Abstract
Purpose
To quantify the radiotherapy dose–response of prostate cancer, adjusted for prognostic factors in a mature cohort of men treated relatively uniformly at a single institution.
Patients and Methods
The study cohort consisted of 1,530 men treated with three-dimensional conformal external-beam radiotherapy between 1989 and 2002. Patients were divided into four isocenter dose groups: <70 Gy (n = 43), 70–74.9 Gy (n = 552), 75–79.9 Gy (n = 568), and ≥80 Gy (n = 367). The primary endpoints were freedom from biochemical failure (FFBF), defined by American Society for Therapeutic Radiology and Oncology (ASTRO) and Phoenix (nadir + 2.0 ng/mL) criteria, and freedom from distant metastases (FFDM). Multivariate analyses were performed and adjusted Kaplan-Meier estimates were calculated. Logit regression dose–response functions were determined at 5 and 8 years for FFBF and at 5 and 10 years for FFDM.
Results
Radiotherapy dose was significant in multivariate analyses for FFBF (ASTRO and Phoenix) and FFDM. Adjusted 5-year estimates of ASTRO FFBF for the four dose groups were 60%, 68%, 76%, and 84%. Adjusted 5-year Phoenix FFBFs for the four dose groups were 70%, 81%, 83%, and 89%. Adjusted 5-year and 10-year estimates of FFDM for the four dose groups were 96% and 93%, 97% and 93%, 99% and 95%, and 98% and 96%. Dose–response functions showed an increasing benefit for doses ≥80 Gy.
Conclusions
Doses of ≥80 Gy are recommended for most men with prostate cancer. The ASTRO definition of biochemical failure does not accurately estimate the effects of radiotherapy at 5 years because of backdating, compared to the Phoenix definition, which is less sensitive to follow-up and more reproducible over time.
Keywords: Prostate cancer, Radiotherapy, Dose
INTRODUCTION
Localized prostate cancer is framed by the bladder and rectum, which potentially limits the radiotherapy (RT) dose. However, RT doses >70 Gy have been delivered safely for over 15 years (1). With continued improvements in radiation delivery and prostate localization, doses of ≥80 Gy have been used with relatively low toxicity (2, 3). Four randomized, controlled studies confirmed the benefit of dose escalation in prostate cancer, but did not allow for extrapolation to higher doses, and were underpowered to account for variations in tumor factors (4–7). Retrospective single-institution and multicenter reviews estimated improvements in freedom from biochemical failure (FFBF) with doses >80 Gy (8, 9). Maximizing local control through the delivery of higher RT doses should translate into reductions in distant metastasis and mortality (10). The characterization of FFBF and freedom from distant metastasis (FFDM) dose–response relationships requires mature follow-up and, in the era of dose escalation, this is now only beginning to become possible. Here, we investigate data from one of the longest single-institution dose-escalation experiences, to construct for the first time adjusted FFBF and freedom from distant metastasis (FFDM) dose–response relationships.
PATIENTS AND METHODS
This report includes 1,530 consecutive patients treated for localized prostate cancer from 1989 to 2002 with three-dimensional conformal radiotherapy (3DCRT) at Fox Chase Cancer Center (Philadelphia, PA). All patients had an initial pretreatment prostate-specific antigen (iPSA). Patients treated with androgen deprivation as part of their initial management were excluded. Dose is reported according to the International Commission on Radiation Units and Measurement (ICRU) (11), with the planned treatment volume (PTV) receiving at least 95% of the prescription dose. Patients were immobilized supine in an alpha cradle cast for CT simulation and treatment.
The technique has been previously described (12). In brief, the clinical target volume (CTV) was defined as the prostate alone for favorable-risk patients (PSA, <10.0 ng/mL; 2002 American Joint Committee on Cancer [AJCC] clinical stage, T1–T2C; Gleason score [GS], 2–6). Clinical T-stage was based on digital rectal examination (DRE) only. For intermediate-risk patients (PSA, 10.0–20.0 ng/mL, or GS 7 in the absence of high-risk features) and high-risk patients (PSA, >20.0 ng/mL; stage T3–T4; or GS, 8–10), CTV1 was defined as the prostate and seminal vesicles, which were treated with the prescription dose, and CTV2 was the periprostatic lymph nodes, which were treated with 46–50 Gy. A 1-cm expansion was made around the CTV to create the PTV, with an additional 5 mm to account for penumbra at the block edge. Between 1989 and 1999, the clinical setup was verified weekly, using megavoltage port film imaging. From 1999, B-mode acquisition and targeting ultrasound alignment (NOMOS, Cranberry Township, PA) were used in addition to the weekly port film imaging.
