Abstract
Rapid and robust methods are required to quantify the effect of hydrodynamic shear on protein conformation change. We evaluated such strategies in this work and found that the binding of the fluorescent probe 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid (bis-ANS) to hydrophobic pockets in the blood protein von Willebrand factor (VWF) is enhanced upon the application of fluid shear to the isolated protein. Significant structural changes were observed when the protein was sheared at shear rates ≥ 6000/s for ∼3.5 min. The binding of bis-ANS to multimeric VWF, but not dimeric VWF or control protein bovine serum albumin, was enhanced upon fluid shear application. Thus, high-molecular-weight VWF is more susceptible to conformation change upon tensile loading. Although bis-ANS itself did not alter the conformation of VWF, it stabilized protein conformation once it bound the sheared molecule. Bis-ANS binding to VWF was reduced when the sheared protein was allowed to relax before dye addition. Taken together with functional data in the literature, our results suggest that shear-induced conformation changes in VWF reported by bis-ANS correlate well with the normal function of the protein under physiological/pathological fluid flow conditions. Further, this study introduces the fluorescent dye bis-ANS as a tool that may be useful in studies of shear-induced protein conformation change.
Introduction
In recent years, several investigators have examined the effect of hydrodynamic forces on protein structure. In this regard, since chaotropic agents (urea and guanidinium), pH, and temperature have been shown to alter or otherwise denature proteins (1,2), an effect of fluid shear on changes in protein conformation may be expected. Although earlier studies suggested that shear in solution can denature or reduce the enzymatic activity of products of biotechnology (3,4), more recent studies have questioned those findings (5,6). Using cytochrome c, a small globular protein, as a model, Jaspe and Hagen (5) detected no changes in protein conformation under shear up to a shear rate of 2 × 105/s. The theoretical calculations of these investigators suggest that extraordinarily high levels of shear (∼107/s) are required to alter the structure of small globular proteins. Using concentrated monoclonal antibody formulations, Bee et al. (6) showed that even prolonged exposure to shear causes only <0.3% reversible aggregation of IgG antibodies. The authors suggested a cooperative role for air entrapment, surface effects, and particulate contamination in causing protein aggregation/loss under shear (6,7). In contrast, using particle image velocimetry and Raman spectroscopy to measure lysozyme structure in situ, other investigators observed rapid, reversible unfolding of this protein when it was subjected to shear (8). In the field of thrombosis research, a large multimeric protein called von Willebrand Factor (VWF) has been shown to undergo structural changes upon application of hydrodynamic shear (9,10). Together, these data suggest that protein conformation changes may take place under specific conditions. Further, the size of the protein may be a critical factor in regulating shear-induced conformation changes.
To determine methods that can reliably and quantitatively report on changes in protein conformation upon application of fluid shear, in this work we revisited many assay techniques that historically have been applied to study protein denaturation (2,11–14). These techniques include circular dichroism (CD), chromatography, fluorescence polarization, ultraviolet absorbance, fluorescence spectroscopy, nuclear magnetic resonance, and differential scanning calorimetry. In particular, we examined the use of CD, chromatography, and fluorescence spectroscopy to detect changes in shear-induced protein conformation. Of the methods we investigated, the most promising strategy involved the application of fluorescence spectroscopy in the context of ANS dyes, 1,8-ANS (1-anilinonaphthalene-8-sulfonic acid) and bis-ANS (4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid) (15,16). These dyes bind noncovalently to hydrophobic surfaces on proteins that are exposed upon fluid shear application. The fluorescence of the dye is highly dependent on the polarity of its microenvironment. Thus, dye binding to protein hydrophobic domains results in a dramatic increase in fluorescence and a substantial blue shift in the emission maxima (15,17). Quantitative data were obtained that demonstrate the reliability and high sensitivity of this assay method.
We performed our investigations with VWF, the largest protein in blood. The biomolecule is found as a linear multimer/polymer in blood, with a radius of gyration, Rg, of ∼100–120 nm (18). VWF was particularly attractive for our investigations because previous biochemical studies suggested that shear-induced conformational changes in VWF may contribute to a variety of physiological and pathological processes in blood, including enhanced VWF binding to platelets (19), self-assembly of VWF into a network of fibers on a collagen matrix (20), substrate immobilization (9), shear-induced platelet aggregation and self-association (21), and proteolysis by a blood protease called ADAMTS-13 (“a disintegrin and metalloprotease with thrombospondin” family member) (22).
We report that bis-ANS binding to VWF in solution is robustly enhanced upon application of fluid shear as a function of both the applied shear rate and the shearing time. Although VWF subjected to fluid shear exhibited higher binding of 1,8-ANS than the unsheared protein, the difference was not as remarkable as the signal detected when bis-ANS was used to probe protein conformation change. VWF subjected to shear in our experiments was functional, and thus the changes in bis-ANS binding reported here are indicators of protein conformation change rather than denaturation. Time-resolved studies also reported some reversibility in VWF conformation change upon cessation of shear application. Studies that compared the effect of fluid shear on multimeric VWF with recombinant dimeric protein showed that multimeric, but not dimeric VWF, undergoes structural change upon fluid shear application. Thus, the largest proteins tested (molecular mass ∼ 0.5–20 MDa) were more prone to shear-induced conformation change. Taken together with data in the literature, our findings indicate that structural changes in VWF conformation by bis-ANS likely correspond to functionally relevant changes in the protein that are associated with atherothrombotic events.
Materials and Methods
Protein isolation and purification
Normal multimeric human VWF was purified from blood plasma cryoprecipitate obtained from the Community Blood Bank (Erie, PA) (10,18). Dimeric VWF (VWF protomer, ΔPro-VWF) was expressed in stably transfected Chinese hamster ovary (CHO) cells (18). The cell culture protocol and purification methods were improved (see the Supporting Material), and this resulted in improved purity and yield. Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in HEPES buffer (30 mM HEPES, 110 mM NaCl, 10 mM KCl, 1 mM MgCl2, pH 7.3) for experimentation. All VWF preparations were also dialyzed against this buffer before fluorescence spectroscopy was conducted.
