Abstract
Rationale
Conditioned behavioral responses to discrete drug-associated cues can be modulated by the environmental context in which those cues are experienced, a process that may facilitate relapse in humans. Rodent models of drug self-administration have been adapted to reveal the capacity of contexts to trigger drug seeking, thereby enabling neurobiological investigations of this effect.
Objectives
We tested the hypothesis that dopamine transmission in the nucleus accumbens, a neural structure that mediates reinforcement, is necessary for context-induced reinstatement of responding for ethanol-associated cues.
Methods
Rats pressed one lever (active) for oral ethanol (0.1 ml; 10% v/v) in operant conditioning chambers distinguished by specific visual, olfactory, and tactile contextual stimuli. Ethanol delivery was paired with a discrete (4 s) light-noise stimulus. Responses on a second lever (inactive) were not reinforced. Behavior was then extinguished by withholding ethanol but not the discrete stimulus in a different context. Reinstatement, expressed as elevated responding for the discrete stimulus without ethanol delivery, was tested by placing rats into the prior self-administration context after administration of saline or the dopamine D1 receptor antagonist, SCH 23390 (0.006, 0.06, and 0.6 μg/side), into the nucleus accumbens core or shell.
Results
Compared with extinction responding, active lever pressing in saline-pretreated rats was enhanced by placement into the prior ethanol self-administration context. SCH 23390 dose-dependently reduced reinstatement after infusion into the core or shell.
Conclusion
These findings suggest a critical role for dopamine acting via D1 receptors in the nucleus accumbens in the reinstatement of responding for ethanol cues triggered by placement into an ethanol-associated context.
Keywords: Addiction, Reward, Relapse, Renewal, Self-administration, SCH 23390, Striatum, Cue, Environment
Introduction
Environmental contexts associated with drug consumption are an integral feature of addiction. Responses to discrete nondrug stimuli (cues) that become associated with the pharmacological effects of drug intake can be strongly modulated by the physical surroundings wherein those cues are experienced, a process that may facilitate relapse in abstinent drug users (Bouton 2002; Conklin and Tiffany 2002; Conklin et al. 2008). In preclinical models of relapse, placement into an environmental context associated with prior drug intake can elicit the reinstatement of instrumental and/or other conditioned behaviors that had previously been extinguished in a different context by withholding the drug (Crombag and Shaham 2002; Zironi et al. 2006; Tsiang and Janak 2006; Chaudhri et al. 2008b). These findings mirror earlier reports that conditioned fear is renewed upon reexposure to a cue in a context where that cue had previously been paired with an aversive event, following extinction of fear in a distinct context (Bouton and King 1983; Bouton 2002). Context-induced renewal has also been demonstrated for cues that predict natural reinforcers such as food pellets (Bouton et al. 1993; Nakajima et al. 2000) and sucrose (Hamlin et al. 2006; Chaudhri et al. 2008b). That contextual stimuli can influence behaviors associated with such a wide array of reinforcers highlights the generality of this phenomenon, and underscores the need for insight into the neurobiological mechanisms that mediate these effects.
Recent studies have identified the nucleus accumbens (NAc) as a primary region in the neural circuitry underlying contextual modulation of drug seeking (Bossert et al. 2006a, b, 2007; Chaudhri et al. 2008a; Fuchs et al. 2008). Located in the ventral forebrain, the NAc is viewed as comprising two functionally and anatomically dissociable subregions, the core and shell, both of which receive dopaminergic projections from the ventral tegmental area (Zahm and Heimer 1988; Brog et al. 1993; Pennartz et al. 1994). Dopamine release in the NAc has been shown in response to discrete drug-conditioned cues (Ito et al. 2000; Bassareo et al. 2007; Di Chiara and Bassareao 2007), and is hypothesized to mediate the influence of salient cues on behavior. NAc dopamine levels in rats increase significantly upon placement into an ethanol self-administration (EtOH-SA) chamber, suggesting that anticipation of access to EtOH, potentially signaled by the context, can activate dopamine systems (Katner and Weiss 1999). Systemic administration of the dopamine D1 family receptor antagonist, SCH 23390 [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepinehydrochloride], attenuates context-induced reinstatement of alcohol seeking (Liu and Weiss 2002; Hamlin et al. 2007), sucrose seeking (Hamlin et al. 2006), and cocaine seeking (Crombag et al. 2002). Furthermore, context-induced reinstatement of heroin seeking is reduced by localized infusions of SCH 23390 in the NAc shell, but not core (Bossert et al. 2007). Unimpaired dopamine neurotransmission therefore appears necessary for the facilitation of conditioned instrumental behavior by contextual stimuli, particularly in the NAc shell.
We tested the hypothesis that dopamine acting via D1 receptors in the NAc is required for context-induced reinstatement of responding for EtOH-associated discrete cues. Rats lever-pressed for access to oral EtOH in a distinctive environmental context where EtOH delivery was consistently paired with a discrete, compound light-noise stimulus. Behavior was then extinguished in a different context by withholding EtOH but not the discrete stimulus. Reinstatement of EtOH seeking was assessed by placing rats back into the prior EtOH-SA context after microinfusions of either saline or the dopamine D1 receptor antagonist SCH 23390 into the NAc core or shell.
Materials and methods
Subjects
Male, Long Evans rats (Harlan, Indianapolis, IN) weighing 260–280 g upon arrival were individually housed in ventilated polycarbonate chambers (colony room temperature, 21 ± 1°C; 12-h light/dark cycle with lights on at 7:00 a.m.). Rats had unrestricted access to standard rat chow and water (except on 4 days; see details below) throughout the study. All procedures were approved by the Gallo Center Institutional Animal Care and Use Committee and are in agreement with recommendations made by the National Research Council (NRC 1996).