Follow-up, which included a history, DRE, and PSA, was performed initially at 3 months and then at 6–12-month intervals thereafter. Radiologic investigation was performed to exclude metastatic disease, as indicated by clinical assessment or rising PSA profile. Outcome measures included PSA failure (BF), initiation of androgen deprivation, distant metastases (DM), and overall mortality (OM). The American Society for Therapeutic Radiology and Oncology (ASTRO) (13) and the Phoenix (nadir + 2.0 ng/mL) (14) definitions were used to define PSA failure.
Four isocenter dose groups were defined: <70 Gy, 70–74.9 Gy, 75–79.9 Gy, and ≥80 Gy. Timing of androgen deprivation was recorded in relationship to BF and the development of DM. Estimates of BF, DM, and OM were calculated using the Kaplan-Meier product limit method. Multivariate Cox proportional hazards regression modeling was used to estimate the outcomes for each dose group. Outcomes were adjusted for iPSA, T-stage, and GSRT. Dose and iPSA were analyzed as continuous variables. Logit regression curves were calculated, using 5- and 8-year FFBF and 5-year and 10-year FFDM from multivariate, adjusted outcome estimates and the median dose points for the four dose groups. For a specified dose, the probability of a response was modeled as p = 1/[eb0 + b1 *dose + 1]. The maximum-likelihood estimates of the parameters b0 and b1 were computed using the modified Newton-Raphson algorithm (15). Probabilities were estimated using the logistic cumulative distribution function, F. Analyses were performed with SAS software, version 9.0 (SAS Institute, Inc., Cary, NC).
RESULTS
Of 1,530 patients in this analysis, 43 were treated with <70 Gy (median follow-up of 85.9 months), 552 patients with 70–74.9 Gy (median follow-up of 67.8 months), 568 patients with 75–79.9 Gy (median follow-up of 54.6 months), and 367 with ≥80 Gy (median follow-up of 45.6 months). The AJCC clinical stage was T1–T2 in 96%, and the Gleason scores were 2–6, 7, or 8–10 in 72%, 24%, and 4% respectively. The iPSA levels were <10 ng/mL in 62%, 10–20 ng/mL in 27%, and >20 ng/mL in 11% of patients. Patients were classified as low-risk in 45%, intermediate-risk in 39%, or high-risk in 16% of cases.
Table 1 shows the distribution of patients by age, T-stage, Gleason score, iPSA, and risk group for the four dose groups. The median age was 73 years for the low-dose cohort, and 68–69 years for the three higher dose cohorts. Median follow-up ranged from 46 to 86 months, reflecting longer follow-up for patients treated with lower doses. The highest dose group was biased toward higher Gleason scores, with 51% of the cohort with a GS of 7, compared to 18% and 15% in the 70–74.9-Gy and 75–79.9 Gy cohorts, respectively. Clinical stage and iPSA were similar between the four dose groups. This difference in GS was also reflected in an increase in intermediate-risk patients in the highest dose group (67%).
Table 1.
Patient characteristics
| Dose group |
|||||
|---|---|---|---|---|---|
| <70 Gy (n = 43) | 70–74.9 Gy (n = 552) | 75–79.9 Gy (n = 568) | ≥80 Gy (n = 367) | All (n = 1,530) | |
| Median age | 73 y | 69 y | 68 y | 69 y | 69 y |
| Median follow-up | 85.9 mo | 67.8 mo | 54.6 mo | 45.6 mo | 55.4 mo |
| Stage T1–T2 | 43 (100%) | 522 (95%) | 545 (96%) | 351 (96%) | 1,461 (96%) |
| Stage T3–T4 | 0 (0%) | 30 (5%) | 23 (4%) | 16 (4%) | 69 (4%) |
| GS 2–6 | 42 (98%) | 441 (80%) | 460 (81%) | 165 (45%) | 1,108 (72%) |
| GS 7 | 1 (2%) | 98 (18%) | 86 (15%) | 188 (51%) | 373 (24%) |
| GS 8–10 | 0 (0%) | 13 (2%) | 22 (4%) | 14 (4%) | 49 (4%) |
| iPSA <10 | 25 (58%) | 362 (66%) | 350 (62%) | 213 (58%) | 950 (62%) |
| iPSA 10–20 | 12 (28%) | 127 (23%) | 152 (27%) | 125 (34%) | 416 (27%) |
| iPSA >20 | 6 (14%) | 63 (11%) | 66 (11%) | 29 (8%) | 164 (11%) |
| Low-risk | 24 (56%) | 299 (54%) | 296 (52%) | 64 (17%) | 683 (45%) |
| Intermediate-risk | 13 (30%) | 164 (30%) | 174 (31%) | 247 (67%) | 598 (39%) |
| High-risk | 6 (14%) | 89 (16%) | 98 (17%) | 56 (16%) | 249 (16%) |
Abbreviations: PSA = prostatic-specific antigen; GS = Gleason score.
