Abstract
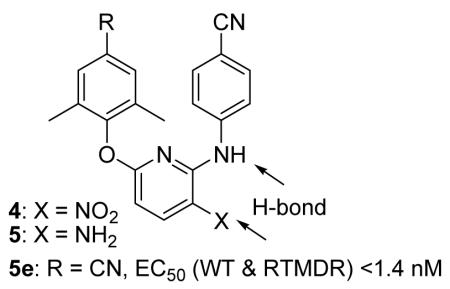
Two series (4 and 5) of diarylpyridine derivatives were designed, synthesized, and evaluated for anti-HIV-1 activity. The most promising compound, 5e, inhibited HIV-1 IIIB, NL4-3, and RTMDR1 with low nanomolar EC50 values and selectivity indexes of >10,000. The results of this study indicate that diarylpyridine can be used as a novel scaffold to derive a new class of potent NNRTIs, active against both wild-type and drug resistant HIV-1 strains.
Keywords: diarylpyridine derivatives, HIV-1 NNRTIs, drug resistance
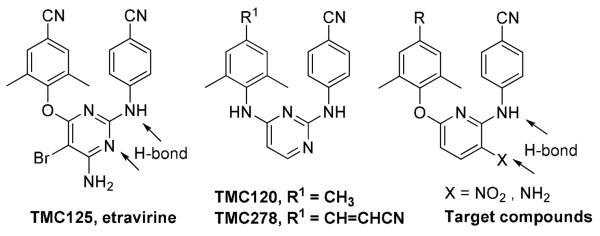
Reverse transcriptase (RT) is one of the most important enzymes in the HIV-1 life cycle. Two major drug target sites in the RT are the substrate binding site and an allosteric site that is distinct from, but closely located to, the substrate binding site.1,2,3 Non-nucleoside reverse transcriptase inhibitors (NNRTIs) interact with the allosteric binding site on HIV-1 RT to cause distortion of the three-dimensional structure of the enzyme and to inhibit RT catalytic function. Currently,NNRTIs approved for AIDS therapy include nevirapine, delavirdine, efavirenz, and etravirine (TMC125). TMC125,4 TMC120,5 a previous clinical candidate, and TMC278,6 a promising new drug candidate in phase III clinical trial, (Fig 1) are compounds belonging to the diarylpyrimidine (DAPY) family.7 These compounds are very potent against both wild-type and many drug-resistant HIV-1 strains. The excellent pharmacological profile of these compounds has encouraged new research to explore a next-generation of NNRTIs with new scaffolds. Prior studies on DAPY derivatives have revealed some pharmacophores,1,8 such as a horseshoe binding conformation, a proper positioning of two phenyl rings in the eastern and western wings of the NNRT binding pockets, a para-cyanoaniline moiety in the eastern wing, and two hydrogen bonds to K101 of HIV-1 RT. Based on this SAR information, we initiated a program to explore new NNRTI leads with new molecular scaffolds and high potency against wild-type and drug-resistant viral strains. By using an isosteric replacement strategy, diarylpyridine compounds were designed, synthesized, and evaluated for anti-HIV-1 activity. Among the tested compounds, new active leads with high potency against both wild-type and drug-resistant HIV-1 strains were discovered. We report our promising results herein.
Figure 1.
TMC125, TMC120, TMC278, and target compounds.
Compared to TMC125, our target compounds (Figure 1) were designed to retain the active para-cyanoaniline moiety, but have a pyridine replacing the pyrimidine ring and various substituents at the para-position of the phenoxy ring. Because the isosteric replacement of pyridine for pyrimidine should not change the molecular topology or flexibility, we hypothesized that the designed compounds would have similar binding orientation, conformation, and comparable activity to that of TMC125 and TMC278 (EC50/SI: 0.00158 μM/63,096 and 0.0005 μM/60,000, respectively, against HIV-1IIIB replication in the MT-4 cell line),9,10 as well as TMC120. However, the substitution of pyridine for pyrimidine resulted in the loss of an H-bond between K101 and the second nitrogen on the pyrimidine ring. Therefore, we incorporated a nitro or amino group at the 3-position on the pyridine ring to provide potential H-bonding with K101, as either an H-bond acceptor or donor.