Apparatus
Experimental sessions were conducted in operant conditioning chambers (Med Associates Inc., St. Albans, VT) located in a different room from where the rats were housed. Chambers were contained within ventilated sound-attenuating cubicles, and comprised of a clear Plexiglas ceiling, front door and back wall, paneled aluminum side walls and a stainless steel bar floor. The right wall featured a central port containing a circular fluid receptacle, into which ethanol was delivered via a 20 ml syringe attached to a pump located outside the sound attenuating cubicle. Chambers were outfitted with retractable levers flanking the port, a white-noise generator (28 V, 10–25 KHz, 3 dB), white stimulus lights (28 V, 100 mA) above each lever, and a white chamber light (28 V, 100 mA) located centrally near the ceiling on the left wall. EtOH delivery and stimulus presentations were controlled by a computer running Med PC IV (Med Associates) software, which also recorded responses on both levers.
Drugs
EtOH (10% v/v) was made by combining 95% EtOH in tap water. Sweetened EtOH was made by dissolving sucrose (10% w/v) in 10% EtOH. SCH 23390 (Sigma) was dissolved in sterile saline to obtain a concentration of 2 mg/ml for the highest dose (0.6 μg/0.3 μl). Additional doses (0.06 and 0.006 μg/0.3 μl) were obtained through serial dilution. Aliquots of each dose were stored at −20°C until use and protected from exposure to light. These doses are comparable to those used in similar behavioral procedures by other laboratories, and have been shown to have no effect on sucrose self-administration when infused into either the NAc core or shell (Bossert et al. 2007).
Ethanol preexposure
One week after arrival, rats were acclimated to the taste and pharmacological effects of EtOH (10% v/v) in their home cages. To ensure that they all sampled EtOH, water was removed and rats were given only EtOH for 3 days. Next, they received concurrent 24-h access to both water and sweetened EtOH (8–15 days), followed by water and EtOH (20 days). Rats were then habituated to limited EtOH access to accustom them to the period of EtOH availability during operant self-administration (EtOH for 1-h/day and water for 23-h/day; 20 days). Body weights and liquid volumes consumed were recorded daily. EtOH intakes (mean ± SEM) over the last 5 days of limited-EtOH access were 0.72 ± 0.06 and 0.93 ± 0.05 g/kg for the final cohorts (after determination of cannula placements) of core (n = 7) and shell (n = 9) implanted rats, respectively.
Surgery
Standard stereotaxic procedures were used to implant bilateral, 26-gauge guide cannulae (Plastics One, Roanoke, VA) targeting the NAc core and shell. Rats were anesthetized with isoflurane, their heads shaved, and placed in a stereotaxic frame (Kopf, Tujunga, CA). The scalp was incised to expose the skull, and bregma and lambda were used to estimate a flat skull position. Coordinates for cannula implantation were based on pilot surgeries: NAc core, AP+1.2, ML ±2.0, DV-3.8; NAc shell, AP+1.6, ML±1.0, DV-4.0. Microinfusions were conducted using 33 gauge injectors that protruded 3 mm below the base of the cannulae (final DV: core, −6.8; shell, −7.0). Cannulae were anchored to the skull surface with dental cement and metal screws, and occluded with metal obdurators of the same length. Rats were treated post-surgically with the analgesic buprenophine (0.1 mg/kg, i.m. single injection), and monitored daily to ensure regular weight gain.
Ethanol self-administration, extinction sessions, and reinstatement testing
One week after surgery, rats were water-restricted for 16 h and placed into the operant conditioning chambers for a single, overnight lever-press training session. Responding on the left (inactive) lever had no consequence, whereas responding on the right (active) lever produced a discrete, compound light-noise stimulus paired with EtOH delivery on a continuous reinforcement schedule (each response that did not occur during EtOH delivery was reinforced). The discrete stimulus comprised of the concurrent onset of white noise and the stimulus lights above both levers for 4 s. EtOH delivery (0.1 ml over 3 s) occurred 1 s after stimulus onset. The session ended upon self-administration of 20-ml EtOH. Contextual stimuli (see subsequent discussion) were not added to the chambers during lever-press training, in case being housed in chambers overnight produced aversive effects that might have become associated with the context.
Subsequently, rats were allowed to acquire lever pressing for EtOH in daily (Monday–Friday; between 0800–1100 h) 60-min EtOH-SA sessions. Session onset was signaled by the illumination of the white chamber light and insertion of both levers into the chamber. Active lever responses produced the compound stimulus and EtOH as described above, first on a continuous reinforcement schedule (days 1–14) and then a fixed-ratio three schedule (reinforcement delivered upon completion of three responses; FR3; days 15–27). Inactive lever responses were recorded but had no consequence. EtOH-SA occurred in either of two contexts that were distinct across visual, tactile, and olfactory domains. One context consisted of clear Plexiglas chamber walls, a green grid floor, and a strawberry-scented air freshener taped to the outside of the chamber door. The second context had black chamber walls, a smooth Plexiglas floor, and a single spray of acetic acid (30%) misted into the bedding below the floor. Assignment to context was counterbalanced across subjects based on EtOH-intake during prior EtOH preexposure, and the context used during EtOH-SA (referred to as Context A) was kept constant across EtOH-SA sessions.
Upon completion of 27 EtOH-SA sessions, rats were given extinction training in the context not used during prior EtOH-SA (referred to as Context B). Active lever responding activated the pump and produced the discrete stimulus on an FR3 schedule, but no EtOH was delivered. Except for the context change and the absence of EtOH, all other session parameters were identical to those used during EtOH-SA. On session 10 of extinction rats were habituated to the micro-infusion procedure with an infusion of sterile saline (0.3 μl/hemisphere; 0.3 μl/min) into the NAc core or shell. Injectors were left in place for 2 min post-infusion, after which rats were put back into their home-cages (located within the behavioral testing room) for 7–9 min, and then placed into the operant conditioning chambers. The same procedure was utilized in subsequent reinstatement tests.
