to symbiotic harmony. J Bacteriol. 2000;182:5641–5652. doi: 10.1128/jb.182.20.5641-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Smith AW. Dormancy and persistence in chronic infection: role of the general stress response in resistance to chemotherapy. J Antimicrob Chemother. 2001;48:141–142. doi: 10.1093/jac/48.1.141. [DOI] [PubMed] [Google Scholar]
- Della M, Palmbos PL, Tseng HM, Tonkin LM, Daley JM, Topper LM, et al. Mycobacterial Ku and ligase proteins constitute a two-component NHEJ repair machine. Science. 2004;306:683–685. doi: 10.1126/science.1099824. [DOI] [PubMed] [Google Scholar]
- Ditta G, Stanfield S, Corbin D, Helsinki DR. Broad host range cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Ferreras A, Pérez-Arnedo R, Becker A, Olivares J, Soto MJ, Sanjuán J. Transcriptome profiling reveals the importance of plasmid pSymB for osmoadaptation of Sinorhizobium meliloti. J Bacteriol. 2006;188:7617–7625. doi: 10.1128/JB.00719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GP, Datta A, Carlson RW, Walker GC. Importance of unusually modified lipid A in Sinorhizobium stress resistance and legume symbiosis. Mol Microbiol. 2005;56:68–80. doi: 10.1111/j.1365-2958.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Washington, DC: American Society for Microbiology Press; 2006. [Google Scholar]
- Furukawa S, Kuchma SL, O’Toole GA. Keeping their options open: acute versus persistent infections. J Bacteriol. 2006;188:1211–1217. doi: 10.1128/JB.188.4.1211-1217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage DJ. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev. 2004;68:280–300. doi: 10.1128/MMBR.68.2.280-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, et al. The composite genome of the legume symbiont Sinorhizobium meliloti. Science. 2001;27:668–672. doi: 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- Gong C, Bongiorno P, Martins A, Stephanou NC, Zhu H, Shuman S, Glickman MS. Mechanism of nonhomologous end-joining in mycobacteria: a low-fidelity repair system driven by Ku, ligase D and ligase C. Nat Struct Mol Biol. 2005;12:304–312. doi: 10.1038/nsmb915. [DOI] [PubMed] [Google Scholar]
- Hefferin ML, Tomkinson AE. Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair (Amst) 2005;4:639–648. doi: 10.1016/j.dnarep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Houtsmuller AB, Vermeulen W. Macromolecular dynamics in living cell nuclei revealed by fluorescence redistribution after photobleaching. Histochem Cell Biol. 2001;115:13–21. doi: 10.1007/s004180000234. [DOI] [PubMed] [Google Scholar]
- Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nat Rev Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski PA, Batut J, Boistard P. A survey of symbiotic nitrogen fixation by rhizobia. In: Spaink HP, Kondorosi A, Hooykaas PJJ, editors. The Rhizobiaceae. Dordrecht, Boston, London: Kluwer Acad Press; 1998. pp. 431–460. [Google Scholar]
- Kenyon CJ, Walker GC. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci USA. 1980;77:2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Sunako M, Hayashi M, Murooka Y. DNA synthesis and fragmentation in bacteroids during Astragalus sinicus root nodule development. Biosci Biotechnol Biochem. 2001;65:510–515. doi: 10.1271/bbb.65.510. [DOI] [PubMed] [Google Scholar]
- Leigh JA, Signer ER, Walker GC. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci USA. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD. Movement of replicating DNA through a stationary replisome. Mol Cell. 2000;6:1321–1330. doi: 10.1016/s1097-2765(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Mari PO, Florea BI, Persengiev SP, Verkaik NS, Bruggenwirth HT, Modesti M, et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci USA. 2006;103:18597–18602. doi: 10.1073/pnas.0609061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc Natl Acad Sci USA. 2006;103:5230–5235. doi: 10.1073/pnas.0600912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- Moeller R, Stackebrandt E, Reitz G, Berger T, Rettberg P, Doherty AJ, et al. Role of DNA repair by non-homologous end joining (NHEJ) in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV and ionizing radiation. J Bacteriol. 2007;189:3306–3311. doi: 10.1128/JB.00018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Elías EJ, Timm J, Botha T, Chan WT, Gomez JE, McKinney JD. Replication dynamics of Mycobacterium tuberculosis in chronically infected mice. Infect Immun. 2005;73:546–551. doi: 10.1128/IAI.73.1.546-551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard S, Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991;19:5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellock BJ, Cheng HP, Walker GC. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J Bacteriol. 2000;182:4310–4318. doi: 10.1128/jb.182.15.4310-4318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret X, Staehelin C, Broughton WJ. Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev. 2000;64:180–201. doi: 10.1128/mmbr.64.1.180-201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher RS, Tonkin LM, Green AJ, Doherty AJ. Domain structure of a NHEJ DNA repair ligase from Mycobacterium tuberculosis. J Mol Biol. 2005;351:531–544. doi: 10.1016/j.jmb.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Pitcher RS, Brissett NC, Doherty AJ. Nonhomologous end-joining in bacteria: a microbial perspective. Annu Rev Microbiol. 2007a;61:259–282. doi: 10.1146/annurev.micro.61.080706.093354. [DOI] [PubMed] [Google Scholar]