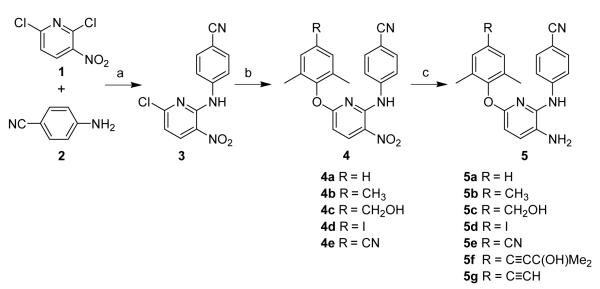
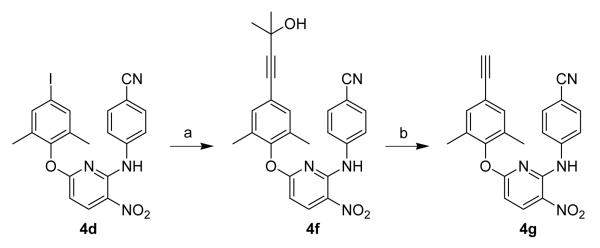
The target compounds were synthesized via the short routes detailed in Schemes 1 and 2. The starting material 2,6-dichloro-3-nitropyridine (1) is inexpensive and commercially available. Reacting 1 with 4-aminobenzonitrile (2) at 140 °C provided 4-(6-chloro-3-nitropyridin-2-ylamino)benzonitrile (3). Compound 3 was coupled with a substituted phenol in DMF in the presence of potassium carbonate at 120 °C for 6 h to afford the target compounds 4a-e. Compound 4f was prepared by Sonogashira coupling11 between 4d and 2-methyl-3-butyn-2-ol in DMF catalyzed by palladium-copper, and was then refluxed in dry toluene in the presence of powdered sodium hydroxide to provide 4g (Scheme 2). Finally, the nitro group on the pyridine ring in 4a-4g was reduced to an amino group by using sodium hydrosulfite dehydrate12 to afford 5a-5g, respectively (Scheme 1). The spectroscopic data of the 14 target compounds were consistent with the structures shown in Scheme 1.13
Scheme 1.
(a) 140 °C, 4 h, N2, 73%; (b) substituted phenol, K2CO3, DMF, 120 °C, 6 h, 65-82%; (c) Na2S2O4, NH3·H2O, THF/H2O = 1/1 (v/v), rt, 3h, 41-70%.
Scheme 2.
(a) 2-methyl-3-butyn-2-ol, Pd(PPh3)2Cl2, Et3N, CuI, DMF, N2, rt, 7 h, 78%; (b) NaOH, toluene, reflux, 16 h, 69%.
The inhibitory activity of nitro-diarylpyridine (4a-4g) and amino-diarylpyridine (5a-5g) derivatives on HIV-1 IIIB replication in MT-2 cells and cytotoxicity against MT-2 cells were determined as previously described.14 As shown in Table 1, except for 4f, most nitro-substituted compounds (series 4) showed significant inhibitory activity against HIV-1 replication (EC50 0.12 – 2.4 μM) and low cytotoxicity (CC50 50 – 503 μM), resulting in selectivity index (SI) values of 61 to 4,264. All the amino-substituted compounds (series 5) displayed greater anti-HIV-1 IIIB activity (EC50 0.001 – 1.58 μM) than the corresponding compounds in series 4. However, the series 5 compounds were also more cytotoxic (CC50 2.19 – 31.78 μM) than the corresponding series 4 compounds. With an SI of 22,700, the most promising compound was 5e.
Table 1.
Inhibitory activity of 4a-4g and 5a-5g on HIV-1IIIB replication in MT-2 cellsa
| Compds | R | CC50 (μM)b | EC50 (μM)c | SId |
|---|---|---|---|---|
| 4a | H | 110.2±0.0 | 1.028±0.139 | 107 |
| 4b | CH3 | 503.2±5.2 | 0.118±0.053 | 4,264 |
| 4c | CH2OH | 115.8±14.1 | 0.192±0.005 | 603 |
| 4d | I | 148.5±31.2 | 2.428±0.309 | 61 |
| 4e | CN | 50.13±2.60 | 0.135±0.286 | 371 |
| 4f | C≡CC(OH)Me2 | 8.91±0.0 | 23.28±10.48 | <1 |
| 4g | C≡CH | 173.6±46.0 | 0.677±0.286 | 256 |
| 5a | H | 29.9±2.3 | 0.576±0.182 | 52 |
| 5b | CH3 | 10.55±0.41 | 0.058±0.026 | 182 |
| 5c | CH2OH | 2.19±0.41 | 0.006±0.002 | 391 |
| 5d | I | 23.49±5.77 | 0.079±0.007 | 297 |
| 5e | CN | 31.78±10.37 | 0.0014±0.0023 | 22,700 |
| 5f | C≡CC(OH)Me2 | 3.54±1.07 | 1.578±0.097 | 2 |
| 5g | C≡CH | 8.25±0.51 | 0.424±0.282 | 20 |
Compounds were tested in triplicate and the data are presented as means ± SD.
