Abstract
Dendritic cells (DCs) play a pivotal role in the interface between immunity and maintenance of peripheral tolerance. The capture of immunoglobulin G (IgG)-containing immune complexes (ICs) by low-affinity Fcγ receptors (FcγRs) expressed on DCs may influence the immunogenicity/tolerogenicity of these cells, depending on the activating/inhibitory potential of FcγRs. Because of the key role that low-affinity FcγRs play in determining the magnitude of the response in IC-driven inflammation, these receptors are likely to play a role in autoimmune diseases, such as systemic lupus erythematosus (SLE). To evaluate if an altered expression of costimulatory molecules and/or FcγRs could account for disease severity, we evaluated the expression of these molecules on immature and mature DCs derived from peripheral blood monocytes of SLE patients and healthy donors. Our results show an increased expression of the costimulatory molecules CD40 and CD86. Furthermore, the ratio of CD86/CD80 is higher in SLE patients compared with healthy donors. Conversely, while the expression of activating FcγRs was higher on DCs from SLE patients, expression of inhibitory FcγRs was lower, compared with DCs obtained from healthy donors. As a result, the activating to inhibitory FcγR ratio was significantly higher in DCs from SLE patients. The altered ratio of activating/inhibitory FcγRs on mature DCs showed a significant correlation with the activity of SLE, as determined by the SLE Disease Activity Index (SLEDAI) score. We postulate that the increased ratio of activating/inhibitory FcγRs expressed on DCs from SLE patients can contribute to the failure of peripheral tolerance in the IC-mediated phase of autoimmune pathogenesis.
Keywords: dendritic cells, Fcγ receptors, systemic lupus erythematosus
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells specialized in the initiation of immune responses and with the unique capacity to activate naïve T cells.1–4 Normally, DCs can be found in two states, as immature DCs (iDCs) with the capacity to induce T-cell tolerance, or as mature DCs (mDCs), which are immunogenic and promote T-cell activation.2,3,5,6 iDCs reside in most peripheral tissues where they take up antigens continuously and transport them to draining lymph nodes for presentation to T cells in a tolerogenic manner. However, during an infection, pathogen-associated molecular pattern (PAMP) recognition promotes DC maturation and upregulation of the surface expression of costimulatory molecules.2,3,5,6 In the lymph nodes, mDCs present processed antigens to specific T cells in the presence of highly expressed surface costimulatory molecules in order to trigger T-cell immunity.
The generation of immune complexes (ICs) occurs as the physiological consequence of the encounter of antibodies with their cognate antigens. This process may occur in the setting of an immune response to an invading pathogen, but when a self-antigen is recognized, IC formation is also believed to underlie autoimmune pathogenesis.7–10 The Fc portion of the antibody component of the IC interacts with Fc receptors (FcγRs), which are expressed by a wide range of immune cells.3,9,11 Two major types of FcγRs have been described, either activating receptors or inhibitory receptors, depending on the immunotyrosine motifs transducing intracellular signalling.9,11,12 The balance of FcγR expression on DCs can determine two alternative phenotypes, the first corresponding to a mature and activating state capable of inducing T-cell immunity, and the second corresponding to an immature state inducing T-cell tolerance.3,9,11 As a result, the predominant receptor type determines the outcome of IC-induced DC response and T-cell activation. FcγRs are important effector molecules of humoral immunity and they are involved in the pathogenesis of autoimmune diseases characterized by the accumulation of ICs, such as systemic lupus erythematosus (SLE).8,10,13,14 Three different classes of FcγRs have been described in humans thus far: FcγRI (CD64); FcγRII (CD32); and FcγRIII (CD16).9 FcγRI is a high-affinity FcγR, while FcγRII and FcγRIII display low affinity for immunoglobulin G (IgG). FcγRII exists as two major isoforms – FcγRIIa (CD32a) and FcγRIIb (CD32b) – which serve divergent functions. While activating FcγRI and FcγRIII associate with the immunoreceptor