Silver staining and Western blot analysis
Multimeric and dimeric VWF purity was characterized by resolving the purified protein under reducing conditions on 4–20% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gradient gels (Thermo Scientific, Waltham, MA) followed by silver staining. Only a single prominent protein band with molecular mass corresponding to monomeric VWF was observed. This suggests that >95% of the purified protein is VWF (Fig. 1 A).
Figure 1.
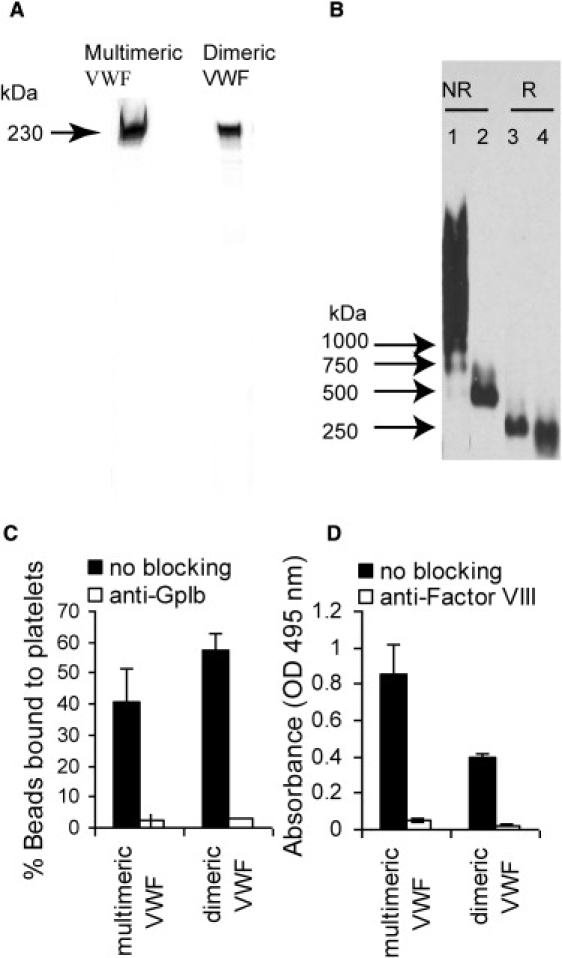
Multimeric and dimeric VWF. (A) Silver staining of multimeric and dimeric VWF. As shown, protein purity is >95%. (B) Western blot analysis of multimeric and dimeric VWF. In this experiment, 5 ng native multimeric VWF (lane 1) and 1 ng dimeric VWF (lane 2) were subjected to electrophoresis under nonreducing (NR) conditions, and 25 ng multimeric VWF (lane 3) and 25 ng dimeric VWF (lane 4) were reduced (R) with 25 mM DTT for 30 min at room temperature before electrophoresis. After the samples were transferred onto nitrocellulose membrane, VWF in all lanes was detected with the use of rabbit-anti-human VWF polyclonal Ab (Dako, Carpinteria, CA). (C) Isolated human platelets were mixed with polystyrene beads bearing immobilized multimeric or dimeric VWF at a shear rate of 1863/s for 60 s. The percentage of beads with bound platelets was quantified by flow cytometry. Both beads bearing dimeric and multimeric VWF bound platelets efficiently. The binding could be specifically blocked by anti-GpIbα blocking mAb AK2. (D) Binding of multimeric and dimeric VWF to Factor VIII was detected by ELISA. Factor VIII-VWF binding interaction was completely blocked by anti-Factor VIII blocking mAb ESH4.
The samples were also analyzed using 0.6% (w/v) agarose-SDS gel electrophoresis followed by Western blot analysis. The purified multimeric VWF protein exhibited a range of molecular masses, suggesting that it was composed of 30–40 monomer units (Fig. 1 B). Dimeric VWF had a molecular mass of 500 kDa under nonreducing conditions. Reduced multimeric and dimeric VWF were both composed of a single band with molecular mass ∼ 250 kDa, as expected (18).
Functional assays with purified and recombinant VWF
The activity of multimeric and dimeric VWF used in our study was confirmed in functional assays that measured the binding of this biomolecule to platelet and Factor VIII. Details regarding the methods used are provided in the Supporting Material.
In flow cytometry-based assays that measured the binding of platelets to beads bearing immobilized VWF, we observed that both multimeric and dimeric VWF bound the platelet GpIbα receptor efficiently (Fig. 1 C). In both cases, >40% of the polystyrene beads with immobilized VWF bound platelets after 60 s of shear application. The binding of VWF-bearing beads to platelets could be completely blocked by anti-GpIbα blocking mAb AK2. Thus, the GpIbα recognizing A1-domain of VWF is functionally active.
In enzyme-linked immunosorbent assay (ELISA) experiments, both immobilized multimeric and dimeric VWF were observed to bind soluble Factor VIII (Fig. 1 D). This binding interaction was abrogated by anti-Factor VIII blocking mAb ESH-4. Thus, the D′D3 domain of VWF that recognizes Factor VIII is functional.
Fluorescence spectroscopy
Bis-ANS (Invitrogen, Eugene, OR) was dissolved at 0.1% w/v in Na-K phosphate buffer (8 mM Na2HPO4, 2 mM KH2PO4, 140 mM NaCl, pH 7.2). This solution was equilibrated at 4°C for 3 days before it was used in experiments. Fluid shear stress to multimeric VWF (60–80 μg/mL), dimeric VWF (100 μg/mL), and BSA (50–60 μg/mL) was applied by means of a cone-and-plate viscometer. Typically, 220 μL of protein solution were subjected to fluid shear. Then 140 μL of the postshear sample were incubated with 15 μL of bis-ANS stock solution in a quartz cell (1 cm path length) for a fixed time before emission spectra were recorded (excitation wavelength (λ) = 375 nm, emission λ = 420–620 nm) at ambient temperature using a QuantaMaster Fluorometer (PTI, Birmingham, NJ). F(G) (counts per second (cps)) denotes the magnitude of the emission signal for protein at a given wavelength after it was subjected to fluid shear at a shear rate of G (/s).