XTT assay was used to determine the 50% cytotoxic concentration (CC50).
ELISA was used to determine p24 production, based on which the 50% effective concentration (EC50) for inhibiting HIV-1 replication was calculated.
Selectivity index (SI) = CC50/ EC50
All target compounds were further tested against HIV-1 NL4-3 and a drug resistant strain HIV-1 RTMDR1, in comparison with TMC120 (Table 2). In agreement with the MT-2 data, 5e was also the most active compound and had the highest SI (13,162) against NL4-3. The potency of 5e in this assay was comparable to that of TMC120. Interestingly, the active compounds in both series 4 and 5 showed similar inhibitory potency against NL4-3 and RTMDR1, which is resistant to many NRTIs and NNRTIs.15 The most potent compound, 5e, had an EC50 value of 0.96 nM against HIV-1 RTMDR1 and 0.68 nM against HIV-1 NL4-3, a difference of only 1.4-fold. These results suggested that the pyridine ring is an acceptable moiety to replace the pyrimidine ring of DAPY compounds and an amino group at the 3-position of the pyridine ring enhances anti-HIV activity against both wild-type (IIIB and NL4-3) and multidrug resistant (RTMDR1) HIV-1 strains.
Table 2.
Inhibitory activity of 4a-4g and 5a-5g on HIV-1NL4-3 and HIV-1RTMDR-1 replication in TZM-bl cells. *
| NL4-3 | RTMR1a | |||||
|---|---|---|---|---|---|---|
| Cmpd | R | CC50 (μM) |
EC50 (μM) |
SI | EC50 (μM) |
SI |
| 4a | H | >55.56 | 0.234±0.058 | >238 | 0.128±0.031 | >433 |
| 4b | CH3 | >53.48 | 0.0229±0.0052 | >2,335 | 0.0552±0.0098 | >969 |
| 4c | CH2OH | >51.28 | 0.477±0.371 | >108 | 0.877±0.562 | >58 |
| 4d | I | >41.15 | 0.061±0.024 | >675 | 0.0943±0.009 | >436 |
| 4e | CN | >51.95 | 0.0221±0.0061 | >2,351 | 0.0211±0.004 | >2,462 |
| 4f | C≡CC(OH)Me2 | >45.25 | 41.346 | >1.09 | 25.480 | >1.78 |
| 4g | C≡CH | >52.08 | 0.111±0.086 | >469 | 0.215±0.0327 | >242 |
| 5a | H | 8.14 | 0.00402±0.00058 | 2,025 | 0.00378±0.00134 | 2,153 |
| 5b | CH3 | 18.63 | 0.00167±0.00014 | 11,156 | 0.00120±0.0003 | 15,525 |
| 5c | CH2OH | 9.34 | 0.0109±0.0037 | 857 | 0.0251±0.0081 | 372 |
| 5d | I | 16.72 | 0.0076±0.0023 | 2,200 | 0.00513±0.00115 | 3,259 |
| 5e | CN | 8.98 | 0.00068±0.00003 | 13,206 | 0.00096±0.00027 | 9,354 |
| 5f | C≡CC(OH)Me2 | 8.73 | 0.241±0.0147 | 36 | 0.531 | 16 |
| 5g | C≡CH | 9.94 | 0.0067±0.0010 | 1,484 | 0.00903±0.00204 | 1,101 |
| TMC120 | >0.304 | 0.00062±0.00012 | >490 | 0.000298±0.00015 | >1,020 | |
The compounds were tested in triplicate and the data are presented as means ± SD.
