Abstract
Fluorescence lifetime (FLT) properties of organic molecules provide a new reporting strategy for molecular imaging in the near infrared (NIR) spectral region. Unfortunately, most of the NIR fluorescent dyes have short FLT typically clustered below 1.5 ns. In this study, we demonstrate that a new class of NIR fluorescent dyes, pyrrolopyrrole cyanine dyes, have exceptionally long FLTs ranging from 3 to 4 ns, both in vitro (dimethyl sulfoxide and albumin/water solutions) and in vivo (mice). These results provide a new window for imaging molecular processes, rejecting backscattered light and autofluorescence, and multiplexing imaging information with conventional NIR fluorescent dyes that absorb and emit light at similar wavelengths.
A variety of near-infrared (NIR) fluorescent molecular probes have been developed for in vivo imaging applications, mostly driven by the deep penetration of NIR light in tissue (1,2). Since optical imaging is often based on fluorescence intensity, requirements for ideal NIR fluorescent dyes include high molar absorptivity, photostability, and fluorescence quantum yield to improve image quality. However, the dependence of fluorescence intensity on many intrinsic and extrinsic variables presents a serious challenge in data analysis from in vivo studies. To overcome these problems, advanced optical imaging systems and image reconstruction algorithms have been developed to provide quantitative fluorescence intensity maps of dye distribution in small animals (3,4). In contrast, the fluorescence lifetimes (FLT) of organic dyes are less dependent on dye concentration and can be sensitive to the dye's microenvironment. In addition, FLT is much less affected by concentration artifacts and photobleaching (5), light scattering (6,7), excitation intensity, or sample turbidity (8,9). These features have motivated the use of FLT as a complementary in vivo optical imaging method (10–13). In combination with intensity maps, changes in FLT can reliably report molecular events in a specific region of interest.
Beyond improving the tissue penetration depth of light, imaging in the NIR region is particularly favorable for FLT measurements. In the visible wavelengths between 500 and 650 nm, autofluorescence FLTs ranges from 1–10 ns (14,15). Consequently, in vivo molecular imaging would require that the lifetime of exogenous probes must be significantly higher than that of autofluorescence (16,17). Since autofluorescence is negligible between 750 and 850 nm, molecular probes with diverse FLTs are suitable for NIR FLT imaging, depending on instrument capability and FLT resolution. While the NIR window facilitates the use of NIR fluorescent dyes with a wide FLT range, virtually all the NIR fluorescent organic dyes currently used for molecular imaging exhibit FLTs of <1.5 ns (18), with very few exceptions such as phthalocyanines (19), thereby confining the range of imaging applications with these probes.
A major obstacle to developing NIR dyes with long FLTs has been attributed to the energy gap law (21,22), which states that radiationless transitions at longer wavelengths increase due to vibrational overlaps between the ground and excited states, thus causing a decrease in quantum yield and fluorescence lifetime (23). Recently, the energy gap law was challenged by new generations of fluorescent dyes based on the pyrrolopyrrole cyanine (PPCy) skeleton (24,25). The new dyes exhibit high quantum yields (>0.50) in the NIR range, rendering them suitable for in vivo imaging. Herein, we report that these dyes have long FLTs, which expand the FLT window for imaging molecular processes. We also demonstrate what to our knowledge are the first applications of these dyes for in vivo imaging.
PPCy compounds are interesting for in vivo use because similar diketopyrrolopyrroles are generally nontoxic by either the oral route (LD50 > 5000 mg/kg) or by skin contact (LD50 > 2000 mg/kg), are not irritating to either the skin or eyes, are not mutagenic (26), and have been approved by the FDA for food contact. Particularly, the NIR PPCy dyes 4a and 4a′ (Fig. 1) were selected for this study because we anticipated that they would have similar toxicity profiles as basic PPCy dyes and their compact structure produced promising steady-state optical properties such as high molar absorptivities, quantum yields and photostability (24,25). For comparative purpose, we used indocyanine green (ICG) dye as a reference dye for this study since it is widely used in molecular imaging and its steady state and lifetime properties in vitro and in vivo are well established (18). Optical spectra of PPCy dyes and ICG in dimethyl sulfoxide (DMSO) are shown in Fig. 2.
Figure 1.
Structures of NIR PPCy dyes 4a and 4a′ and heptamethine cyanine dye ICG used in this work.
Figure 2.