Reinstatement tests occurred after a minimum of 13 extinction sessions, once the level of active lever responding averaged across three consecutive sessions had decreased to 12% of individual response levels averaged across the last 4 days of EtOH-SA. Using a within-subject, Latin-square design rats received intra-NAc saline or SCH 23390 (0.006, 0.06, and 0.6 μg/side) before being placed into the prior EtOH-SA context (Context A). Active lever responses generated the compound stimulus and activated the pump on an FR3 schedule, but no EtOH was delivered. Rats received at least five extinction sessions between consecutive tests. Table 1 indicates the Mean ± SEM number of extinction sessions before the first test and between consecutive tests.
Table 1.
Mean ± SEM number of extinction sessions before the first reinstatement test and between consecutive tests
| Test 1 | Test 2 | Test 3 | Test 4 | |
|---|---|---|---|---|
| Core (n = 7) | 23.86 ± 3.45 | 9.71 ± 1.13 | 7.43 ± 0.84 | 7.00 ± 0.76 |
| Shell (n = 9) | 16.22 ± 1.16 | 11.00 ± 1.33 | 10.00 ± 0.76 | 9.86 ± 1.12 |
Histology
Rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with 0.9% saline followed by 10% formalin. Brains were removed, post-fixed in formalin and 25% sucrose, sectioned (60 µm, coronal) and stained with cresyl violet to verify placement of cannulae and injector tips using light microscopy.
Statistical analyses
EtOH-SA was analyzed using data averaged across the last full week of EtOH-SA (5 days, FR3), and compared with extinction data averaged across three sessions before the first test. Analysis of variance (ANOVA) utilized phase (EtOH-SA, Extinction) and lever (active or inactive) as within-subject repeated measures and context type (see Methods for descriptions of two contexts used for EtOH-SA) and region (core, shell) as between-subject factors.
In addition to a manipulation of context, reinstatement tests involved a microinfusion that did not also occur during extinction. Thus, planned comparisons (t tests for paired-samples) were used to compare extinction baselines with behavioral results obtained when saline-pretreated rats were tested in the prior EtOH-SA context (Chaudhri et al. 2008a). There were no differences across extinction baselines before each of the four reinstatement tests for any of the dependent measures reported below (data not shown, F < 2, p = ns for all comparisons). The effect of SCH 23390 on reinstatement was analyzed using repeated-measures ANOVA with dose (0, 0.006, 0.06, or 0.6 μg/side) and lever as within-subject factors, and context type and region as between-subject factors. Separate analyses were conducted on the number of stimulus presentations earned, using ANOVA with dose as the within-subject factor, and context type and region as between-subject factors. The Huynh-Feltd test was used to correct for significant violations of homogeneity as determined by the Mauchly sphericity test. Significant main effects and interactions were subjected to targeted 2-way ANOVAs and paired-samples t tests. Analyses were conducted using SPSS (V11) with a significance level of α = 0.05.
Results
Histology
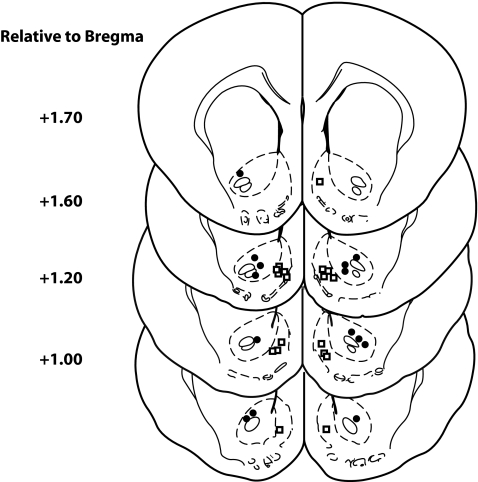
Figure 1 illustrates the placement of injector tips in the NAc core and shell. Rats were excluded if injector tips were not situated bilaterally within the core (n = 2 excluded, final n = 7) or shell (n = 1 excluded, final n = 9) as defined by the atlas of Paxinos and Watson (Paxinos and Watson 1997).
Fig. 1.
Placement of injector tips within the NAc core (n = 7) and shell (n = 9), according to the rat brain atlas of Paxinos and Watson (1997)
Ethanol self-administration
Behavioral data obtained during EtOH-SA sessions for core- and shell-implanted rats are summarized in Table 2. Responding was higher during prior EtOH-SA, compared with extinction responding before the first reinstatement test [Phase, F(1, 12) = 9.95, p < 0.01], and elevated on the active lever compared with the inactive lever [Lever, F(1, 12) = 85.17, p < 0.001]. There were no main effects or interactions with context type or region [F < 2, p = ns for all comparisons]. There was a significant Phase x Lever interaction [Phase x Lever, F(1, 12) = 69.57, p < 0.0001], indicating that active lever responding decreased in extinction compared with EtOH-SA (p < 0.001), with no change in responding on the inactive lever (p = ns). Active lever responding was also lower before the first test compared with day 1 of extinction [Table 2: Phase, F(1, 12) = 51.44, p < 0.00; Lever, F(32.33, p < 0.001; Phase x Lever, F(1, 12) = 28.92, p < 0.001; F < 2, p = ns for main effects and interactions involving Context Type or Region].
Table 2.
Behavioral measures (mean ± SEM) averaged across the last 5 days of EtOH self-administration (EtOH-SA), the first day of extinction (extinction day 1) and three extinction sessions before the first reinstatement test (extinction pretest)
| Active lever (FR3) | Inactive lever | EtOH intake (mls) | EtOH intake (g/kg) | ||
|---|---|---|---|---|---|
| Core n = 7 | EtOH-SA | 116.40 ± 7.53 | 6.46 ± 0.87** | 3.19 ± 0.20 | 0.51 ± 0.03 |
| Extinction (day 1) | 62.86 ± 14.62^ | 13.86 ± 3.42*,^ | – | – | |
| Extinction (pretest) | 8.95 ± 0.61^^,# | 8.33 ± 2.06 | – | – | |
| Shell n = 9 | EtOH-SA | 134.51 ± 10.37 | 6.98 ± 1.12** | 3.87 ± 0.29 | 0.61 ± 0.04 |
| Extinction (day 1) | 59.44 ± 8.78^ | 22.44 ± 4.67* | – | – | |
| Extinction (pretest) | 7.81 ± 0.88^^,# | 1.89 ± 0.37*,^,# | – | – |
Symbols denote significant outcomes from paired-samples t test comparisons
*p < 0.05; **p < 0.001 significantly different from corresponding active lever; ^p < 0.05; ^^p < 0.001 significantly different from same lever during EtOH-SA; #p < 0.05 significantly different from same lever on day 1 of extinction
The EtOH intake values achieved during self-administration (Table 2) are comparable to previous reports from our laboratory (Chaudhri et al. 2008a), and similar doses have been shown to produce detectable levels of EtOH in the NAc (Nurmi et al. 1999; 0.40 g/kg/h, voluntary oral consumption) and stimulate dopamine release in the NAc of rats (Bassareo et al. 2003; 0.33 g/kg, delivered via intraoral infusion).