Salvage androgen deprivation was initiated in 151 of 338 patients with an ASTRO biochemical failure. There were 61 patients with distant metastases. Androgen deprivation was not started before the diagnosis of distant disease in 39 patients (64%). The numbers of patients treated with androgen deprivation before distant metastases, compared to those who did not receive androgen deprivation before distant metastases, were 0:1, 9:24, 11:7, and 2:7 for the <70-Gy, 70–74.9-Gy, 75–79.9-Gy, and ≥80-Gy dose groups, respectively.
Multivariate analyses of biochemical failure are shown in Table 2. For the ASTRO definition, increasing dose (relative risk [RR] = 0.92, continuous variable), T-stage (RR = 2.27, dichotomous variable), Gleason score (RR = 2.07, dichotomous variable), and increasing iPSA (RR = 1.02, continuous variable) were significant independent predictors of BF. For the Phoenix definition, dose (RR = 0.94), T-stage (RR = 2.37), Gleason score (RR = 2.07), and iPSA (RR = 1.02) were significant independent predictors of BF. When the median dose in each cohort was used to define categorical variables, dose remained a significant predictor of FFBF with both the ASTRO and Phoenix definitions.
Table 2.
Multivariate analyses of biochemical failure
| ASTRO BF |
Nadir + 2 BF |
||||
|---|---|---|---|---|---|
| Variable | Type | RR (95%CI) | p value | RR (95%CI) | p value |
| Dose | Continuous | 0.92 (0.89–0.94) | <0.0001 | 0.94 (0.91–0.97) | 0.0002 |
| T3–T4 | Dichotomous | 2.27 (1.58–3.26) | <0.0001 | 2.37 (1.85–3.03) | 0.0003 |
| GS 7–10 | Dichotomous | 2.07 (1.63–2.61) | <0.0001 | 2.07 (1.63–2.61) | <0.0001 |
| iPSA | Continuous | 1.02 (1.02–1.03) | <0.0001 | 1.02 (1.02–1.02) | <0.0001 |
Abreviations: BF = biochemical failure; RR = relative risk, with 95% confidence interval (CI) in parentheses.
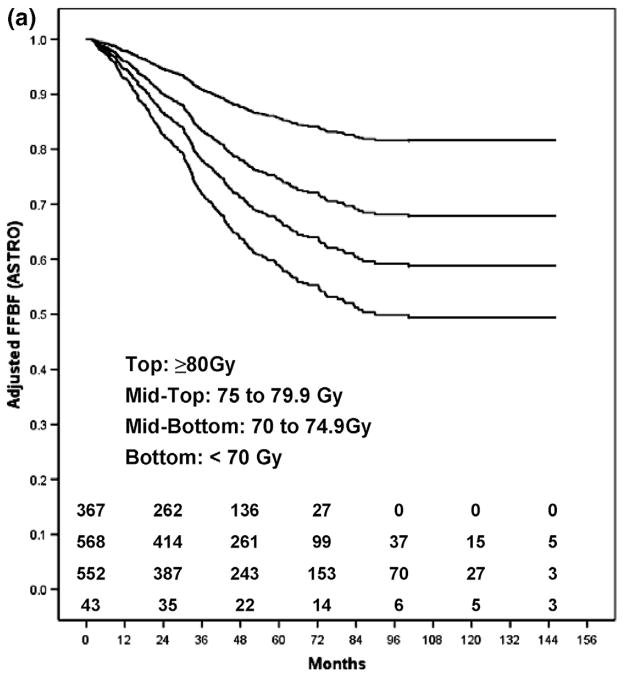
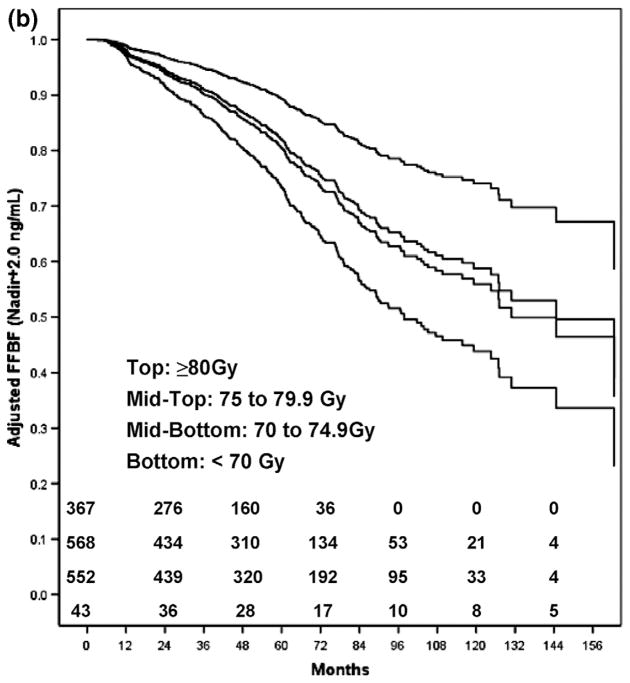
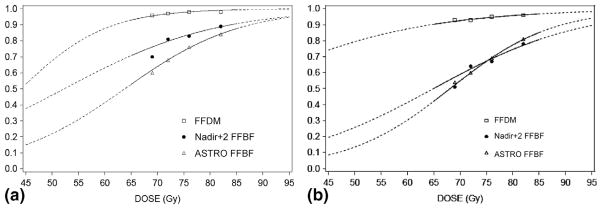