A 10–20 μL sample was also withdrawn for protein concentration determination before and after shear application using the Coomassie/Bradford protein assay kit (Pierce Biotechnology, Rockford, IL). To minimize the time between sample collection and concentration analysis, protein withdrawn from the viscometer was directly pipetted into the 96-well plates used for concentration assays. Quantitative amino acid analysis (18) was used to validate the Bradford assay.
A variety of parameters were varied as part of this investigation, including the 1) concentration of protein/probe; 2), shearing time (tsh); 3), applied shear rate (G); 4), time of probe addition after shear stoppage (tadd); and 5), time of sample measurement after bis-ANS addition to protein (incubation time, tinc). In addition, in some runs, bis-ANS was added to VWF prior to shear application. In all cases, when testing the effect of the experimental parameters, both VWF studies and control studies including runs with BSA and dimeric VWF, were performed under the same conditions on the same day. The nonsheared samples, noted as samples at G = 0/s, were left touching the viscometer plates for the same time as the tsh of their corresponding sheared samples.
The raw cps data (absolute fluorescence, F(G)) were used for major qualitative interpretations, and the following additional indices were developed to aid quantitative comparison:
-
1.
Peak absolute fluorescence, F(G)max (cps): peak absolute emission fluorescence at fixed wavelength.
-
2.
Normalized fluorescence, Fc(G) (cps.mL/μg): concentration-normalized emission spectra after accounting for fluorescence due to free bis-ANS in HEPES buffer (F(G)HEPES+bis-ANS), intrinsic protein fluorescence in buffer (F(G)protein+HEPES), and fluorescence of HEPES buffer alone (F(G)HEPES), i.e., Fc(G) = (F(G)protein+HEPES+bis-ANS-F(G)HEPES+bis-ANS-F(G)protein+HEPES + F(G)HEPES)/protein concentration. In this expression, F(G)protein+HEPES+bis-ANS is the F(G) measured in experiments containing protein and bis-ANS in HEPES buffer.
-
3.
Peak normalized fluorescence, Fc(G)max (cps.mL/μg): peak concentration-normalized emission fluorescence at fixed wavelength.
Statistics
All data are presented as the mean ± SD. Student's t-test (two-tailed) was performed, and p < 0.05 was considered to be statistically significant.
Results
We quantified the effect of fluid shear on multimeric VWF from human blood (see Figs. 3–6), and dimeric recombinant VWF (see Fig. 6). All proteins were fully functional (18,21) (Fig. 1).
Figure 3.
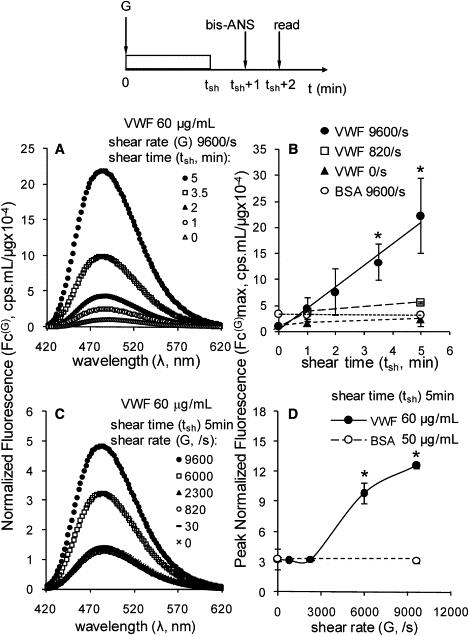
Shear and time dependence. (A) Fluid shear was applied to 60 μg/mL multimeric VWF using a cone-plate viscometer at 9600/s for various shearing times (tsh). Bis-ANS was added 1 min after shear stoppage, and fluorescence spectra were measured 1 min thereafter as illustrated in the schematic. Normalized fluorescence due to bis-ANS-VWF interaction Fc(G) was calculated. This parameter accounts for background signal due to free/unbound bis-ANS and the concentration of multimeric VWF in the measured sample. (B) Peak Fc(G) and Fc(G)max values at a fixed wavelength (484–492 nm for multimeric VWF and 480–482 nm for BSA) are presented. (C) Studies similar to those shown in panel A were performed in which the time of shear (tsh) was fixed at 5 min and the magnitude of fluid shear (G) was varied. (D) Peak Fc(G) and Fc(G)max values. Error bars represent the SD for three independent experiments; ∗p < 0.05 with respect to nonsheared sample (G = 0/s; tsh = 0 min).
Figure 4.
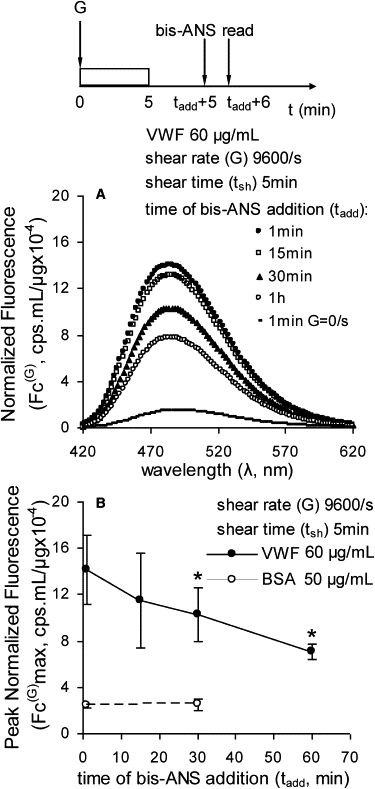
Bis-ANS signal reversibility. (A) Fluid shear was applied to 60 μg/mL multimeric VWF using a cone-plate viscometer at 9600/s for 5 min. Bis-ANS was added at various times after shear stoppage (tadd), and fluorescence spectra were measured 1 min thereafter as illustrated in the schematic. Normalized fluorescence (Fc(G)) data are presented. (B) Fc(G)max at a fixed wavelength (484–492 nm for multimeric VWF and 480–482 nm for BSA). Error bars represent the SD for three independent experiments with either multimeric VWF or BSA; ∗p < 0.05 with respect to the run in which bis-ANS was added 1 min after shear stoppage (i.e., tadd = 1 min).
Figure 5.