Context-induced reinstatement of ethanol-seeking
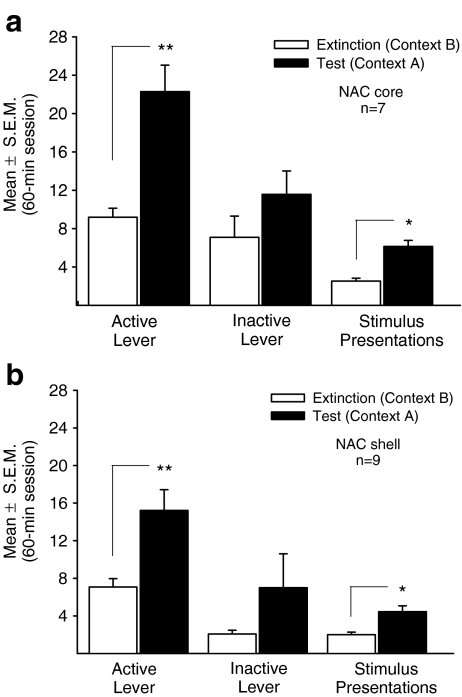
Figure 2 depicts behavioral measures obtained during extinction in Context B and at test upon placement into the prior EtOH-SA context (Context A) after a microinfusion of saline into the NAc core (Fig. 2a) or NAc shell (Fig. 2b). Planned t tests for paired-samples revealed a significant increase in active lever responding at test compared with extinction for core (p < 0.01) and shell (p < 0.01) implanted rats, indicative of context-induced reinstatement of EtOH seeking. The number of stimulus presentations earned was also enhanced by placement into the prior EtOH-SA context (core, p < 0.05; shell, p < 0.01). There was no change in responding on the inactive lever at test for either region, compared with extinction (p = ns for both comparisons).
Fig. 2.
Reinstatement of ethanol-seeking is triggered by placement into the prior ethanol self-administration context. Behavioral measures (Mean ± SEM) during extinction (open bars) and at test (filled bars) are depicted for nucleus accumbens core (a) and shell (b) implanted rats. Responding on the active lever and inactive lever, and number of stimulus presentations earned are shown. Extinction baselines were obtained using data averaged across three extinction sessions in Context B before the test. As test rats were infused with saline and placed into the prior ethanol self-administration context (Context A) where active lever responding produced the light-noise stimulus without ethanol delivery. **p < 0.01, *p < 0.05 test significantly different from corresponding extinction baseline
Effects of SCH 23390 in the NAC on context-induced reinstatement of ethanol seeking
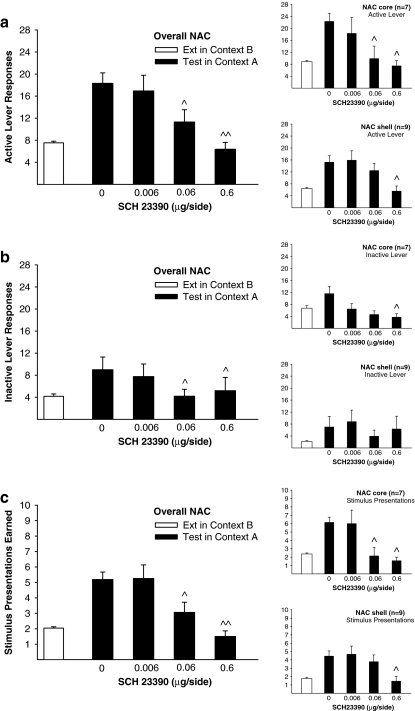
Blocking dopamine D1 receptors in the NAc dose-dependently reduced context-induced reinstatement of EtOH-seeking, relative to saline (Fig. 3). ANOVA revealed main effects of Dose [F(3, 36) = 10.13, p < 0.0001] and lever [F(1, 12) = 9.99, p < 0.01] and a significant Dose x Lever interaction [F(3, 36) = 4.40, p < 0.05]. There were no main effects or interactions involving Context Type and Region [F < 2, p = ns for all comparisons]. Therefore, the main panels in Fig. 3 present the combined findings from core and shell implanted rats. Collapsed across brain region, the main panel of Fig. 3a depicts a dose-dependent reduction in active lever responding after pretreatment with 0.06 (p < 0.05) and 0.6 (p < 0.001) µg/side of SCH 23390, compared with saline. Responding after pretreatment with 0.6 μg/side of SCH 23390 was also significantly reduced compared with 0.006 (p < 0.001) and 0.06 (p < 0.05) µg/side.
Fig. 3.