tyrosine-based activation motif (ITAM)-containing γ-chain, FcγRIIa, another activating receptor, contains an ITAM in its cytoplasmic tail.9,15–17 Engagement of these activating FcγRs by ICs results in src and syk kinase-mediated activation of immune responses and IC internalization.9,15–17 Conversely, the cytoplasmic tail of FcγRIIb contains an immunotyrosine inhibitory motif (ITIM) capable of mediating inhibitory functions.9,15–17 Whereas the high-affinity FcγRI is able to bind monomeric IgG, low-affinity FcγRII and FcγRIII bind mostly IgG aggregated as immune complexes. The downstream signalling events that are triggered by FcRs differ according to subtype, in that IC engagement of either FcγRIIa or FcγRIII promotes an increased capability of DCs to stimulate T cells.2,3,9 By contrast, targeting of the inhibitory low-affinity FcγRIIb on DCs promotes inhibition of signalling triggered via activating FcγRs.3,16,17 Thus, the balance of activating/inhibitory low-affinity FcγRs on DCs may determine their immunogenic/tolerogenic potential.2,3
SLE is an autoimmune disorder characterized by autoantibody production and IC formation and deposition, which results in immunologically mediated tissue injury.13,18–20 Because DCs play a pivotal role at the interface of immunity and peripheral tolerance,2,3 and because of the key role that low-affinity FcγRs play in determining the magnitude of the response in IC-driven inflammation and autoimmune diseases,7,9,13,21,22 we set out to examine the expression of low-affinity FcγRs on DCs derived from peripheral blood monocytes of SLE patients and healthy donors. In addition, we addressed the question as to whether the expression of costimulatory molecules is altered in DCs from SLE patients. We found that DCs derived from SLE patients (SLE-DC) show an increase in the expression of costimulatory molecules, which is consistent with previous studies.23,24 In addition, while activating FcγRs also show an increase in expression, inhibitory FcγRs are expressed at a lower level when compared with healthy donors (healthy-DC). Remarkably, the ratios of CD86/CD80 and of activating/inhibitory FcγRs are higher on SLE-DC than on healthy-DC and show a significant correlation with the SLE Disease Activity Index (SLEDAI) score. Our results highlight a potential mechanism by which DCs play a pivotal role in the progression of SLE.
Materials and methods
Antibodies and reagents
Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) against human leucocyte antigen (HLA)-DR, CD16 (clone 3G8), CD32 (clone FLI8·26) and anIgG-γ1 isotype-matched-control; phycoerythrin (PE)-conjugated mAbs against CD11c, CD80 (clone L307·4) and an IgG-γ1 isotype-matched-control; and allophycocyanin (APC)-conjugated mAbs against CD86 and an IgG-γ1 isotype-matched control were all purchased from Becton Dickinson (San Jose, CA). FITC-conjugated and PE-conjugated mAb anti-CD32 (clone 7·3) were respectively obtained from Research Diagnostics and from Fitzgerald Industries International Inc. (Concord, MA). AlexaFluor488-conjugated mAb anti-CD32a (clone IV.3) and anti-CD32b (clone 2B6) were obtained from MacroGenics, Inc. (Rockville, MD). Lipopolysaccharide (LPS) was purchased from Sigma (St Louis, MO). Recombinant human interleukin-4 (IL-4) and recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) were purchased from Prospec-Tany Technogene Ltd (Rehovot, Israel).
Patients
In this cross-sectional study, 31 non-selected SLE patients treated at Hospital Clínico de la Pontificia Universidad Católica de Chile were analyzed. Exclusion criteria were pregnancy; patients undergoing dialysis or who were severely ill (such as those in the intensive care unit or who were haemodynamically unstable); patients with infections; and patients with drug-induced leucopoenia or anaemia. Patient characteristics are summarized in Table 1. As controls we included 20 healthy donors. In both the SLE and healthy-donor groups, 90% were women and the average age was 39 ± 14 years and 28 ± 5 years, respectively. In addition, we included three male liver-transplanted patients undergoing treatment with prednisone and cyclosporine (average age 58 ± 4 years). An informed consent form, obtained in accordance with the Ethics Committee of the School of Medicine, was signed by each patient before the collection of peripheral blood samples.
Table 1.