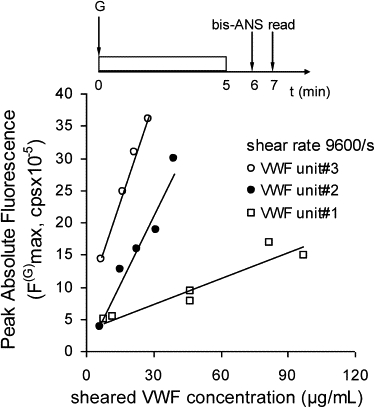
Protein-protein interaction. Fluid shear was applied to various concentrations of multimeric VWF using a cone-plate viscometer at 9600/s for 5 min. Bis-ANS was added 1 min after shear stoppage, and fluorescence spectra were measured 1 min thereafter. VWF purified from three different blood plasma cryoprecipitate units was used.
Figure 6.
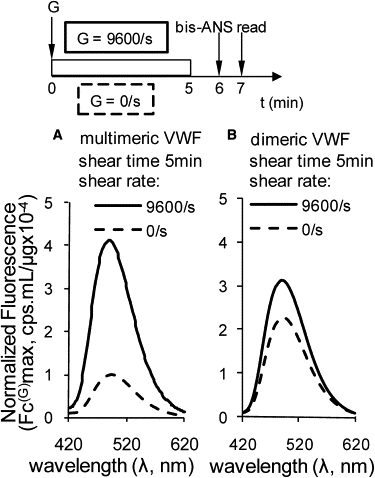
Sheared dimeric versus multimeric VWF. Fluid shear at 9600/s and 0/s for 5 min was applied to (A) 97 μg/mL multimeric VWF and (B) 104 μg/mL dimeric VWF. Bis-ANS was added 1 min after shear stoppage, and fluorescence spectra were measured 1 min thereafter. Although bis-ANS binding to multimeric VWF is enhanced upon fluid shear application, this is not the case for the dimeric protein.
Fluorescence signal due to bis-ANS interaction with VWF increases with protein and probe concentration
Fluorescence due to bis-ANS binding to purified VWF was used to assay protein conformation change in solution. To calibrate the system, the concentrations of VWF (unsheared protein, G = 0/s) and bis-ANS were varied (Fig. 2).
Figure 2.
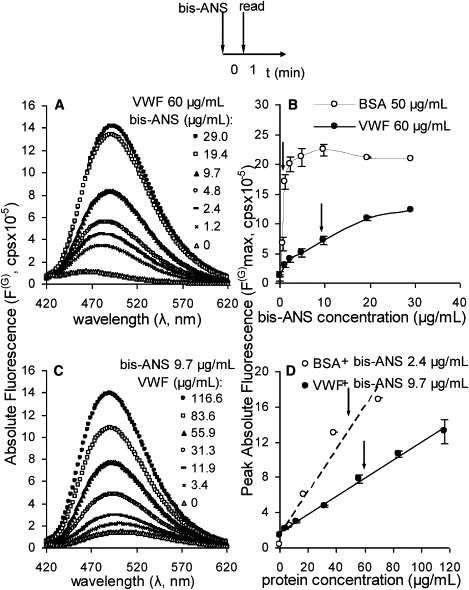
Bis-ANS fluorescence. (A) Representative absolute fluorescence spectra (F(G), cps) when 60 μg/mL multimeric VWF was incubated with varying concentrations of bis-ANS (0–29 μg/mL) for precisely 1 min before fluorescence measurement (excitation λ = 375 nm; emission λ = 420–620 nm). (B) Peak absolute fluorescence intensity (F(G)max) for 60 μg/mL multimeric VWF and 50 μg/mL BSA at various bis-ANS concentrations. (C) Absolute fluorescence spectra when multimeric VWF concentration was varied (0–116.6 μg/mL) in the presence of a fixed amount of bis-ANS (9.7 μg/mL). (D) Peak fluorescence intensity for studies in which multimeric VWF or BSA concentration was varied in the presence of a fixed amount of bis-ANS (9.7 μg/mL for runs with multimeric VWF and 2.4 μg/mL for BSA runs). Arrows in panels B and D indicate the concentrations of bis-ANS and protein, respectively, used in most of the experiments. The sketch at the top of the figure is a schematic of the experimental protocol. Error bars represent the SD for three independent runs.
When VWF was kept constant at ∼60 μg/mL and bis-ANS was varied from 0 to 29 μg/mL (Fig. 2 A), we observed an increase in fluorescence signal with probe concentration. Fluorescence signal did not saturate even at the highest bis-ANS concentration (29 μg/mL; Fig. 2 B). Peak fluorescence (F(G)max) was observed at 482 nm at the lowest probe concentration (1.2 μg/mL), and there was a shift to higher wavelengths (494 nm) when bis-ANS concentration was increased to 29 μg/mL. This red shift in fluorescence with probe concentration suggests that VWF may contain a range of sites for bis-ANS binding, with the probe binding to the more hydrophobic site(s) when applied at lower concentrations. When bis-ANS concentration was increased, the probe also recognized less hydrophobic sites, resulting in the red shift. A similar increase in fluorescence with bis-ANS dose was observed in studies performed with control protein BSA (Fig. 2 B and Fig. S1). Here, a lower concentration of bis-ANS (9.7 μg/mL) was sufficient to saturate the hydrophobic binding sites on BSA. The peak fluorescence wavelength varied from 484 nm when bis-ANS was at 0.6 μg/mL to 490 nm at 9.7 μg/mL.
When VWF (Fig. 2 C and D) or BSA (Fig. 2 D and Fig. S2) concentration was varied in the presence of fixed amounts of bis-ANS, a linear increase in fluorescence intensity was observed with protein concentration. Bis-ANS alone in the absence of protein exhibited low fluorescence (fivefold lower than that of the same sample containing ∼60 μg/mL VWF). Further, a blue shift from 502 nm to 490 nm was noted when VWF concentration was increased from 3.4 μg/mL to 116 μg/mL (Fig. 2 C). This shift is consistent with the proposition that when the probe/VWF ratio is high (i.e., at low VWF concentration), a sufficient amount of probe is available to bind the range of hydrophobic pockets in VWF. When this ratio falls (i.e., when VWF concentration is high), the probe prefers to bind the more hydrophobic regions. This then results in a blue shift.