Reinstated ethanol-seeking triggered by placement into the prior ethanol self-administration context is dose-dependently attenuated by blocking dopamine D1 receptors in the nucleus accumbens (NAC). Main panels depict Mean (± SEM) responses on the active lever (a), inactive lever (b), and number of stimulus presentations earned (c) after pretreatment in NAC core and shell subregions combined (Overall NAC). Data from each subregion are shown in corresponding insets. There were no significant differences across 3-day extinction baselines before each of the four reinstatement tests. Therefore, open bars represent an extinction baseline in Context B obtained by collapsing data across extinction baselines for individual tests. At test (filled bars), rats were pretreated with saline or SCH 23390 and placed into the prior ethanol self-administration context (Context A) where active lever responding produced the light-noise stimulus without ethanol. ^^ p < 0.01, ^ p < 0.05 significantly different from saline
Overall responding on the inactive lever collapsed across the brain region is depicted in the main panel of Fig. 3b. Compared with saline, inactive lever responding was reduced after pretreatment with 0.06 μg/side (p < 0.05) and 0.6 μg/side (p < 0.05) of SCH 23390. Responding after pretreatment with 0.06 μg/side was also significantly reduced compared with the lowest dose of SCH 23390 (p < 0.05). Collapsed across brain region, active lever responding was greater than inactive lever responding after pretreatment with saline (p < 0.01), 0.006 μg/side (p < 0.01) and 0.06 μg/side (p < 0.01), but not after 0.6 μg/side (p = ns) of SCH 23390.
As expected from the lack of a main effect of region, separate ANOVAs conducted on data from core and shell subjects each indicate an effect of SCH23390 infusion on responding during the reinstatement test. For core implanted rats (Fig. 3a. inset), collapsed across Context Type, there were main effects of Dose [F(3, 18) = 5.19, p < 0.01] and Lever [F(1, 6) = 12.89, p < 0.05] but no Dose by Lever interaction [F(3, 18) = 2.25, p = ns]. Active lever responding was reduced after pretreatment with 0.06 (p < 0.05) or 0.6 μg/side (p < 0.001) of SCH 23390, compared with saline. Pretreatment with 0.6 μg/side of the dopamine D1 anatagonist in the core caused a reduction in responding on the inactive lever (Fig. 3b. inset), compared with saline (p < 0.05). Within the core, active lever responding was elevated compared with the inactive lever after pretreatment with saline (p < 0.05) and 0.006 μg/side (p < 0.05), but not at the two remaining doses of SCH 23390 (p = ns for each comparison).
Separate ANOVA conducted on test data from shell implanted rats (Fig. 3a. inset) collapsed across Context Type indicated a main effect of Dose [F(3, 24) = 6.09, p < 0.01] and Dose x Lever interaction [F(3, 24) = 3.52, p < 0.05], but no main effect of Lever [F(1, 8) = 3.23, p=ns]. Active lever responding was not different after pretreatment with saline, 0.006 or 0.06 μg/side of SCH 23390 (p > 0.05 for each comparison), but reduced after 0.6 μg/side of SCH 23390 compared with saline (p < 0.01). There were no dose-dependent changes in responding on the inactive lever after pretreatment with saline or SCH 23390 in the NAc shell (Fig. 3b. inset). Within the shell, active lever responding was higher than the inactive lever after pretreatment with 0.06 μg/side (p < 0.01) of SCH 23390, but not at the remaining doses (p = ns for each comparison). Further analyses revealed that the lack of discrimination in lever pressing at these doses was caused by one rat that responded substantially more on the inactive lever compared with the group average (± SEM) at each dose without that subject (saline, 35 vs. 3.50 ± 0.98; 0.006 μg/side, 37 vs. 5.25 ± 1.85; 0.06 μg/side, 19 vs. 2.00 ± 0.98; 0.6 μg/side, 40 vs. 2.13 ± 0.93). Upon excluding this subject, who was identified as a statistical outlier, active lever pressing was higher than inactive responding after pretreatment with saline (p < 0.01), 0.006 (p < 0.05) and 0.06 (p < 0.01) µg/side of SCH 23390, but not after the highest dose tested.
Importantly, there were no differences in the latency to press the active lever after pretreatment with saline or 0.6 μg/side of SCH 23390 for either core or shell implanted rats (Table 3), indicating that blocking dopamine D1 receptors did not affect locomotor activity in this paradigm.
Table 3.
Median latency (in minutes) to respond on the active lever during extinction (no infusion) and at test in the prior ethanol self-administration context (after pretreatment with saline or 0.6 ug/side of SCH 23390). There were no significant differences across extinction baselines (averaged across 3 days before the test) and between extinction and test data as indicated by the Wilcoxon’s signed-rank test
| Extinction (Context B) | Test (Context A) | |
|---|---|---|
| Core n = 7 | 1.08 | Saline: 0.66 |
| 1.47 | 0.6 μg/side: 0.26 | |
| Shell n = 9 | 2.25 | Saline: 1.14 |
| 4.45 | 0.6 μg/side: 1.30 |
Blocking dopamine D1 receptors in the NAc also caused a dose-dependent reduction in the number of stimulus presentations earned upon placement into the prior EtOH-SA context (Fig. 3c, main panel). ANOVA indicated a main effect of Dose [F(3, 36) = 11.36, p < 0.001], but not main effects or interactions with Context Type and Region [F < 2, p = ns for all comparisons]. The overall ANOVA is supported by separate analyses of each region. Within the core (Fig. 3c. inset) fewer stimulus presentations were earned after pretreatment with 0.06 (p < 0.05) and 0.6 (p < 0.001) µg/side of SCH 23390, compared with saline. There was no difference in the number of stimulus presentations earned after saline or 0.006 μg/side (p > 0.05) of SCH 23390 in the core. Within the shell (Fig. 3c. inset), earned stimulus presentations were reduced after pretreatment with 0.6 μg/side of SCH 23390, compared with saline, 0.006 and 0.06 μg/side of SCH 23390 (p < 0.01 for each comparison).