Clinical data of patients tested
| Organ involvement |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Age (years) | Disease duration (years) | Criteria1 | SLEDAI-2K | Treatment2 | ↑ DNA binding | Low complement | CNS | Kidney | Skin | Blood |
| P1 | F | 41 | 1 | 4 | 6 | Pred, HCQ | − | + | − | − | − | − |
| P2 | F | 57 | 3 | 3 | 12 | Pred, HCQ | − | − | + | − | + | − |
| P3 | F | 38 | 9 | 6 | 4 | NSAID, Pred, HCQ | − | + | − | − | + | − |
| P4 | F | 40 | 5 | 6 | 25 | Pred, HCQ, Aza | − | + | − | − | + | + |
| P5 | F | 51 | 7 | 4 | 15 | NSAID, HCQ | + | − | + | − | + | + |
| P6 | F | 22 | 1 | 4 | 12 | Pred | + | + | + | − | − | − |
| P7 | F | 27 | 0 | 5 | 16 | No | − | + | − | − | + | − |
| P8 | F | 27 | 1 | 4 | 29 | Pred, AZT | − | − | + | + | − | + |
| P9 | F | 34 | 20 | 5 | 4 | Pred | + | + | − | − | − | − |
| P10 | F | 65 | 8 | 6 | 9 | Pred, Mtx | − | − | − | − | + | + |
| P11 | F | 22 | 7 | 3 | 2 | Pred, AZT, HCQ | − | − | − | − | − | − |
| P12 | F | 26 | 1 | 5 | 0 | Pred, AZT, HCQ | − | − | − | − | − | − |
| P13 | F | 48 | 20 | 6 | 20 | Pred | + | + | − | + | + | − |
| P14 | F | 38 | 24 | 6 | 8 | Pred, HCQ | + | + | − | + | − | − |
| P15 | F | 33 | 0·4 | 4 | 10 | Pred | + | + | − | − | + | − |
| P16 | F | 23 | 9 | 6 | 28 | Pred, Ciclo, HCQ | + | + | + | + | − | − |
| P17 | M | 46 | 22 | 6 | 27 | NSAID, Pred, Lfm | + | + | − | + | + | + |
| P18 | F | 24 | 1 | 5 | 2 | HCQ | − | − | − | − | + | − |
| P19 | F | 45 | 6 | 5 | 12 | Pred | − | − | + | − | + | + |
| P20 | M | 46 | 0·6 | 4 | 4 | Pred, HCQ | − | − | − | − | − | − |
| P21 | F | 39 | 12 | 6 | 14 | NSAID, Pred, Ritux | − | − | − | − | − | − |
| P22 | F | 53 | 15 | 4 | 3 | Pred, HCQ | + | + | − | − | − | − |
| P23 | F | 68 | 18 | 4 | 4 | No | + | + | − | − | − | − |
| P24 | F | 25 | 10 | 4 | 12 | HCQ | − | − | + | − | − | − |
| P25 | F | 45 | 10 | 5 | 10 | Pred, HCQ | − | − | + | − | + | − |
| P26 | F | 64 | 11 | 7 | 10 | NSAID, Pred | − | − | − | − | + | − |
| P27 | F | 56 | 30 | 5 | 8 | Pred, HCQ | − | − | − | − | + | − |
| P28 | F | 18 | 0·2 | 4 | 8 | NSAID | − | − | − | − | + | + |
| P29 | F | 18 | 0·2 | 6 | 13 | No | + | + | − | − | + | − |
| P30 | F | 50 | 20 | 4 | 2 | Pred, HCQ | + | − | − | − | − | − |
| P31 | F | 23 | 0·1 | 4 | 16 | Pred, HCQ | − | − | + | + | − | − |
| Summary | ||||||||||||
| Gender (M/F) % | Age | Disease duration (years) | SLEDAI-2K | |||||||||
| 90/10 | 39 ± 14 | 9 ± 8 | 11 ± 8 | |||||||||
American College of Reumatology criteria for SLE.
AZA, azacortine; AZT, azathioprine; Ciclo, ciclophosphamide; HCQ, hydroxychloroquine; Lfm, leflunomide; Mtx, methotrexate; NSAID, non-steroidal anti-inflammatory drug; Pred, prednisone; Ritux, rituximab.
CNS, central nervous system; SLEDAI, systemic lupus erythematosus Disease Activity Index.
Generation of monocyte-derived DCs
Human peripheral blood mononuclear cells (PBMCs) were separated from whole blood using the standard Ficoll centrifugation method. Monocytes were obtained using the adherence method.25 Briefly, PBMCs (3 × 106 cells/ml) were incubated in serum-free XVIVO-15 medium (Bio-Whittaker, Walkersville, MD) supplemented with 1% autologous serum and 50 μg/ml of gentamycin (Calbiochem, San Diego, CA) (DC-medium) for 2 hr at 37°. Adherent cells were washed four times with prewarmed serum-free XVIVO-15 medium (Bio-Whittaker) and were then cultured in DC-medium at 37°. Monocytes were differentiated to iDCs over 6 days by the addition of 1000 U/ml of IL-4 and 1000 U/ml of GM-CSF on days 0, 3 and 5. DC maturation was triggered by the addition of LPS (5 μg/ml) for an additional 48 hr. DC immunophenotypes were confirmed by flow cytometry using specific antibodies against surface markers. The iDCs obtained were CD4−, CD8−, CD14−, CD40−, CD11c+, HLA-DR+, CD80+, CD83+, CD86+ and CD209+. After stimulation with LPS, the expression of HLA-DR, CD40, CD80, CD83 and CD86 was significantly increased in mDCs compared with iDCs.