Overall, the results reveal that VWF-bis-ANS interactions result in distinctly higher fluorescence signals compared to either VWF or bis-ANS alone. The fluorescence signal varies approximately linearly with protein concentration. Finally, based on these dose-dependence studies, and to ensure that the experiments were in the linear range of fluorescence signal increase, we incubated 9.7 μg/mL (14 μM) bis-ANS with 60 μg/mL VWF to probe protein conformation changes. Similarly, 2.4 μg/mL bis-ANS was added to 50 μg/mL BSA in control runs.
Time- and shear-dependent changes in VWF conformation
The ability of bis-ANS to differentially bind unsheared VWF versus VWF subjected to fluid shear was quantified. In these studies, the fluorescence signal due to bis-ANS binding to VWF increased in both a time- (Fig. 3, A and B) and shear- (Fig. 3, C and D) dependent manner.
When we varied the time of shear application to VWF at a fixed high shear rate (G) of 9600/s, we observed an increase in fluorescence signal above background values at the earliest sampling time point (1 min), and the increase became statistically significant at 3.5 min (Fig. 3, A and B). Fluorescence was three- to fourfold higher than background at 5 min (Fig. 3 B). At lower shear rates (820/s) and for unsheared protein (0/s), the extent of bis-ANS binding to VWF at all times was similar to that of the unsheared sample at 0 min. In comparison, a 4.7-fold increase in bis-ANS signal was noted when VWF was heat-denatured at 95°C for 10 min. Taken together, the data suggest that >60% of potential bis-ANS sites in VWF may be exposed upon application of shear at 9600/s.
When we varied the applied shear rate on VWF (Fig. 3, C and D), we observed that the bis-ANS signal did not increase at shear rates up to 2300/s. Application of G ≥ 6000/s, however, increased bis-ANS fluorescence. The fluorescence signal was threefold higher than that of the unsheared sample at 6000/s, and fourfold higher at 9600/s. Independent functional studies performed with VWF that had been subjected to shear at 9600/s for 5 min confirmed that VWF is active after the application of shear. In these assays, we detected the efficient binding of postsheared VWF to platelets by flow cytometry. A previous shear-induced platelet activation assay (21) also revealed that proteins that had been sheared for 5 min were able to activate human blood platelets (Fig. S3).
Although normalized fluorescence values are plotted in Fig. 3 after correction for background due to unbound/free bis-ANS and protein concentration, similar results were also seen when the sample was analyzed without normalization (Fig. S4). In this case also, the absolute fluorescence due to bis-ANS-VWF interaction was significantly higher after application of fluid shear ≥ 6000/s. Overall, the data suggest that bis-ANS binding to VWF is markedly augmented upon application of fluid shear to the protein.
Fluorescence signal due to bis-ANS-VWF interaction is reversible after stoppage of shear
We sought to determine whether the changes observed in VWF structure detected using bis-ANS as a probe were transient (Fig. 4). To this end, fluid shear was applied to VWF for 5 min at 9600/s. Sheared protein was then allowed to relax for varying amounts of time before bis-ANS addition. As shown in Fig. 4 A, a progressive decrease in fluorescence occurred when the time between shear stoppage and bis-ANS addition was increased. No change in peak emission fluorescence wavelength (∼486 nm) was noted. A 19% reduction in bis-ANS signal was observed when the protein was allowed to relax for 15 min, and this increased to 50% at 1 h (Fig. 4 B). Overall, the VWF conformation changes upon shear application were transient and relaxed upon stoppage of shear over the course of minutes. It remains to be determined whether the decrease in fluorescence was due to reversion of VWF to its native unsheared conformation or to another state.
Bis-ANS locks protein in altered conformation
We next sought to determine whether bis-ANS itself influences the conformation of VWF (Fig. S5). Here, VWF sheared at 9600/s for 5 min was incubated with bis-ANS for varying periods of time before fluorescence signal measurements were obtained. Fluorescence due to bis-ANS was measured at 1 min, followed by a second measurement at either 5 min (Fig. S5 A) or 30 min (Fig. S5 B). Fluorescence measured at both 5 and 30 min resembled that obtained at the 1 min time point in terms of both peak fluorescence and peak emission wavelength. There was negligible change in measured fluorescence signal over the duration of probe incubation (Fig. S5 C). Thus, bis-ANS itself may not dramatically alter the solution conformation of VWF.
We performed studies to determine the degree to which the fluorescence signal measured in our assays report on the structure of VWF while shear is being applied (Fig. S6). Thus, bis-ANS was added to VWF before fluid shear application (Fig. S6 A) and the resulting fluorescence was compared with that of a paired control to which bis-ANS was added 1 min after stoppage of fluid shear under identical shear conditions (Fig. S6 B). In both cases, the fluorescence was measured 2 min after shear stoppage. The measured fluorescence signal was higher at 9600/s than at 30/s, both when bis-ANS was added before shear (fivefold) and when it was added after shear stoppage (sevenfold). Thus, the time of probe addition does not affect the fluorescence signal.
Taken together, the results of the above experiments suggest that the hydrophobic domains of VWF that are revealed upon shear application reduce their exposure on the order of minutes. The presence of bis-ANS, however, has the appearance of locking the hydrophobic domains in their exposed configuration, because the measured fluorescence does not decrease with time after addition of the fluorescent dye.
Bis-ANS signal is not influenced by protein-protein interaction
The collision frequency between particles subjected to linear shear flow varies linearly with the applied shear rate and as a square of the particle concentration (23,24). The application of fluid shear under the conditions described in this work is also likely to contribute to the self-association or aggregation of VWF (21). To determine the degree to which the enhanced fluorescence signal due to bis-ANS binding can be attributed to shear-induced change in individual protein conformation versus the effects of protein-protein interaction, we performed studies in which the concentration of VWF subjected to fluid shear was varied (Fig. 5). Our supposition was that, if particle-particle collision is the dominant feature contributing to bis-ANS signal, then the fluorescence signal measured in our assay must also vary as a square of protein concentration. On the other hand, the signal should vary linearly with protein concentration if the bis-ANS signal is primarily dependent on changes in individual protein conformation. As can be seen in Fig. 5, the fluorescence signal varied linearly with protein concentration. Thus, the fluorescence signal observed in this study was not markedly influenced by VWF-VWF interaction or protein self-association under shear.