Effects of SCH 23390 in the NAc on the pattern of active lever responding during reinstatement
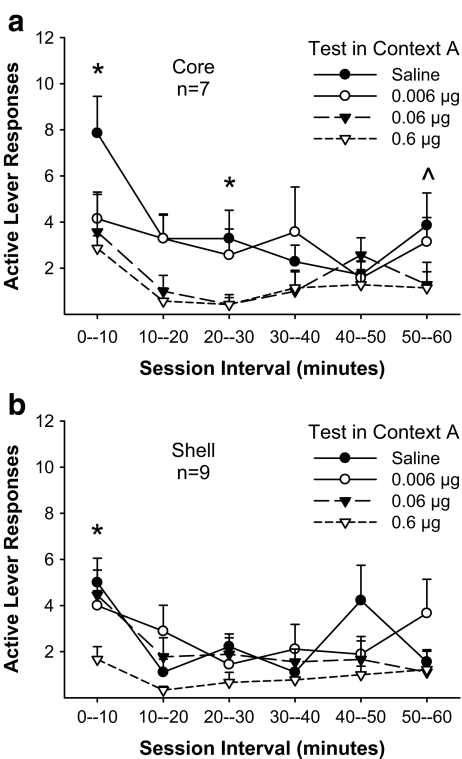
It is possible that the similar effect of SCH23390 in the core and shell resulted from diffusion of the drug from one region to the other. If that were the case, one might predict the onset of the drug effect to occur later in one region as compared with the other. Therefore, we analyzed responding within the test session across 10-min bins, separately for core and shell. Figure 4 illustrates active lever response patterns in 10-min intervals across test sessions in which rats received either saline or SCH 23390 (0.006, 0.06, or 0.6 μg/0.3 μl/side) before placement into the prior EtOH-SA context. Core implanted rats (Fig. 4a) showed a dose-dependent reduction in responding [Dose, F(5, 30) = 5.54, p < 0.01] across Time [F(3, 18) = 4.25, p < 0.05] with no Dose x Time interaction [F(15, 90) = 1.25, p = ns]. Follow-up analyses revealed lower response levels compared with saline after pretreatment with 0.06 [Dose, F(1, 6) = 6.31, p < 0.05] and 0.6 [Dose, F (1, 6) = 54.26] µg/side of SCH 23390. Importantly, the highest dose of SCH 23390 significantly attenuated responding within the first 10 min of testing (p < 0.05).
Fig. 4.
Blocking dopamine D1 receptors in the NAC core (a) or shell (b) reduces active lever responding within the first 10 min of testing. Data are Mean (± SEM) active lever responses in 10-min intervals across reinstatement tests where rats were infused with SCH 23390 (0, 0.006, 0.06, and 0.6 μg/side) and allowed to respond for the light-noise stimulus in the prior ethanol self-administration context (Context A). *p < 0.05, significant difference between saline and 0.6 μg/side of SCH 23390; ^p < 0.05 significant difference between saline and 0.06 μg/side of SCH 23390
Similarly, ANOVA on data obtained from shell implanted rats (Fig. 4b) indicated main effects of Dose [F(3, 24) = 5.99, p < 0.01] and Time [F(5, 40) = 3.71, p < 0.01] with no Dose x Time interaction [F(15, 120) = 1.58, p < 0.05]. Follow-up comparisons indicated less active lever responding after 0.6 μg/side of SCH 23390 compared with saline [Dose, F(1, 8) = 10.57, p < 0.05], caused by a reduction in responding during the first 10 min of testing (p < 0.05). Therefore, responding is decreased by 0.6 μg/side of SCH 23390 in the first 10 min of the test session after infusion into either the core or the shell.
Effects of SCH 23390 in the NAc on port entries made immediately after stimulus presentations
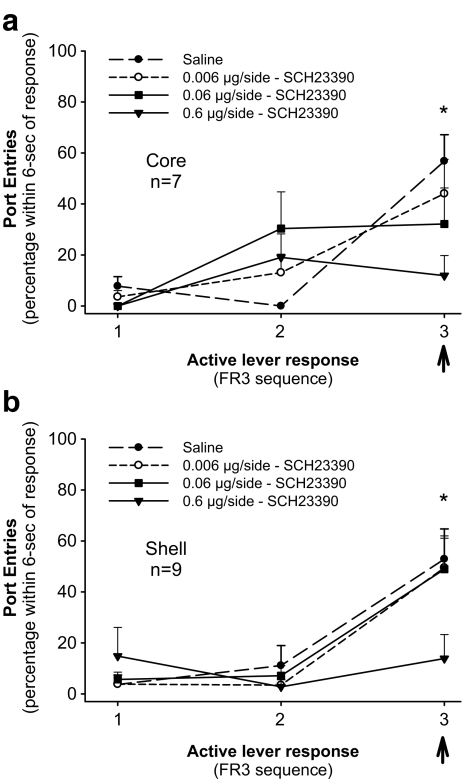
In addition to its effects on instrumental EtOH seeking, blocking dopamine D1-like receptors may also affect entries into the fluid delivery receptacle made in response to the EtOH-associated light-noise stimulus. To investigate this hypothesis post-hoc, we examined port entries made into the fluid-receptacle ≤6 s (rapid port entry) after each active lever press in a sequence of three active lever presses in which the third response produced the discrete light-noise stimulus (Fig. 5). Data are expressed as a percentage of the number of opportunities that a rat had at each point in the FR3 sequence (i.e., after the first press, second press, or third press of the sequence) to make a rapid port entry. Percentages were used in place of absolute values to account for both between-subject variability in the number of stimulus presentations earned and the dose-dependent decreases in active lever pressing and earned stimulus presentations. A duration of 6 s was chosen to allow subjects 2 s to transition between the active lever and the fluid receptacle after the offset of the 4 s light-noise stimulus. Data from each reinstatement test are depicted.
Fig. 5.