Table 3 shows FFBF and FFDM rates by dose group, adjusted for T-stage, GS, and iPSA. The adjusted ASTRO FFBF rates were lower than the Phoenix FFBF rates at 5 years, and higher at 8 years. Figure 1 shows the adjusted Kaplan-Meier estimates of FFBF for the ASTRO definition (Fig. 1a) and the Phoenix definition (Fig. 1b) for each dose group. A comparison of adjusted curves reveals that the ASTRO definition has a steeper initial slope, and plateaus at approximately 7 years, compared to the Phoenix definition. This reflects the effect of backdating failures in the ASTRO definition. By the Phoenix definition, there is a higher FFBF rate initially, and a steady rate of decline over time, without a noticeable plateau. These patterns are reflected in the dose–response functions for FFBF at 5 and 8 years shown in Figs. 2a and b.
Table 3.
Patient outcomes adjusted for T-stage, iPSA, and GS
| Dose group |
||||||||
|---|---|---|---|---|---|---|---|---|
| <70 Gy (n = 43) | 70–74.9 Gy (n = 552) | 75–79.9 Gy (n = 568) | ≥80 Gy (n = 367) | |||||
| ASTRO adjusted | 5-year | 8-year | 5-year | 8-year | 5-year | 8-year | 5-year | 8-year |
| FFBF | 60% | 54% | 68% | 60% | 76% | 69% | 84% | 81%* |
| No. at risk | 17 | 5 | 208 | 69 | 155 | 37 | 72 | NA |
| Nadir + 2 adjusted | 5-year | 8-year | 5-year | 8-year | 5-year | 8-year | 5-year | 8-year |
| FFBF | 70% | 51% | 81% | 64% | 83% | 67% | 89% | 78%* |
| No. at risk | 25 | 9 | 264 | 94 | 205 | 50 | 97 | NA |
| Adjusted | 5-year | 10-year | 5-year | 10-year | 5-year | 10-year | 5-year | 10-year |
| FFDM | 96% | 93% | 97% | 93% | 98% | 95% | 98% | 96%* |
| No. at risk | 32 | 16 | 413 | 72 | 319 | 47 | 119 | NA |
| Adjusted | 10-year | 10-year | 10-year | 10-year | ||||
| OM | 49% | 37% | 31% | 36% | ||||
| No. at risk | 16 | 78 | 48 | 9 | ||||
Abreviations: ASTRO = American Society for Therapeutic Radiology and Oncology; FFBF = freedom from biochemical failure; FFDM = freedom from distant metastases; NA = not available; OM = overall mortality.