Bis-ANS binding to multimeric, but not dimeric, VWF is augmented by fluid shear
We examined the role of VWF size in augmenting bis-ANS binding. To that end, dimeric VWF was expressed and purified from CHO cells. The binding of bis-ANS to dimeric VWF versus multimeric VWF subjected to fluid shear was compared (Fig. 6). The fluorescence signal due to bis-ANS binding increased by fourfold upon application of shear to multimeric VWF (Fig. 6 A), whereas the increase was only 0.4-fold in the case of the dimeric protein (Fig. 6 B). Thus, the bis-ANS signal and protein conformation changes measured in our study may be primarily attributed to changes in the conformation of large, multimeric VWF molecules.
Discussion
We report that bis-ANS can be applied as a fluorescence probe to quantify shear-induced conformational changes in proteins. Although previous studies used such fluorescence dyes to probe changes in the surface hydrophobicity of proteins subjected to denaturation (25), pH (26), and hydrostatic pressure (27), this study is the first one (to our knowledge) in which this dye was used to study the effects of hydrodynamic shear on protein structure. In general, we observed that fluorescence spectroscopy was superior to the other methods we tested to study changes in protein conformation change. Notably, CD did not reveal a quantitative and reproducible change in molar ellipticity when fluid shear was applied to VWF at 9600/s compared to unsheared protein. Similarly, attempts to resolve different conformations of VWF using chromatography were largely qualitative due to the multimeric nature of VWF.
Structural changes in VWF detected using bis-ANS may have functional relevance
A significant increase in bis-ANS binding to VWF was observed at G ≥ 6000/s. Bis-ANS fluorescence increased at the earliest sampling time point after shear application (1 min), and the signal was statistically higher at 3.5 min at 9600/s. VWF was functional even after it was subjected to shear at 9600/s for 5 min, because it efficiently bound platelet GpIbα and stimulated cell activation. Thus, the bis-ANS signal measured in this study reports on protein conformation change rather than protein denaturation. Table 1 presents an estimate of the peak forces applied to various biomolecules in our experiments. The peak force is tensile in nature, as described previously (28). Our analysis suggests that small forces (on the order of 5–10 pN) are sufficient to alter the conformation of VWF.
Table 1.
Peak normal force estimates
| Biomolecule dimensions |
||||
|---|---|---|---|---|
| Protein name | Sphere radius a1 | Separation distance, d | Force coefficient∗, αn | Peak force at G = 6000/s Fn= αnμGa12 |
| BSA | 2.3 nm | 1 nm | 21.03 | 0.0006 pN |
| Dimeric VWF | 13 nm | 94 nm | 51.56 | 0.05 pN |
| Multimeric VWF (200-mer) | 13 nm | 94 nm | 51.56 | 11 pN |
BSA and dimeric VWF are modeled as dimeric proteins with sphere size a1 and separation distance d (28). BSA data were taken from previous studies that used small-angle scattering and light scattering (18,39). VWF dimensions are based on electron microscopy (40). Forces applied to multimeric VWF relied on the force calculations of Shankaran and Neelamegham (28), and extrapolations by Zhang et al. (38). Solvent viscosity (μ) is 1 cp.
For details, see Shankaran and Neelamegham (28).
Although the effect of surfaces cannot be clearly discerned in any mixing/shearing device, several aspects suggest that the effects we observe are due to the effect of fluid flow on the protein in solution. First, we specifically design our experiments to minimize secondary or radial flow in the viscometer by using a low cone angle (0.5°) and by minimizing the volume of sample being sheared (29,30). Second, we sample protein from the bulk of the viscometer for the spectroscopy measurements. Third, the range of shear rates in which we observe structural transitions in VWF is remarkably similar to that reported by Schneider and colleagues (9,20). Those authors used microfluidics and fluorescence microscopy to investigate changes in the structure of individual, fluorescently labeled VWF, and our work complements that strategy by focusing on the volume-averaged behavior of this protein. By studying the dynamics of individual proteins at resolution of ∼2 μm, these authors (9,20) showed that a threshold shear rate of ≥5000/s is necessary for fluorescently-tagged VWF to undergo a large-scale structural change from a compact molecule to an extended/elongated structure in solution. Given the similarity in the magnitude of shear rate required for protein conformation change in our study compared to that in previous reports (9,20), it appears possible that the changes we observed may also be due to the unraveling/extension of VWF in solution.
Although the increase in bis-ANS fluorescence appears to be directly related to a change in individual protein conformation, the persistence of this conformation change over a period of minutes may be due to the entanglement or self-association of VWF under shear (21). In this regard, the linear increase in fluorescence signal with VWF concentration in the shear assay suggests that the magnitude of the measured fluorescence is likely due to the effect of fluid shear on single protein conformation, rather than to the effects of protein-protein interaction. However, studies that measured the relaxation of bis-ANS signal after stoppage of shear revealed a 50% reduction in fluorescence signal over a 60 min time course. Similar slow relaxation was previously observed in polymeric systems (31,32). According to the “reptation” model (31,32), the time required for a single polymer chain to disengage from a mesh-like network, i.e., the disengagement time (τd), varies as a third power of the number of monomer segments, N, according to τd = τ1 × N3. Here, τ1 is a characteristic time on the order of 10−11s. Thus, for a polymer like VWF with 2050 amino acids and 40 monomer units, τd ∼ 1.5 h [= 10−11 × (2050 × 40)3 s].
Bis-ANS reports on shear-induced conformation changes, and the dye alone minimally affects VWF conformation. In this regard, changing the incubation time for unsheared (Fig. 2) or sheared VWF (Fig. S5) in bis-ANS did not result in a different signal as a function of the incubation time. Studies that compared bis-ANS signals obtained when the probe was present during shear application and when it was added immediately after shear stoppage (Fig. S6) also reveal that the time of probe addition does not affect the measured fluorescence signal. Thus, this dye does not stabilize any states that might not exist in its absence.