Port entries into the fluid receptacle are most frequently made upon completion of an FR3 sequence of active lever pressing, which results in presentation of the ethanol-associated discrete stimulus. Data are the percentage of times a port entry is made within 6 s after each active lever press in a reinforced FR3 sequence, at test after pretreatment with saline, 0.006, 0.06, or 0.6 μg/side of SCH 23390 into the nucleus accumbens core (a) or shell (b). The arrow indicates presentation of the EtOH-associated discrete stimulus. *p < 0.05, significant difference between saline and 0.6 μg/side dose of SCH 23390
For core implanted rats (Fig. 5a) ANOVA indicated a significant effect of Active Lever Response [F(2, 12) = 10.98, p < 0.01] and Active Lever Response x Dose interaction [F(6, 36) = 5.37, p < 0.0001], with no main effect of Dose [F(3, 18) = 1.96, p = ns]. After pretreatment with saline or 0.006 μg/side of SCH 23390, rats checked the fluid receptacle most often after the third press, compared with the first or second response (p < 0.01–p < 0.05). Compared with saline or 0.006 µg/side of SCH 23390, port entries made after the third response were significantly suppressed by pretreatment with 0.6 μg/side (p < 0.05), but not 0.06 μg/side of SCH 23390 (p > 0.05).
ANOVA conducted on data from shell implanted rats (Fig. 5b) indicated a significant effect of Active Lever Response [F(2, 16) = 20.39, p < 0.001] and Active Lever Response x Dose interaction [F(6, 48) = 2.90, p < 0.05], with no main effect of Dose [F(3, 24) = 0.92, p = ns]. Port entries were most frequently made after the third press compared with the first or second responses, after pretreatment with saline, 0.006 or 0.06 μg/side of SCH 23390 (p < 0.01 for all comparisons). Significantly fewer port entries were made after the third press after pretreatment with 0.6 μg/side of SCH 23990, compared with saline (p < 0.05) or to 0.006 μg/side of SCH 23390 (p < 0.05).
Discussion
Placement into an environmental context associated with prior EtOH-SA reinstated operant responding for an EtOH-associated stimulus following saline infusion into the NAc core or shell. Reinstated EtOH seeking was dose-dependently attenuated by blocking dopamine D1 receptors in the NAc, with similar effects in both core and shell subregions. These findings are the first to demonstrate a role for NAc dopamine neurotransmission in reinstated responding for EtOH cues, triggered explicitly by the return to an EtOH-associated context.
It is conceivable that SCH 23390 had similar effects when infused into the core or shell because of diffusion from one subregion to the other. However, SCH 23390 infused into the core or shell has been shown to produce distinct behavioral effects at volumes and concentrations similar to those used in the present study, including the high dose of 0.6 μg/0.3 μl/side (Bossert et al. 2007). Moreover, whereas diffusion would likely have influenced responding towards the middle or end of a test session, we observed a deficit in reinstatement within the first 10 min of testing for both subregions. Lastly, in separate studies, we have observed a significant decrease in EtOH-SA caused by SCH 23390 in the NAc core (0.6 μg/0.3 μl/side) but not shell (Chaudhri and Janak, unpublished data), indicating that we are able to achieve region-specific effects of blocking intra-accumbal dopamine in different behavioral paradigms. Thus, it is likely that the present results demonstrate a requirement for dopamine in the NAc core and shell. One intriguing interpretation, consistent with the literature, is that dopamine in the NAc core and shell is important for mediating the incentive salience of EtOH-associated discrete cues and environmental contexts, respectively.
Current research advocates that the NAc shell is particularly important for mediating the influence of contextual stimuli on conditioned behavior (Ito et al. 2008). Neurons in this subregion are activated by context-induced reinstatement of alcohol or sucrose seeking, and both reinstatement and neuronal activation are reduced by systemically administered SCH 23390 (Hamlin et al. 2006, 2007). The present finding that blocking dopamine D1 receptors in the NAc shell reduced reinstated EtOH seeking triggered by an EtOH-SA context is consistent with this literature, and with evidence that similar doses of SCH 23390 administered into the medial or lateral NAc shell decrease context-induced reinstatement of heroin seeking (Bossert et al. 2007).
In the present study, SCH 23390 administered into the NAc core also dose-dependently reduced EtOH seeking elicited by placement in the prior EtOH-SA context. In contrast, Bossert et al. (2007) showed that SCH 23390 in the core had no effect on context-induced reinstatement of heroin seeking. Rather, blocking dopamine D1 receptors in the core but not shell reduced cue-induced reinstatement of heroin seeking, elicited by response-contingent presentations of a discrete light-tone stimulus following extinction during which both heroin and the stimulus were withheld. Importantly, cue-induced reinstatement was tested in a context where heroin had not previously been self-administered, thereby enabling the effects of dopamine on cue-driven behavior to be explicitly assessed. Results from Bossert et al. (2007) support the hypothesis that contextual influences on behavior require dopamine acting on D1 receptors in the shell, and suggest that the associative properties of discrete drug-conditioned cues are mediated by D1 receptors in the core. Additional studies support a role for the core in responding to and mediating the incentive-salience of discrete conditioned cues (Fuchs et al. 2004; Di Ciano et al. 2008; Hollander and Carelli 2007; Ito et al. 2008; Blaiss and Janak 2009).
The reduction in reinstatement caused by blocking dopamine D1 receptors in the NAc core in the present study may also be attributable to an effect on responding for the discrete stimulus. To investigate this hypothesis we examined the effects of SCH 23390 in the core and shell on entries made into the fluid port where EtOH had previously been delivered. The analysis indicated that saline-treated rats more frequently checked the fluid receptacle after the third active lever press, compared with the first or second response in an FR3 sequence. This pattern of behavior suggests that port checking was driven by the EtOH-associated light-noise cue, although rats may also have learned to check the port after the third active lever response, regardless of the cue. A reduction in rapid port checking was observed after infusion of 0.6 μg/side of SCH 23390 in the NAc core, whereas infusion with 0.06 μg/side of SCH 23390 caused a nonsignificant trend towards increased port checking following the second lever press. We also found a similar reduction in rapid port entries after the third active lever press following infusion with 0.6 μg/side of SCH 23390 in the NAc shell. While these specific effects of SCH 23390 warrant further exploration, there were no clear differences in the decrease in port entries produced by the D1 antagonist after infusion into the core or shell.