Estimated.
Fig. 1.
(a) The ASTRO FFBF adjusted Kaplan-Meier estimates for each dose group.
(b) The nadir +2 FFBF adjusted Kaplan-Meier estimates for each dose group. ASTRO = American Society for Therapeutic Radiology and Oncology; FFBF = freedom from biochemical failure.
Fig. 2.
(a) Adjusted dose–response functions at 5 years for FFBF and FFDM. (b) Adjusted dose–response functions at 8 years for FFBF and 10 years for FFDM. FFBF = freedom from biochemical failure; FFDM = freedom from distant metastases.
Table 4 displays the results of multivariate analysis using FFDM as the endpoint. Dose (RR = 0.92, p = 0.0224), T-stage (RR = 2.42, p = 0.0152), GS (RR 4.41, p < 0.0001), and iPSA (RR = 1.02, p < 0.0001) were independent predictors of DM. For median doses of 69 Gy, 72 Gy, 76 Gy, and 82 Gy, the adjusted 5- and 10-year FFDM estimates were 96% and 93%, 97% and 93%, 98% and 95%, and 98% and 96%, respectively. Figure 2a and Fig. 2b display the 5- and 10-year dose–response function for FFDM. The number of events remains low, even at 10 years, but the relationship of FFDM to dose is nonetheless robust.
Table 4.
Multivariate analysis of distant metastasis
| Variable | Type | DM RR (95% CI) | p value |
|---|---|---|---|
| Dose | Continuous | 0.92 (0.85–0.99) | 0.0224 |
| T3–T4 | Dichotomous | 2.42 (1.19–4.94) | 0.0152 |
| GS 7–10 | Dichotomous | 4.41 (2.62–7.42) | <0.0001 |
| iPSA | Continuous | 1.02 (1.01–1.03) | <0.0001 |
Abreviations: DM = distant metastasis; RR = relative risk, with 95% confidence interval (CI) in parentheses.
The effect of higher RT doses on improved FFDM appeared to translate into a survival advantage. Overall mortality, when adjusted for age, T-stage, iPSA, and GS at 10 years, was 49%, 37%, 31%, and 26% for the <70-Gy, 70–74.9-Gy, 75–79.9-Gy, and ≥80-Gy dose groups, respectively (p = 0.0023).
DISCUSSION
The ASTRO definition of biochemical failure is suboptimal for estimating dose–response, primarily because of the effects of backdating. Because of backdating, there is an inaccurate registration of failures early after RT, a flattening of Kaplan-Meier curves, and an underestimation of biochemical failure when follow-up is brief. In contrast, BF by the Phoenix definition registers failures at the times they occur during the follow-up period, does not result in an artificial flattening of Kaplan-Meier curves after definitive RT, and is much less influenced by length of follow-up (16–19). The results in Fig. 1 and Fig. 2 are illustrative of this effect. As a consequence, the ASTRO FFBF RT dose–response estimates were lower at 5 years than at 8 years, relative to the Phoenix estimates (Fig. 2).
Although both definitions show improved FFBF with increasing dose, the dose–response at 5 years using the ASTRO definition appears to overestimate the effect of dose. Prior studies showed that at approximately 7 years, FFBF estimates by the Phoenix definition cross and become lower than those using the ASTRO definition (16, 18, 20). This explains why the dose–response results at 8 years using the ASTRO definition approximate those with the Phoenix definition. Over time, the FFBF estimates of dose–response using the ASTRO definition are expected to deviate from those using the Phoenix definition, because failures will be falsely ascribed to an earlier time.
Cheung et al. (21, 22) also found a descrepancy in the dose–repsonse results using the Phoenix and ASTRO definitions. They compared the unadjusted FFBF in low-, intermediate-, and high-risk patients for doses between 66 Gy and 78 Gy. Comparisons at 3 and 5 years showed an overestimation of BF using the ASTRO definition by risk group.