HIV-1RTMDR1 (obtained from AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH), which contains mutations in RT amino acid residues 74V, 41L, 106A, and 215Y, is resistant to AZT, ddI, nevirapine, and other non-nucleoside RT inhibitors.
Similar to TMC125, the most active new compound, 5e, has a para-cyano group on the phenoxy ring. Compounds with methyl, hydroxymethyl, ethynyl, iodo, and no para-substituent were also active. However, compounds 4f and 5f with a bulky 2-methylbut-3-yn-2-ol [C≡C C(OH)Me2] group at the para-position of the phenoxy ring lost anti-HIV potency, although 4g and 5g with only a simple ethynyl substituent were active. These results suggested that substituents at this position of the phenoxy ring also directly affect anti-HIV potency, and a bulky group might not fit well into the hydrophobic cleft on the west wing of the NNRTI binding site.16
In summary, two series (4 and 5) of diarylpyridine derivatives were designed, synthesized, and evaluated for anti-HIV-1 activity, resulting in the discovery of a new class of NNRTI leads, diarylpyridine-3-amine derivatives (5a — 5e, 5g), with highly potent anti-HIV-1 activity against both RTI-sensitive (IIIB and NL4-3) and -resistant (RTMDR1) HIV-1 strains. The most promising compound was 5e, which inhibited HIV-1 IIIB, NL4-3, and RTMDR1 with low nM EC50 values. The results of this study indicated that diarylpyridine could be used as a novel scaffold to derive a new class of potent NNRTIs with activity against both wild-type and drug resistant HIV-1 strains. In addition, the pyridine ring replacement provides a more convenient and shorter synthetic route, using inexpensive commercial reagents, compared to the synthesis of DAPYderivatives TMC125 and TMC278.17,10 Our current preliminary structure-activity relationship (SAR) studies have revealed that (1) the pyridine is an acceptable isosteric replacement for the pyrimidine ring in DAPY derivatives, (2) an amino group at the 3-position on the pyridine is crucial for enhancing anti-HIV activity, and (3) the R group on the phenoxy ring is also an important moiety affecting anti-HIV activity. In light of the promising anti-HIV-1 activity of the diarylpyridine derivatives, further structural optimization is likely to yield novel NNRTIs with greatly improved potency.
Acknowledgments
This investigation was supported by grants 20472114 and 7052057 from the National Natural Science Foundation of China and Beijing government, respectively, awarded to L. Xie, as well as NIH grants AI46221 to S. Jiang, AI33066 to K. H. Lee, and AI65310 to C. H. Chen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.De Corte BL. J. Med. Chem. 2005;48:1689. doi: 10.1021/jm040127p. [DOI] [PubMed] [Google Scholar]