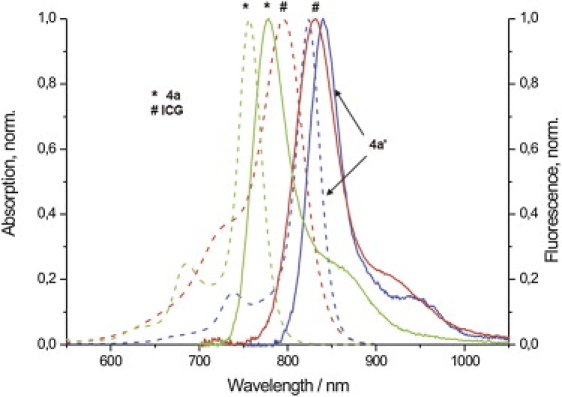
Steady-state normalized absorption (dashed line) and emission (solid line) spectra of 4a (∗), 4a′, and ICG (#) in DMSO (see footnote (20)). Absorption/emission maxima: 4a: 757 nm/779 nm; 4a′: 824 nm/840 nm; and ICG: 794 nm/831 nm. Fluorescence emission was excited at 690 nm.
In accordance with our primary interest in identifying NIR dyes with long FLTs, we first evaluated the FLT properties of both PPCy dyes in solution. Fig. 3 shows representative fluorescence decays for 4a and ICG. The compounds exhibited unusually long FLTs that ranged from 3–4 ns in vitro and 2.5–3.8 ns in vivo for 4a and 4a′. These FLTs were 3–4 times longer than that of ICG (Table 1) in either DMSO or albumin/water solution. The FLTs of organic compounds in these media have been shown to simulate their values in tissues in vivo (18). Although small organic NIR fluorescent molecules with such long FLTs have not been reported, this finding was hardly surprising. The extremely rigid structures of 4a and 4a′ achieved by the difluoro- or diphenylboron “clamp” ensured high quantum yields and subsequently long fluorescence lifetimes for both compounds.
Figure 3.
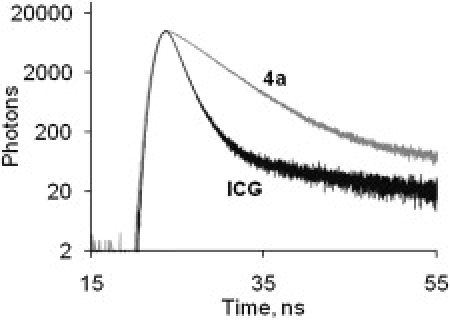
Decay curves in vitro: decays of 4a and ICG in DMSO. Ex/em. 773/830 nm.
Table 1.
Fluorescence lifetimes in vitro (Ex/em: 773/830 nm, 2-exponential fit)
| Entry | Solvent | Lifetime, ns | % | χ2 |
|---|---|---|---|---|
| 4a | DMSO | 4.02 | 97 | 1.21 |
| albumin/water | 3.89 | 92 | 1.80 | |
| 4a′ | DMSO | 3.35 | 97 | 1.55 |
| albumin/water | 2.95 | 96 | 1.32 | |
| ICG | DMSO | 1.11 | 96 | 1.15 |
| albumin/water | 0.80∗ | 98 | 1.22 |
Encouraged by the long FLTs of the NIR PPCy dyes in vitro, we determined the FLT map of these molecular probes as well as that of ICG in mice (28), as shown in Fig. 4. The fluorescent intensity maps (Fig. 4, top) showed that 4a′ displayed high levels of fluorescence that remained stable for several hours post injection (not shown). Our in vivo imaging system used a fixed 780 nm excitation wavelength, which is suboptimal for dye 4a, resulting in the observed low fluorescence intensity map for this compound. Fluorescence lifetimes values determined from the lifetime imaging experiments (Fig. 4, bottom) were in accordance with our measurements in vitro and ranged from 3.05 to 3.8 ns for 4a and 2.50 to 2.88 ns for 4a′. These average FLT values are several times longer than that of ICG (Table 2). However, the FLT images were generally more uniform than their intensity counterparts because they are less dependent on the dye concentration. The fixed excitation and detection in vivo system was suboptimal for 4a, resulting in the pronounced edge-effect artifacts (blue edges) seen in Fig. 4. Finally, we did not observe any adverse effects in mice several days post-injection of the dyes.
Figure 4.
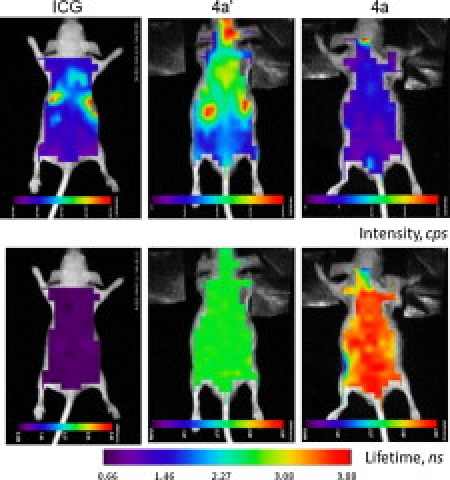
Intensity (top panel) and lifetime (bottom panel) maps of mice 5 min after injections of the studied NIR compounds. 4a and 4a′: 60 μM of each dye in a 15% DMSO and 2.5% Tween-80/water solution (see footnote (20)), ICG: 60 μM in 20% DMSO/water. Each mouse received 100 μL. Ex/em. 780/>830 nm.