Dose escalation improves outcome, as reported in large retrospective studies and four prospective, randomized, controlled trials (2–7, 21–25). Hanks et al. (9) plotted dose–response curves for 618 patients, and showed a benefit of between 22% and 40% with an increase of dose to >80 Gy. Although intermediate-risk patients appeared to have the greatest gain in FFBF with higher doses, low- and high-risk patients also experienced a benefit (21–26). Comparison of dose escalation in subgroups based on iPSA and GS may be too simplistic, due to the differential dose–response within these subgroups, as shown by Thames et al. (27). Adjusted dose–response curves account for these differences. Moreover, when the Phoenix definition is used, the results are less influenced by length of follow-up (a potential bias of the higher dose cohort). Another potential bias of dose–response analyses relates to unaccounted for factors that change over time. We did not include year of treatment as a covariate in this analysis because, as we showed previously, year of treatment is strongly related to radiotherapy dose (10), and inclusion of year of treatment would therefore mask the full impact of dose on outcome.
The adjusted BF curves in our study imply that current standard doses are still insufficient to obtain local disease eradication in many patients. Biochemical failure is the result of micrometastatic disease before RT and/or local persistence after RT. Because there is no evidence of a leveling-off of FFBF curves using the Phoenix definition, local persistence likely remains a problem. Pathologically confirmed responses are in agreement with these observations. With doses between 70–78 Gy, biopsies >2 years after treatment were positive in 23%–51% of patients (4, 28–30). A single-institution, dose-escalation study from the Memorial Sloane-Kettering Cancer Center (New York, NY) included biopsy data in 252 patients at a minimum of 2.5 years following radiotherapy (30). Positive biopsies were reported in 54%, 34%, 23%, and 10% of patients treated with 64.8 Gy, 70.2 Gy, 75.6 Gy, and 81 Gy, respectively. Although these investigators classified cases with residual tumor cells showing severe radiation effects as negative, which is not entirely accurate, the pathologic dose–response correlates with BF results.
The dose–response curves produced from our cohort of 1,530 patients included 367 patients treated with ≥80 Gy (median dose, 82 Gy), and 568 patients treated with 75–79.9 Gy (median dose, 76 Gy). At these high doses, there was an estimated improvement in FFBF from 67% to 78% at 8 years, for a relative gain of >16%. Using the 8-year dose–response estimates, for every added 1 Gy in this range, there was a 2.2% gain in long-term FFBF. These data confirm that local persistence is an important mechanism of failure, and that higher doses are paramount in eradicating the disease.
Biochemical failure after definitive treatment for prostate cancer is a predictor of subsequent metastatic disease (31–33). A decrease in BF secondary to dose escalation should translate into a reduction in distant spread (10). Our results more precisely define this relationship, showing that RT dose causes an 8% reduction in the risk of distant metastases for each 1 Gy delivered. We anticipate that as our median follow-up increases, the benefit of dose escalation will strengthen, because higher initial doses will proportionally increase local control and prevent the late wave of distant metastasis due to persistent local disease (34, 35). Follow-up >10 years is required to fully characterize the RT dose–response for DM. Although confounding factors, such as GS shift (36), changes in RT delivery and target-localization techniques, length of follow-up, and timing of salvage androgen deprivation, could alter FFDM curves, the results are notably consistent.
The effect of androgen deprivation on dose escalation is not answered in this analysis; our cohort of patients was hormone-naive until biochemical or distant failure. It is currently unknown whether the addition of androgen deprivation will substitute for higher doses or improve outcomes beyond what is achievable with radiotherapy alone. Androgen deprivation may enhance local tumor eradication through at least additive cell-killing with RT (37–39). There could be other mechanisms involved (distant effects) with long-term androgen-deprivation therapy. We continue to use long-term androgen deprivation for ≥2 years with dose-escalated RT for men with high-risk disease. Short-term androgen-deprivation therapy for men with intermediate-risk disease who are treated with ≥80 Gy is debatable (40, 41).
In conclusion, a pronounced RT dose–response by FFBF was identified after adjusting for iPSA, GS, and T-stage, suggesting that the vast majority of patients should receive ≥80 Gy. There may be a subgroup of favorable patients who are adequately treated with <80 Gy, although the results from randomized dose-escalation trials are discordant on this point. Using image guidance and intensity-modulated radiation therapy, doses ≥80 Gy are now deliverable with minimal toxicity, and therefore might be appropriate for all patients. The Phoenix definition of BF more accurately described changes over the follow-up period. A dose–response for DM was also seen, confirming that higher RT doses reduce tumor persistence and subsequent distant spread.