- 2.Tantillo C. J. Mol. Biol. 1994;243:369. doi: 10.1006/jmbi.1994.1665. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels R. Curr. Opin. Pharmacol. 2004;4:437. doi: 10.1016/j.coph.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Andries K, Azijn H, Thielemans T, Ludovici D, Kukla M, Heeres J, Janssen P, De Corte B, Vingerhoets J, Pauwels R, de Be’thuneet MP. Antimicrobl Agents and Chemother. 2004;48(12):4680. doi: 10.1128/AAC.48.12.4680-4686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruzdev B, Horban A, Boron-Kaczmarska A, Gille D, Van’t Klooster G, Pauwels R. 8th Conference on Retroviruses and Opportunistic Infections; Chicago, IL. February 4-8, 2001; Abstr. 13. [Google Scholar]
- 6.De Clercq E. Int. J. Antimicrob. Agents. 2009;33:307. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Janssen PAJ, Lewi PJ, Arnold E, Daeyaert F, de Jonge M, Heeres J, Koymans L, Vinkers M, Guillemont J, Pasquier E, Kukla M, Ludovici D, Andries K, de Bethune MP, Pauwels R, Das K, Clark AD, Frenkel YV, Hughes SH, Medaer B, De Knaep F, Bohets H, De Clerck F, Lampo A, Williams P, Stoffels P. J. Med. Chem. 2005;48:1901. doi: 10.1021/jm049534r. [DOI] [PubMed] [Google Scholar]
- 8.Das K, Lewi PJ, Hughes SH, Arnold E. Prog. Biophys. Mol. Biol. 2005;88:209. doi: 10.1016/j.pbiomolbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Van Herrewege Y, Vanham G, Michiels J, Fransen K, Kestens L, Andries K, Janssen P, Lewi P. Antimicrob. Agents Chemother. 2004;48:3684. doi: 10.1128/AAC.48.10.3684-3689.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillemont J, Pasquier E, Palandjian P, Vernier D, Gaurrand S, Lewi PJ, Heeres J, de Jonge MR, Koymans LMH, Daeyaert FFD, Vinkers MH, Arnold E, Das K, Pauwels R, Andries K, de Bethune MP, Bettens E, Hertogs K, Wigerinck P, Timmerman P, Janssen PAJ. J. Med. Chem. 2005;48:2072. doi: 10.1021/jm040838n. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez JG, Tejedor JL, La Parra T, Diaz C. Tetrahedron. 2006;62:3355. [Google Scholar]
- 12.Redemann CT, Redemann CE. Org. Synth. 1949;29:8. [Google Scholar]
- 13.Target compound 4a: Yield 67%; yellow solid, mp 200-203 °C; 1H NMR (CDCl3) δ 10.67 (1H, br s), 8.62 (1H, d, J = 8.8 Hz), 7.23 (7H, m), 6.65 (1H, d, J = 8.8 Hz), 2.12 (6H, s); MS m/z: 361 (M+). 4b: Yield 72%; yellow solid, mp 167-169 °C; 1H NMR (DMSO-d6) δ 10.38 (1H, br s), 8.65 (1H, d, J = 8.8 Hz), 7.39 (4H, m), 7.04 (2H, s), 6.78 (1H, d, J = 8.8 Hz), 2.36 (3H, s), 2.00 (6H, s); MS m/z: 375 (M+). 4c: Yield 73%; yellow solid, mp 188-190 °C; 1H NMR (DMSO-d6) δ 10.41 (1H, br s), 8.67 (1H, d, J = 8.8 Hz), 7.45 (2H, d, J = 8.8 Hz), 7.37 (2H, d, J = 8.8 Hz), 7.19 (2H, s), 6.81 (1 H, d, J = 8.8 Hz), 5.39 (1H, t, J = 6.4 Hz), 4.55 (2H, d, J = 6.4 Hz), 2.04 (6H, s); MS m/z: 391 (M+). 4d: Yield 82%; yellow solid, mp 162-165°C; 1H NMR (CDCl3) δ 10.65 (1H, br s), 8.63 (1H, d, J=8.8 Hz), 7.54 (2H, s), 7.38 (2H, d, J= 8.8 Hz), 7.21 (2H, d, J = 8.8 Hz), 6.65 (1 H, d, J = 8.8 Hz), 2.06 (6H, s); MS m/z: 487 (M+). 4e: Yield 65%; yellow solid, mp 240-241 °C; 1H NMR (CDCl3) δ 10.68 (1H, br s), 8.66 (1H, d, J = 8.8 Hz), 7.53 (2H, s), 7.32 (2H, d, J = 8.3 Hz), 7.19 (2H, d, J = 8.3 Hz), 6.67 (1H, d, J = 9.2 Hz), 2.16 (6H, s); MS m/z: 386 (M+). 