Table 2.
Effective lifetime of studied compound in vivo
| Entry | Lifetime range, ns | Average lifetime, ns |
|---|---|---|
| 4a | 3.05–3.80 | 3.46 |
| 4a′ | 2.50–2.88 | 2.64 |
| ICG | 0.60–0.84 | 0.75 |
In summary, we demonstrated that NIR PPCy dyes exhibit remarkably long FLTs in vitro and in vivo. These findings will serve as the foundation for a number of studies capitalizing on FLT imaging. Specifically, we envisage utilizing the information to i), enhance contrast via elimination of the backscattered light, ii), perform functional time-gated imaging by designing the analogous environment-sensitive FLT probes, and iii), multiplex fluorescence imaging when two or more NIR fluorescent probes are used.
Acknowledgments
This study was funded in part by grants from the National Institutes of Health (R01 EB008111 and R01 CA109754).
References and Footnotes
- 1.Achilefu S., Dorshow R.B., Bugaj J.E., Rajagopalan R. Novel receptor-targeted fluorescent contrast agents for in vivo tumor imaging. Invest. Radiol. 2000;35:479–485. doi: 10.1097/00004424-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Mahmood U., Tung C.H., Bogdanov A., Weissleder R. Near-infrared optical imaging of protease activity for tumor detection. Radiology. 1999;213:866–870. doi: 10.1148/radiology.213.3.r99dc14866. [DOI] [PubMed] [Google Scholar]
- 3.Eppstein M.J., Hawrysz D.J., Godavarty A., Sevick-Muraca E.M. Three-dimensional, Bayesian image reconstruction from sparse and noisy data sets: near-infrared fluorescence tomography. Proc. Natl. Acad. Sci. USA. 2002;99:9619–9624. doi: 10.1073/pnas.112217899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milstein A.B., Oh S., Webb K.J., Bouman C.A., Zhang Q. Fluorescence optical diffusion tomography. Appl. Opt. 2003;42:3081–3094. doi: 10.1364/ao.42.003081. [DOI] [PubMed] [Google Scholar]
- 5.Lichtman J.W., Conchello J.A. Fluorescence microscopy. Nat. Methods. 2005;2:910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- 6.Kuwana E., Sevick-Muraca E.M. Fluorescence lifetime spectroscopy in multiply scattering media with dyes exhibiting multiexponential decay kinetics. Biophys. J. 2002;83:1165–1176. doi: 10.1016/S0006-3495(02)75240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerussi A.E., Maier J.S., Fantini S., Franceschini M.A., Mantulin W.W. Experimental verification of a theory for the time-resolved fluorescence spectroscopy of thick tissues. Appl. Opt. 1997;36:116–124. doi: 10.1364/ao.36.000116. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary M.A., Boas D.A., Li X.D., Chance B., Yodh A.G. Fluorescence lifetime imaging in turbid media. Opt. Lett. 1996;21:158–160. doi: 10.1364/ol.21.000158. [DOI] [PubMed] [Google Scholar]
- 9.Ntziachristos V., Weissleder R. Charge-coupled-device based scanner for tomography of fluorescent near-infrared probes in turbid media. Med. Phys. 2002;29:803–809. doi: 10.1118/1.1470209. [DOI] [PubMed] [Google Scholar]
- 10.Bloch S., Lesage F., McIntosh L., Gandjbakhche A., Liang K.X. Whole-body fluorescence lifetime imaging of a tumor-targeted near-infrared molecular probe in mice. J. Biomed. Opt. 2005;10:054003–054008. doi: 10.1117/1.2070148. [DOI] [PubMed] [Google Scholar]
- 11.Hassan M., Riley J., Chernomordik V., Smith P., Pursley R. Fluorescence lifetime imaging system for in vivo studies. Mol. Imaging. 2007;6:229–236. [PMC free article] [PubMed] [Google Scholar]
- 12.Almutairi A., Akers W.J., Berezin M.Y., Achilefu S., Frechet J.M.J. Monitoring the biodegradation of dendritic near-infrared nanoprobes by in vivo fluorescence imaging. Mol. Pharmaceutics. 2008;5:1103–1110. doi: 10.1021/mp8000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakabayashi T., Wang H.P., Kinjo M., Ohta N. Application of fluorescence lifetime imaging of enhanced green fluorescent protein to intracellular pH measurements. Photochem. Photobiol. Sci. 2008;7:668–670. doi: 10.1039/b800391b. [DOI] [PubMed] [Google Scholar]
- 14.Becker W., Bergmann A., Biskup C. Multispectral fluorescence lifetime imaging by TCSPC. Microsc. Res. Tech. 2007;70:403–409. doi: 10.1002/jemt.20432. [DOI] [PubMed] [Google Scholar]