- Pitcher RS, Brissett NC, Picher AJ, Andrade P, Juarez R, Thompson D, et al. Structure and function of a mycobacterial NHEJ DNA repair polymerase. J Mol Biol. 2007b;366:391–405. doi: 10.1016/j.jmb.2006.10.046. [DOI] [PubMed] [Google Scholar]
- Pitcher RS, Green AJ, Brzostek A, Korycka-Machala M, Dziadek J, Doherty AJ. NHEJ protects mycobacteria in stationary phase against the harmful effects of desiccation. DNA Repair (Amst) 2007c;6:1271–1276. doi: 10.1016/j.dnarep.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Prell J, Poole P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 2006;14:161–168. doi: 10.1016/j.tim.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Quandt J, Hynes MF. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- Renzette N, Gumlaw N, Nordman JT, Krieger M, Yeh SP, Long E, et al. Localization of RecA in Escherichia coli K-12 using RecA-GFP. Mol Microbiol. 2005;57:1074–1085. doi: 10.1111/j.1365-2958.2005.04755.x. [DOI] [PubMed] [Google Scholar]
- Riera J, Fernandez de Henestrosa AR, Garriga X, Tapias A, Barbe J. Interspecies regulation of recA gene of gram-negative bacteria lacking an E. coli like SOS operator. Mol Gen Genet. 1994;245:523–527. doi: 10.1007/BF00302266. [DOI] [PubMed] [Google Scholar]
- Rocha EP, Cornet E, Michel B. Comparative and evolutionary analysis of the bacterial homologous recombination systems. PLoS Genet. 2005;1:e15. doi: 10.1371/journal.pgen.0010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbour, NY: Cold Spring Harbour University Press; 1989. [Google Scholar]
- Simmons LA, Grossman AD, Walker GC. Replication is required for the RecA localization response to DNA damage in Bacillus subtilis. Proc Natl Acad Sci USA. 2007;104:1360–1365. doi: 10.1073/pnas.0607123104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BT, Grossman AD, Walker GC. Visualization of mismatch repair in bacterial cells. Mol Cell. 2001;8:1197–1206. doi: 10.1016/s1097-2765(01)00402-6. [DOI] [PubMed] [Google Scholar]
- Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJ. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- Stephanou NC, Gao F, Bongiorno P, Ehrt S, Schnap-pinger D, Shuman S, Glickman MS. Mycobacterial nonhomologous end joining mediate mutagenic repair of chromosomal repair of chromosomal double-strand DNA breaks. J Bacteriol. 2007;189:5237–5246. doi: 10.1128/JB.00332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias A, Barbé J. Regulation of divergent transcription from the uvrA-ssb promoters in Sinorhizobium meliloti. Mol Gen Genet. 1999;262:121–130. doi: 10.1007/s004380051066. [DOI] [PubMed] [Google Scholar]
- de Vries E, van Driel W, Bergsma WG, Arnberg AC, van der Vliet PC. HeLa nuclear protein recognizing DNA termini and translocating on DNA forming a regular DNA-multimeric protein complex. J Mol Biol. 1989;208:65–78. doi: 10.1016/0022-2836(89)90088-0. [DOI] [PubMed] [Google Scholar]
- Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- Wang ST, Setlow B, Conlon EM, Lyon JL, Imamura D, Sato T, et al. The forespore line of gene expression in Bacillus subtilis. J Mol Biol. 2006;358:16–37. doi: 10.1016/j.jmb.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Weller GR, Kysela B, Roy R, Tonkin LM, Scanlan E, Della M, et al. Identification of a DNA nonhomologous end-joining complex in bacteria. Science. 2002;297:1686–1689. doi: 10.1126/science.1074584. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Topper LM, Palmbos PL. Non-homologous end-joining: bacteria join the chromosome breakdance. Trends Biochem Sci. 2003;28:62–66. doi: 10.1016/S0968-0004(03)00005-7. [DOI] [PubMed] [Google Scholar]
- Yakovleva L, Shuman S. Nucleotide misincorporation, 3′-mismatch extension, and responses to abasic sites and DNA adducts by the polymerase component of bacterial DNA ligase D. J Biol Chem. 2006;281:25026–25040. doi: 10.1074/jbc.M603302200. [DOI] [PubMed] [Google Scholar]
- Yaneva M, Kowalewski T, Lieber MR. Interaction of DNA-dependent protein kinase with DNA and with Ku: biochemical and atomic-force microscopy studies. EMBO J. 1997;16:5098–5112. doi: 10.1093/emboj/16.16.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Shuman S. Novel 3′-ribonuclease and 3′-phosphatase activities of the bacterial non-homologous end-joining protein, DNA ligase D. J Biol Chem. 2005a;280:25973–25981. doi: 10.1074/jbc.M504002200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shuman S. A primer-dependent polymerase function of pseudomonas aeruginosa ATP-dependent DNA ligase (LigD) J Biol Chem. 2005b;280:418–427. doi: 10.1074/jbc.M410110200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shuman S. Substrate specificity and structure-function analysis of the 3′-phosphoesterase component of the bacterial NHEJ protein, DNA ligase D. J Biol Chem. 2006;281:13873–13881. doi: 10.1074/jbc.M600055200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shuman S. Characterization of Agrobacterium tumefaciens DNA ligase C and D. Nucleic Acids Res. 2007;35:3631–3645. doi: 10.1093/nar/gkm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang LK, Shuman S. Essential constituents of the 3′-phosphoesterase domain of bacterial DNA ligase D, a nonhomologous end-joining enzyme. J Biol Chem. 2005;280:33707–33715. doi: 10.1074/jbc.M506838200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Nandakumar J, Aniukwu J, Wang LK, Glickman MS, Lima CD, Shuman S. Atomic structure and nonhomologous end-joining function of the polymerase component of bacterial DNA ligase D. Proc Natl Acad Sci USA. 2006;103:1711–1716. doi: 10.1073/pnas.0509083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.