Immunostaining
Cells were washed with phosphate-buffered saline (PBS), resuspended at 2 × 106 cells/ml (50 μl/tube), and incubated with FITC-, PE- and APC-conjugated antibodies for 30 min at 4°. FITC-, PE- and APC-conjugated isotype-matched antibodies were used as negative controls. Cells were washed with PBS, fixed with 1% formaldehyde in PBS and analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Data analyses
Data analyses and statistical analysis were performed using prism 4 software (Graph Pad Software, Inc., San Diego, CA). For statistical analyses we used the unpaired Student’s t-test. P values below 0·05 were considered statistically significant. Correlation analyses were performed using the Spearman two-tailed correlation test with a confidence interval of 95%. Flow cytometry data were analyzed using cellquest Pro Software (BD Biosciences) and WinMDI 2·9 (http://facs.scripps.edu/software.html).
Results
DCs derived from SLE patients display altered expression of costimulatory molecules
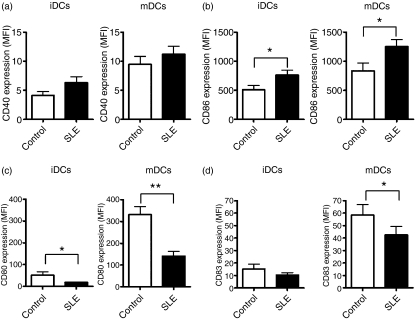
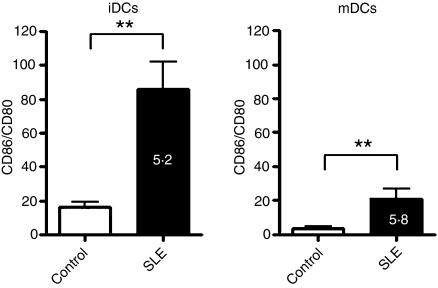
Given that the expression of costimulatory molecules on the surface of DCs critically determines whether an interacting T cell is activated or tolerized,2,3,26,27 we analyzed the expression of CD40, CD80, CD83 and CD86 on the surface of immature and LPS-matured DCs obtained from 31 SLE patients (Table 1) and from 20 healthy donors. As expected, expression of these surface molecules was augmented, after LPS-promoted maturation, on the surface of both iDCs and mDCs (Fig. 1). Although this trend did not reach statistical significance, CD40 was more highly expressed on iDCs and mDCs from SLE patients than on iDCs and mDCs from healthy donors (Fig. 1a). However, another important costimulatory molecule, CD86, was expressed at a significantly higher level on both iDCs and mDCs of SLE patients, which is consistent with previous studies.23,24 Conversely, the levels of expression of CD80 and CD83 were significantly decreased on mDCs from SLE patients (Fig. 1c,d), while only the level of expression of CD80 was significantly decreased on iDCs from SLE patients, compared with healthy donors (Fig. 1c,d). Although CD80 and CD86 share structural similarities, it was recently suggested that these two molecules have contrasting effects on regulatory T cells (Tregs),28 in that CD86 inhibits, while CD80 enhances, their suppressive effect. We hypothesized that in the setting of SLE, the Treg-suppressive function would be diminished, in part because of an unbalanced ratio of CD86/CD80 expression. As shown in Fig. 2, the ratio of CD86/CD80 expression was approximately fivefold and sixfold higher on iDCs and mDCs, respectively, derived from SLE patients compared with healthy donors.
Figure 1.
Dendritic cells derived from patients with systemic lupus erythematosus (SLE) have an altered expression of costimulatory molecules on their surface. Immature dendritic cells (iDCs) or dendritic cells matured with 5 μg/ml of lipopolysaccharide (LPS) (mDCs) obtained from SLE patients or from healthy donors were labelled with specific conjugated monoclonal antibodies directed against CD11c and CD40, CD83, CD86 or CD80 and analyzed using flow cytometry. The mean fluorescence intensity (MFI) of human leucocyte antigen (HLA)-DR+ CD11c+ cells from 20 healthy donors and from 31 SLE patients were plotted for CD40 (a), CD86 (b), CD80 (c) and CD83 (d) expression. White bars represent healthy donors (Control) and black bars represent SLE patients (SLE). Data represent mean ± standard error of the mean (SEM). *P < 0·05; **P < 0·01.