- 15.Uehlinger P., Gabrecht T., Glanzmann T., Ballini J.P., Radu A. In vivo time-resolved spectroscopy of the human bronchial early cancer autofluorescence. J. Biomed. Opt. 2009;14:024011. doi: 10.1117/1.3088100. [DOI] [PubMed] [Google Scholar]
- 16.Wagnieres G.A., Star W.M., Wilson B.C. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem. Photobiol. 1998;68:603–632. [PubMed] [Google Scholar]
- 17.Marriott G., Clegg R.M., Arndt-Jovin D.J., Jovin T.M. Time resolved imaging microscopy. Phosphorescence and delayed fluorescence imaging. Biophys. J. 1991;60:1374–1387. doi: 10.1016/S0006-3495(91)82175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akers W.J., Berezin M.Y., Lee H., Achilefu S. Predicting in vivo fluorescence lifetime behavior of near-infrared fluorescent contrast agents using in vitro measurements. J. Biomed. Opt. 2008;13:0540421–0540429. doi: 10.1117/1.2982535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waddell E., Wang Y., Stryjewski W., McWhorter S., Henry A.C. High-resolution near-infrared imaging of DNA microarrays with time-resolved acquisition of fluorescence lifetimes. Anal. Chem. 2000;72:5907–5917. doi: 10.1021/ac0009705. [DOI] [PubMed] [Google Scholar]
- 20.Sample preparations. 4 and 4a′ were dissolved in chloroform at the concentration of 1.2 mM as measured by absorption spectra (ɛoo = 205,000 M−1 cm−1 for 4a and 256,000 M−1 cm−1 for 4a′ correspondingly. Chloroform solutions (50 μL) were diluted with 50 μL of DMSO and exposed to flow of nitrogen for ∼30 min to remove chloroform. For in vitro measurements the aliquots of DMSO solutions were further diluted with DMSO, or slowly diluted with 2.9% Tween-80/water followed by 3% albumin/water solution. The final solvent mixture was 1% Tween-80/1.4% albumin/1.5% DMSO/water. For in vivo measurements, the obtained DMSO solutions were slowly diluted with 2.9% of Tween-80/water solution (330 mL) to prevent precipitation of the dyes. The final solvent mixture contained 60 μM of the dye in a 15% DMSO, 2.5% Tween-80/water solution. ICG solutions were prepared as 60 μM in 20% DMSO/water.
- 21.Englman R., Jortner J. Energy gap law for radiationless transitions in large molecules. Mol. Phys. 1970;18:145–164. [Google Scholar]
- 22.Caspar J.V., Kober E.M., Sullivan B.P., Meyer T.J. Application of the energy gap law to the decay of charge-transfer excited states. J. Am. Chem. Soc. 1982;104:630–632. [Google Scholar]
- 23.Chynwat V., Frank H.A. The application of the energy gap law to the S1 energies and dynamics of carotenoids. Chem. Phys. 1995;194:237–244. [Google Scholar]
- 24.Fischer G.M., Ehlers A.P., Zumbusch A., Daltrozzo E. Near-infrared dyes and fluorophores based on diketopyrrolopyrroles. Angew. Chem. Int. Ed. Engl. 2007;46:3750–3753. doi: 10.1002/anie.200604763. [DOI] [PubMed] [Google Scholar]
- 25.Fischer G.M., Isomaki-Krondahl M., Gottker-Schnetmann I., Daltrozzo E., Zumbusch A. Pyrrolopyrrole cyanine dyes: a new class of near-infrared dyes and fluorophores. Chem. Eur. J. 2009;15:4857–4864. doi: 10.1002/chem.200801996. [DOI] [PubMed] [Google Scholar]
- 26.Zillgitt M. Neumann Druck; Heidelberg, Germany: 2002. Colourants for Food Contact Plastics. [Google Scholar]
- 27.Berezin M.Y., Lee H., Akers W., Achilefu S. Near infrared dyes as lifetime solvatochromic probes for micropolarity measurements of biological systems. Biophys. J. 2007;93:2892–2899. doi: 10.1529/biophysj.107.111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.In vivo imaging. After injections, the animals were positioned prone on the heated imaging platform and a 2D scanning region of interest was selected by top-view charge-coupled device camera to include the area from the neck to the pelvis. The 780-nm pulsed diode laser was set to 0.4 μW for excitation scans and adjusted for optimal signal strength, in the range 5–100 μW for fluorescence detection. Regions of interest were raster-scanned in 3-mm increments for contrast agent imaging with 0.3 s integration time per pixel. For more details see Akers et al. (18).