4f: Yield: 78%; yellow solid, mp 114-116 °C; 1H NMR (DMSO-d6) δ 10.37 (1H, br s), 8.67 (1H, d, J = 8.8 Hz), 7.40 (2H, d, J = 8.8 Hz), 7.34 (2H, d, J = 8.8 Hz), 7.31 (2H, s), 6.83 (1 H, d, J = 8.8 Hz), 5.47 (1H, s), 2.00 (6H, s), 1.53 (6H, s); MS m/z: 465 (M+Na+). 4g: Yield 69%; yellow solid, mp 186-188 °C; 1H NMR (CDCl3) δ 10.66 (1H, br s),8.63 (1H, d, J = 8.8 Hz), 7.35 (2H, d, J = 8.8 Hz), 7.34 (2H,s), d, 7.19 (2H, d, J = 8.8 Hz), 6.66 (1 H, d, J = 8.8 Hz), 3.17(1H, s), 2.09(6H, s); MS m/z: 385 (M+). 5a: Yield 51%; brown solid, mp 140-142 °C; 1H NMR (DMSO-d6) δ 8.31 (1H, br s), 7.35 (2H, d, J = 8.8 Hz), 7.29 (2H, d, J = 8.8 Hz), 7.15 (3H, m), 7.12 (1H, d, J = 8.4 Hz), 6.40 (1H, d, J = 8.4 Hz), 4.79 (2H, br s), 2.05 (6H, s); MS m/z: 331 (M+). 5b: Yield 41%; brown solid, mp 206-208 °C; 1H NMR (DMSO-d6) δ 8.32 (1H , br s), 7.39 (2H, d, J = 8.8 Hz), 7.30 (2H, d, J = 8.8 Hz), 7.11 (1H, d, J = 8.0 Hz), 6.96 (2H, s), 6.36 (1H, d, J = 8.0 Hz), 4.78 (2H, br s), 2.31 (3H, s), 2.00 (6H, s); MS m/z: 345 (M+). 5c: Yield 70%; red solid, mp 278-281 °C; 1H NMR (DMSO-d6) δ 8.27 (1H, br s), 7.34 ((4H, s), 7.11 (1H, d, J = 8.0 Hz), 7.10 (2H, s), 6.38 (1H, d, J = 8.0 Hz), 5.22 (1H, t, J = 6.4 Hz), 4.76 (2H, br s), 4.49 (2H, d, J = 6.4 Hz), 2.03 (6H, s); MS m/z: 361 (M+). 5d: Yield 49%; brown solid, mp 186-188 °C; 1H NMR (CDCl3) δ 7.50 (2H, s), 7.33 (2H, d, J = 8.8 Hz), 7.22 (2H, d, J = 8.8 Hz), 6.98 (1H, d, J = 8.4 Hz), 6.44 (1H, d, J = 8.4 Hz), 2.09 (6H, s); MS m/z: 457 (M+). 5e: Yield 66%; brown solid, mp 222-225 °C; 1H NMR (DMSO-d6) δ 8.36 (1H, br s), 7.73 (2H,S), 7.32 (2H, d, J = 9.2 Hz), 7.26 (2H, s), 7.14 (1H, d, J = 8.0Hz), 6.49 (1H, d, J = 8.0Hz), 4.88 (2H, br s), 2.08 (6H, s); MS m/z: 356 (M+). 5f: Yield 48%; brown solid, mp 155-157 °C; 1H NMR (DMSO-d6) δ 8.33 (1H, br s), 8.01 (1H, br s), 7.41 (1H, d, J = 8.8 Hz), 7.29 (4H, m), 7.23 (2H, s), 6.55 (1 H, d, J = 8.8 Hz), 4.83 (2H, br s), 2.08 (6H, s), 1.51 (6H, s); MS m/z: 413 (M+). 5g: Yield 61%; brown solid, mp 79-82 °C; 1H NMR (DMSO-d6) δ 8.34 (1H, br s), 7.37 (2H, s), 7.03 (4H, m), 6.56 (1H, d, J = 8.4 Hz), 6.44 (1H, d, J = 8.4 Hz), 4.84 (2H, br s), 3.36 (1H, s), 2.02 (6H, s); MS m/z: 355 (M+).
- 14.Xie L, Guo HF, Lu H, Zhuang XM, Zhang AM, Wu G, Ruan JX, Zhou T, Yu D, Qian K, Lee KH, Jiang S. J. Med. Chem. 2008;51:7689. doi: 10.1021/jm8003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larder BA, Kellam P, Kemp SD. Nature. 1993;365:451. doi: 10.1038/365451a0. [DOI] [PubMed] [Google Scholar]
- 16.Das K, Bauman JD, Clark AD, Frenkel YV, Lewi PJ, Shatkin AJ, Hughes SH, Arnold E. Proc. Natl. Acad. Sci. USA. 2008;105:1466. doi: 10.1073/pnas.0711209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludovici DW, De Corte BL, Kukla MJ, Ye H, Ho CY, Lichtenstein MA, Kavash RW, Andries K, de Bethune MP, Azijn H, Pauwels R, Lewi PJ, Heeres J, Koymans LMH, de Jonge MR, Van Aken KJA, Daeyaert FFD, Das K, Arnold E, Janssen PAJ. Bioorg. Med. Chem. Lett. 2001;11:2235. doi: 10.1016/s0960-894x(01)00412-7. [DOI] [PubMed] [Google Scholar]