Changes in the solution structure of VWF have been shown to enhance a number of physiological functions of this protein. Shear rates ≥ 2000/s have been shown to enhance the cleavage of the A2 domain of this protein by the blood metalloprotease ADAMTS-13 (22,33). This shear-dependent proteolysis regulates VWF size and function in human blood. Shear rates on the order of 5000/s were also shown to cause changes in the structure of VWF such that this enhanced its deposition on immobilized collagen substrates in the form of fibrils (9,20). In our previous studies (21), we also demonstrated that VWF-GpIbα interaction-dependent shear-induced platelet activation (SIPAct) is augmented above a threshold shear stress of 75 dyn/cm2 (equivalent to a shear rate of 6800/s in media with viscosity 1.1 cp). The striking correlation between the hydrodynamic shear conditions required to enhance the binding of bis-ANS to VWF observed here and those reported in our previous work suggests that protein conformation changes detected by fluorescence spectroscopy likely correlate with shear-induced changes in VWF function.
Larger VWF multimers, but not the dimeric protein, are more prone to undergo structural change
Numerous studies have highlighted the importance of VWF size in regulating protein activity and human physiological function. Ultralarge VWF derived from endothelial cells have been shown to be more effective than the multimeric plasma VWF in supporting shear-induced platelet aggregation (34) whereas the higher multimericity of ultralarge VWF compared to plasma VWF may partially account for the enhanced function; however, Arya et al. (35) also attributed this difference to the presence of high-affinity binding sites in endothelial VWF for platelet GpIbα. In another example, abnormal VWF multimer distribution in von Willebrand disease (VWD), particularly VWD types 2A and 2B, results in loss of high-molecular mass multimers (36). Loss of these higher-molecular mass fractions is associated with prolonged bleeding in VWD patients. Finally, it is thought that the vessel wall constrictions and high shear stresses associated with arterial stenosis may enhance VWF conformation change. Such structural changes increase the susceptibility of VWF to proteolysis by plasma ADAMTS13 (22). In the context of these observations, our study, which compares bis-ANS binding to multimeric VWF with dimeric VWF under fluid shear, shows that higher-molecular mass VWF is likely to be more susceptible to shear-induced conformation change. When the VWF molecular mass is 500 kDa, as in the case of the dimeric protein, protein conformation changes were not clearly discerned. The possibility that protein conformation change is highly regulated by size in nature, and is limited to a relatively few high-molecular mass proteins is interesting. This hypothesis may be addressed in future studies using a wider panel of proteins and environment-sensitive probes such as bis-ANS.
A variety of structural features may contribute to conformation changes in VWF under flow, especially molecular and hydrodynamic interactions between various protein components (37). One possibility is that there are molecular interactions between VWF domains that stabilize the protein solution structure (18), and these domain level interactions may be disrupted by fluid shear. Another possibility is that individual domains within VWF, particularly the A2 domain, may unfold upon shear application (38).
In conclusion, this work demonstrates the utility of fluorescence spectroscopy and bis-ANS for studying shear-induced protein/biomaterial conformation change. The technique described is simple to implement and yields quantitative information on the effect of shear forces and shearing time in regulating the solution conformation of the largest protein in blood. Such structural regulation of multimeric VWF by fluid flow may also influence other protein-protein interactions in blood, and regulate thrombosis and hemostasis in the vasculature.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (No. HL77258).
Supporting Material
References
- 1.Chi E.Y., Krishnan S., Randolph T.W., Carpenter J.F. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm. Res. 2003;20:1325–1336. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 2.Fan H., Vitharana S.N., Chen T., O'Keefe D., Middaugh C.R. Effects of pH and polyanions on the thermal stability of fibroblast growth factor 20. Mol. Pharm. 2007;4:232–240. doi: 10.1021/mp060097h. [DOI] [PubMed] [Google Scholar]
- 3.Charm S.E., Wong B.L. Enzyme inactivation with shearing. Biotechnol. Bioeng. 1970;12:1103–1109. doi: 10.1002/bit.260120615. [DOI] [PubMed] [Google Scholar]
- 4.Oliva A., Santovena A., Farina J., Llabres M. Effect of high shear rate on stability of proteins: kinetic study. J. Pharm. Biomed. Anal. 2003;33:145–155. doi: 10.1016/s0731-7085(03)00223-1. [DOI] [PubMed] [Google Scholar]
- 5.Jaspe J., Hagen S.J. Do protein molecules unfold in a simple shear flow? Biophys. J. 2006;91:3415–3424. doi: 10.1529/biophysj.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bee J.S., Stevenson J.L., Mehta B., Svitel J., Pollastrini J. Response of a concentrated monoclonal antibody formulation to high shear. Biotechnol. Bioeng. 2009;103:936–943. doi: 10.1002/bit.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biddlecombe J.G., Smith G., Uddin S., Mulot S., Spencer D., Gee C., Fish B.C., Bracewell D.G. Factors influencing antibody stability at solid-liquid interfaces in a high shear environment. Biotechnol. Prog. 2009 doi: 10.1002/btpr.211. (Epub. [DOI] [PubMed] [Google Scholar]
- 8.Ashton L., Dusting J., Imomoh E., Balabani S., Blanch E.W. Shear-induced unfolding of lysozyme monitored in situ. Biophys. J. 2009;96:4231–4236. doi: 10.1016/j.bpj.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider S.W., Nuschele S., Wixforth A., Gorzelanny C., Alexander-Katz A. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA. 2007;104:7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh I., Themistou E., Porcar L., Neelamegham S. Fluid shear induces conformation change in human blood protein von Willebrand factor in solution. Biophys. J. 2009;96:2313–2320. doi: 10.1016/j.bpj.2008.12.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly S.M., Price N.C. The use of circular dichroism in the investigation of protein structure and function. Curr. Protein Pept. Sci. 2000;1:349–384. doi: 10.2174/1389203003381315. [DOI] [PubMed] [Google Scholar]