In addition to effects on the active lever, pretreatment in the core with 0.6 μg/side of the dopamine D1 anatagonist caused a reduction in responding on the inactive lever, compared with saline. While this observation suggests that blocking D1-like receptors in the core may reduce general motor behavior, we did not observe a difference in latency to respond on the active lever after SCH 23390 pretreatment, compared to saline. In addition, 0.6 μg/side of SCH 23390 in the core does not affect high rates of responding maintained by sucrose (Bossert et al. 2007). Importantly, in the present study the reduction of active but not inactive lever-pressing after pretreatment with a lower dose of SCH 23390 (0.06 μg/side) in the NAc core indicates selectivity, at this dose, in the effects of blocking dopamine D1-like receptors in the core on reinstated EtOH seeking.
A recent study found that inactivation of both the NAc core and shell blocks context-induced reinstatement for cocaine in rats trained to lever press for i.v. cocaine in the absence of a response contingent cue (Fuchs et al. 2008). Although this study used reversible inactivation rather than blockade of dopamine receptors, it strongly suggests that further work is required to understand the specific contribution of the NAc core and shell subregions to context-modulated instrumental drug-seeking.
Irrespective of the distinct role of either subregion, a potential mechanism by which dopamine acting on D1 receptors in the NAc could mediate the influence of contexts on conditioned behavior is suggested by neuroanatomical findings indicating that the terminals of dopamine neurons within the NAc are located in close apposition to glutamatergic afferents from the hippocampus and basolateral amygdala (Sesack and Pickel 1990; French and Totterdell 2003). The basolateral amygdala (BLA) complex is critical for assigning motivational significant to discrete stimuli (Ito et al. 2006; Tye and Janak 2007; Tye et al. 2008), whereas the hippocampus is well-recognized for its role in contextual (Maren and Holt 2000) and spatial processing (Ito et al. 2006). Stimulation of afferents from these regions to the NAc can cause a prolonged increase in tonic dopamine release in the NAc, which is independent of dopamine neuron firing in the ventral tegmental area, and is thought to result from direct presynaptic activation of dopaminergic terminals by glutamate (Blaha et al. 1997; Floresco et al. 1998; Taepavarapruk et al. 2000). Dopamine released in this manner is reported to act in concert with glutamate to facilitate a robust, short-term potentiation in the firing of NAc medium spiny neurons evoked by the stimulation of excitatory hippocampal or BLA afferents (Floresco et al. 2001a), which requires activation of D1, but not D2 dopamine receptors (Floresco et al. 2001b, and see Floresco 2007 for review). Thus, the timely release of dopamine may selectively enhance the efficacy of subsets of glutamatergic inputs to the NAc that are simultaneously active.
If information about contextual and discrete stimuli is transmitted to the NAc via excitatory projections from the hippocampus and BLA, respectively, then dopamine acting on D1 receptors could regulate behavioral responding to these stimuli, either independently or when both occur in combination. In support of this hypothesis, roles for both the BLA and hippocampus have been identified in contextual modulation of reinstatement (Fuchs et al. 2005; Dayas et al. 2007; Fuchs et al. 2007; Marinelli et al. 2007; Atkins et al. 2008; Hamlin et al. 2008). Thus, if blocking dopamine D1 receptors in the NAc prevents the potentiation of excitatory inputs from the hippocampus and/or BLA onto medium spiny NAc neurons, then that process may impede the reinstatement of behavioral responding.
In conclusion, dopamine acting via D1 receptors in the NAc core and shell is required for reinstated responding for EtOH cues, triggered by the return to an EtOH-associated context. The current findings cannot address whether the similar effects of the D1 antagonist in the NAc shell and core affect distinct behavioral mechanisms. Therefore, future research should focus on more specifically isolating the independent and combined effects of discrete cues and contexts in EtOH seeking, in an effort to better isolate the neurochemical underpinnings of relapse.
Acknowledgements
This research was supported by NIAAA grant AA014925.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 2007;191(3):689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Aresu M, Aste A, Ariu T, Di Chiara G. Differential adaptive properties of accumbens shell dopamine responses to ethanol as a drug and as a motivational stimulus. Eur J NeuroSci. 2003;17:1465–1472. doi: 10.1046/j.1460-9568.2003.02556.x. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J NeuroSci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res. 2009;200:22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006a;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006b;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/S0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process. 1983;9:248–265. doi: 10.1037/0097-7403.9.3.248. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Rosengard C, Achenbach GG, Peck CA, Brooks DC. Effects of contextual conditioning and unconditional stimulus presentation on performance in appetitive conditioning. Q J Exp Psychol B. 1993;46:63–95. [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the "accumbens" part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J NeuroSci. 2008a;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008b;64:203–210. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037/0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33(6):1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Dopaminergic regulation of limbic-striatal interplay. J Psychiatry Neurosci. 2007;32:400–411. [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J NeuroSci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci. 2001a;21:6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001b;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/S0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J NeuroSci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200(4):545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, McNaughton BL, Everitt BJ. Selective excitotoxic lesions of the hippocampus and basolateral amygdala have dissociable effects on appetitive cue and place conditioning based on path integration in a novel Y-maze procedure. Eur J NeuroSci. 2006;23:3071–3080. doi: 10.1111/j.1460-9568.2006.04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28:6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20(19):7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. doi: 10.1016/S0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J NeuroSci. 2007;26:2815–2823. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Tanaka D, Urushihara K, Imada H. Renewal of extinguished lever-press responses upon return to the training context. Learn Motiv. 2000;31:416–431. doi: 10.1006/lmot.2000.1064. [DOI] [Google Scholar]
- NRC . Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nurmi M, Kiianmaa K, Sinclair JD. Brain ethanol levels after voluntary ethanol drinking in AA and Wistar rats. Alcohol. 1999;19:113–118. doi: 10.1016/S0741-8329(99)00022-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151:242–251. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH. Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol. 2006;38:81–88. doi: 10.1016/j.alcohol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci. 2007;27:3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity. J Comp Neurol. 1988;272:516–535. doi: 10.1002/cne.902720406. [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]