Acknowledgments
This study was supported in part by Grants CA-006927 and CA101984-01 from the National Cancer Institute, and by a grant from Varian Medical Systems.
Footnotes
The contents of this article are solely the responsibility of the authors, and do not necessarily represent the official views of the National Cancer Institute.
Conflict of interest: none.
References
- 1.Hanks GE, Hanlon AL, Epstein B, et al. Dose response in prostate cancer with 8–12 years’ follow-up. Int J Radiat Oncol Biol Phys. 2002;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 3.Pollack A, Hanlon AL, Horwitz EM, et al. Prostate cancer radiotherapy dose response: an update of the Fox Chase experience. J Urol. 2004;171:1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 4.Shipley WU, Verhey LJ, Munzenrider JE, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;32:3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 5.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 6.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs. high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. J Am Med Assoc. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 7.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 8.Fowler JF, Ritter MA, Chappell RJ, et al. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–1104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 9.Hanks GE, Hanlon AL, Pinover WH, et al. Dose selection for prostate cancer patients based on dose comparison and dose response studies. Int J Radiat Oncol Biol Phys. 2000;46:823–832. doi: 10.1016/s0360-3016(99)00498-8. [DOI] [PubMed] [Google Scholar]
- 10.Jacob R, Hanlon AL, Horwitz EM, et al. The relationship of increasing radiotherapy dose to reduced distant metastases and mortality in men with prostate cancer. Cancer. 2004;100:538–543. doi: 10.1002/cncr.11927. [DOI] [PubMed] [Google Scholar]
- 11.International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy. Washington, DC: International Commission on Radiation Units and Measurements; 1993. ICRU report no. 50. [Google Scholar]
- 12.Horwitz EM, Hanlon AL, Pinover WH, et al. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001;92:1281–1287. doi: 10.1002/1097-0142(20010901)92:5<1281::aid-cncr1449>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Cox J, Grignon D, Kaplan R, et al. Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 14.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Dennis JE, Schnabel RB. Numerical methods for unconstrained optimization and nonlinear equations. Englewood Cliffs, NJ: Prentice-Hall; 1996. [Google Scholar]
- 16.Pickles T, Kim-Sing C, Morris WJ, et al. Evaluation of the Houston biochemical relapse definition in men treated with prolonged neoadjuvant and adjuvant androgen ablation and assessment of follow-up lead-time bias. Int J Radiat Oncol Biol Phys. 2003;57:11–18. doi: 10.1016/s0360-3016(03)00439-5. [DOI] [PubMed] [Google Scholar]
- 17.Williams SG. Characterization of the behavior of three definitions of prostate-specific antigen-based biochemical failure in relation to detection and follow-up biases: comparison with the American Society for Therapeutic Radiology and Oncology consensus definition. Int J Radiat Oncol Biol Phys. 2006;64:849–855. doi: 10.1016/j.ijrobp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Kuban D, Thames H, Levy L, et al. Failure definition-dependent differences in outcome following radiation for localized prostate cancer: can one size fit all? Int J Radiat Oncol Biol Phys. 2005;61:409–414. doi: 10.1016/j.ijrobp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Buyyounouski MK, Hanlon AL, Eisenberg DF, et al. Defining biochemical failure after radiotherapy with and without androgen deprivation for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1455–1462. doi: 10.1016/j.ijrobp.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 20.Buyyounouski MK, Hanlon AL, Horwitz EM, et al. Biochemical failure and the temporal kinetics of prostate-specific antigen after radiation therapy with androgen deprivation. Int J Radiat Oncol Biol Phys. 2005;61:1291–1298. doi: 10.1016/j.ijrobp.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Cheung R, Tucker SL, Dong L, et al. Dose-response for biochemical control among high-risk prostate cancer patients after external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:1234–1240. doi: 10.1016/s0360-3016(03)00278-5. [DOI] [PubMed] [Google Scholar]