- 12.Luykx D.M., Goerdayal S.S., Dingemanse P.J., Jiskoot W., Jongen P.M. HPLC and tandem detection to monitor conformational properties of biopharmaceuticals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;821:45–52. doi: 10.1016/j.jchromb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Panda M., Ybarra J., Horowitz P.M. Dissociation of the single-ring chaperonin GroEL by high hydrostatic pressure. Biochemistry. 2002;41:12843–12849. doi: 10.1021/bi020366j. [DOI] [PubMed] [Google Scholar]
- 14.Pelton J.T., McLean L.R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000;277:167–176. doi: 10.1006/abio.1999.4320. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz P.M., Bowman S. Conformational changes accompany the oxidative inactivation of rhodanese by a variety of reagents. J. Biol. Chem. 1987;262:8728–8733. [PubMed] [Google Scholar]
- 16.Luduena R.F., Roach M.C., Horowitz P. The effects of the anilinonaphthalenesulfonates on the alkylation of tubulin: correlation between the appearance of sulfhydryl groups and apolar binding sites. Biochim. Biophys. Acta. 1986;873:143–146. doi: 10.1016/0167-4838(86)90200-1. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz P., Criscimagna N.L. Low concentrations of guanidinium chloride expose apolar surfaces and cause differential perturbation in catalytic intermediates of rhodanese. J. Biol. Chem. 1986;261:15652–15658. [PubMed] [Google Scholar]
- 18.Singh I., Shankaran H., Beauharnois M.E., Xiao Z., Alexandridis P. Solution structure of human von Willebrand factor studied using small angle neutron scattering. J. Biol. Chem. 2006;281:38266–38275. doi: 10.1074/jbc.M607123200. [DOI] [PubMed] [Google Scholar]
- 19.Choi H., Aboulfatova K., Pownall H.J., Cook R., Dong J.F. Shear-induced disulfide bond formation regulates adhesion activity of von Willebrand factor. J. Biol. Chem. 2007;282:35604–35611. doi: 10.1074/jbc.M704047200. [DOI] [PubMed] [Google Scholar]
- 20.Barg A., Ossig R., Goerge T., Schneider M.F., Schillers H. Soluble plasma-derived von Willebrand factor assembles to a haemostatically active filamentous network. Thromb. Haemost. 2007;97:514–526. [PubMed] [Google Scholar]
- 21.Shankaran H., Alexandridis P., Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101:2637–2645. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 22.Tsai H.M., Sussman I.I., Nagel R.L. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83:2171–2179. [PubMed] [Google Scholar]
- 23.Neelamegham S., Taylor A.D., Hellums J.D., Dembo M., Smith C.W. Modeling the reversible kinetics of neutrophil aggregation under hydrodynamic shear. Biophys. J. 1997;72:1527–1540. doi: 10.1016/S0006-3495(97)78801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smoluchowski M.V. Versuch einer mathematischen theorie der koagulationskinetik kolloider losungen. Z. Phys. Chem. 1917;92:129–168. [Google Scholar]
- 25.Almstedt K., Lundqvist M., Carlsson J., Karlsson M., Persson B. Unfolding a folding disease: folding, misfolding and aggregation of the marble brain syndrome-associated mutant H107Y of human carbonic anhydrase II. J. Mol. Biol. 2004;342:619–633. doi: 10.1016/j.jmb.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Poklar N., Lah J., Salobir M., Macek P., Vesnaver G. pH and temperature-induced molten globule-like denatured states of equinatoxin II: a study by UV-melting, DSC, far- and near-UV CD spectroscopy, and ANS fluorescence. Biochemistry. 1997;36:14345–14352. doi: 10.1021/bi971719v. [DOI] [PubMed] [Google Scholar]
- 27.Martins S.M., Chapeaurouge A., Ferreira S.T. Folding intermediates of the prion protein stabilized by hydrostatic pressure and low temperature. J. Biol. Chem. 2003;278:50449–50455. doi: 10.1074/jbc.M307354200. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran H., Neelamegham S. Hydrodynamic forces applied on intercellular bonds, soluble molecules, and cell-surface receptors. Biophys. J. 2004;86:576–588. doi: 10.1016/S0006-3495(04)74136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankaran H., Neelamegham S. Effect of secondary flow on biological experiments in the cone-plate viscometer: methods for estimating collision frequency, wall shear stress and inter-particle interactions in non-linear flow. Biorheology. 2001;38:275–304. [PubMed] [Google Scholar]
- 30.Shankaran H., Neelamegham S. Nonlinear flow affects hydrodynamic forces and neutrophil adhesion rates in cone-plate viscometers. Biophys. J. 2001;80:2631–2648. doi: 10.1016/S0006-3495(01)76233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Gennes P.-G. Cornell University Press; Ithaca, NY: 1979. Scaling Concepts in Polymer Physics. [Google Scholar]
- 32.Teraoka I. John Wiley & Sons; New York: 2002. Polymer Solutions—An Introduction to Physical Properties. [Google Scholar]
- 33.Shim K., Anderson P.J., Tuley E.A., Wiswall E., Sadler J.E. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood. 2008;111:651–657. doi: 10.1182/blood-2007-05-093021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moake J.L., Turner N.A., Stathopoulos N.A., Nolasco L.H., Hellums J.D. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J. Clin. Invest. 1986;78:1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arya M., Anvari B., Romo G.M., Cruz M.A., Dong J.F. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood. 2002;99:3971–3977. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- 36.Sadler J.E., Budde U., Eikenboom J.C., Favaloro E.J., Hill F.G. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J. Thromb. Haemost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 37.Szymczak P., Cieplak M. Proteins in a shear flow. J. Chem. Phys. 2007;127:155106. doi: 10.1063/1.2795725. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X., Halvorsen K., Zhang C.Z., Wong W.P., Springer T.A. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 2009;324:1330–1334. doi: 10.1126/science.1170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen J., Arakawa T., Philo J.S. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal. Biochem. 1996;240:155–166. doi: 10.1006/abio.1996.0345. [DOI] [PubMed] [Google Scholar]
- 40.Fowler W.E., Fretto L.J., Hamilton K.K., Erickson H.P., McKee P.A. Substructure of human von Willebrand factor. J. Clin. Invest. 1985;76:1491–1500. doi: 10.1172/JCI112129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