- 22.Cheung R, Tucker SL, Lee AK, et al. Dose-response characteristics of low- and intermediate-risk prostate cancer treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:993–1002. doi: 10.1016/j.ijrobp.2004.07.723. [DOI] [PubMed] [Google Scholar]
- 23.Kupelian PA, Kuban D, Thames H, et al. Improved biochemical relapse-free survival with increased external radiation doses in patients with localized prostate cancer: the combined experience of nine institutions in patients treated in 1994 and 1995. Int J Radiat Oncol Biol Phys. 2005;61:415–419. doi: 10.1016/j.ijrobp.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Kupelian PA, Buchsbaum JC, Reddy CA, et al. Radiation dose response in patients with favorable localized prostate cancer (Stage T1–T2, biopsy Gleason < or = 6, and pretreatment prostate-specific antigen < or = 10) Int J Radiat Oncol Biol Phys. 2001;50:621–625. doi: 10.1016/s0360-3016(01)01466-3. [DOI] [PubMed] [Google Scholar]
- 25.Kupelian PA, Mohan DS, Lyons J, et al. Higher than standard radiation doses (> or = 72 Gy) with or without androgen deprivation in the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2000;46:567–574. doi: 10.1016/s0360-3016(99)00455-1. [DOI] [PubMed] [Google Scholar]
- 26.Zietman AL, DeSilvio M, Slater JD, et al. A randomized trial comparing conventional dose (70.2GyE) and high-dose (79.2GyE) conformal radiation in early stage adenocarcinoma of the prostate: results of an interim analysis of PROG 95–09. Int J Radiat Oncol Biol Phys. 2004;60(Suppl):S131–S132. [Google Scholar]
- 27.Thames HD, Kuban DA, DeSilvio ML, et al. Increasing external beam dose for T1–T2 prostate cancer: effect on risk groups. Int J Radiat Oncol Biol Phys. 2006;65:975–981. doi: 10.1016/j.ijrobp.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Nichol A, Chung P, Lockwood G, et al. A phase II study of localized prostate cancer treated to 75.6 Gy with 3D conformal radiotherapy. Radiother Oncol. 2005;76:11–17. doi: 10.1016/j.radonc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Pollack A, Zagars GK, Antolak JA, et al. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002;54:677–685. doi: 10.1016/s0360-3016(02)02977-2. [DOI] [PubMed] [Google Scholar]
- 30.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 31.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. J Am Med Assoc. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 32.Zagars GK, Pollack A. Kinetics of serum prostate-specific antigen after external beam radiation for clinically localized prostate cancer. Radiother Oncol. 1997;44:213–221. doi: 10.1016/s0167-8140(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 33.Pollack A, Hanlon AL, Movsas B, et al. Biochemical failure as a determinant of distant metastasis and death in prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:19–23. doi: 10.1016/s0360-3016(03)00538-8. [DOI] [PubMed] [Google Scholar]
- 34.Coen JJ, Zietman AL, Thakral H, et al. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 35.Morgan PB, Hanlon AL, Horwitz EM, et al. Radiotherapy dose and late failures in prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1074–1081. doi: 10.1016/j.ijrobp.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chism DB, Hanlon AL, Troncoso P, et al. The Gleason score shift: score four and seven years ago. Int J Radiat Oncol Biol Phys. 2003;56:1241–1247. doi: 10.1016/s0360-3016(03)00268-2. [DOI] [PubMed] [Google Scholar]
- 37.Zietman AL, Nakfoor BM, Prince EA, et al. The effect of androgen deprivation and radiation therapy on an androgen-sensitive murine tumor: an in vitro and in vivo study. Cancer J Sci Am. 1997;3:31–36. [PubMed] [Google Scholar]
- 38.Joon DL, Hasegawa M, Sikes C, et al. Supraadditive apoptotic response of R3327-G rat prostate tumors to androgen ablation and radiation. Int J Radiat Oncol Biol Phys. 1997;38:1071–1077. doi: 10.1016/s0360-3016(97)00303-9. [DOI] [PubMed] [Google Scholar]
- 39.Pollack A, Salem N, Ashoori F, et al. Lack of prostate cancer radiosensitization by androgen deprivation. Int J Radiat Oncol Biol Phys. 2001;51:1002–1007. doi: 10.1016/s0360-3016(01)01750-3. [DOI] [PubMed] [Google Scholar]
- 40.D’Amico AV, Manola J, Loffredo M, et al. Six-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. J Am Med Assoc. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 41.Martinez AA, Demanes DJ, Galalae R, et al. Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regime. Int J Radiat Oncol Biol Phys. 2005;62:1322–1331. doi: 10.1016/j.ijrobp.2004.12.053. [DOI] [PubMed] [Google Scholar]