Figure 2.
The ratio of CD86/CD80 expression is abnormally high on dendritic cells (DCs) from patients with systemic lupus erythematosus (SLE). The ratio of CD86/CD80 expression was analyzed on immature and mature DCs from SLE patients and from healthy donors. The bars show the ratios of CD86 : CD80 expression, calculated from the data presented in Fig. 1b,c. White bars represent healthy donors and black bars represent SLE patients. The results represent the mean ± standard error of the mean (SEM). *P < 0·05; **P < 0·01.
FcγRs on DCs derived from SLE patients display an overactivation expression pattern
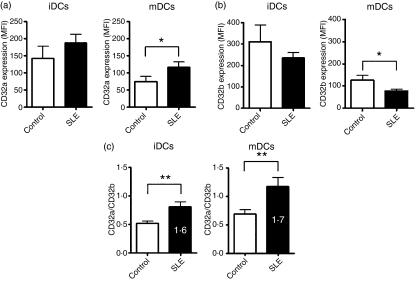
The expression of low-affinity FcγRs is tightly linked to the maturation status of DCs,3,9 and these phenotypic changes have important functional consequences on the ability of DCs to stimulate either immunity or tolerance. We therefore compared the expression of low-affinity FcγRs, including FcγRIII (CD16), FcγRIIa (CD32a) and FcγRIIb (CD32b), on the surface of DCs from SLE patients and healthy donors. In both SLE patients and healthy donors, CD16 expression was very low on the surface of DCs (data not shown). Conversely, both CD32a and CD32b were highly expressed on the surface of iDCs obtained from SLE patients and controls, and in both groups, this expression was significantly decreased after LPS-induced maturation (Fig. 3a,b). As shown in Fig. 3a, the expression of the activating receptor CD32a was slightly increased on iDCs and significantly increased on mDCs from SLE patients, whereas the expression of the inhibitory receptor CD32b was slightly lower on iDCs and significantly lower on mDCs from SLE patients compared with healthy donors (Fig. 3b). It has been reported that the balance between activating and inhibitory low-affinity FcγRs is critical in determining the ability of DCs to promote immunity or tolerance.29,30 In order to explore this concept in our model system, we evaluated the CD32a/CD32b ratio on iDCs and mDCs. Our analysis showed that this ratio was significantly increased in both iDCs and mDCs from SLE patients compared with healthy donors (Fig. 3c).
Figure 3.
Fcγ receptors (FcγRs) on dendritic cells (DCs) from patients with systemic lupus erythematosus (SLE) present an expression pattern skewed towards an overactivated DC phenotype. Expression of the activating receptor CD32a (a) and of the inhibitory receptor CD32b (b) were analyzed on immature DCs (iDCs) or on DCs matured with 5 μg/ml of lipopolysaccharide (LPS) (mDCs) obtained from SLE patients or from healthy donors. Graphs represent the mean fluorescence intensity for each antibody staining (c) The ratio of expression between CD32a and CD32b was plotted for iDCs and mDCs, for SLE patients and healthy donors. White bars represent healthy donors (Control) and black bars represent SLE patients (SLE). The results show the mean ± standard error of the mean (SEM). *P < 0·05; **P < 0·01.
The observation that DCs obtained from liver-transplanted patients treated with immunosuppressive drugs showed no significant differences regarding the expression of FcγRs or costimulatory molecules compared with the DCs from healthy donors (Fig. S1), supports the notion that the altered phenotype shown by DCs derived from SLE patients was not caused by an unspecific effect of the immunosuppressive treatment.
The altered ratio of activating/inhibitory FcγRs is correlated with SLE disease activity
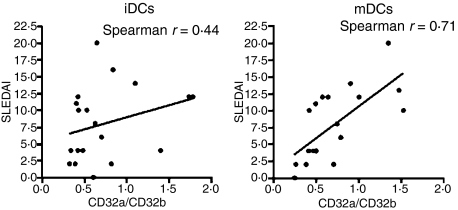
To investigate, in more detail, the relevance of the altered ratio between the activating receptor CD32a and the inhibitory receptor CD32b, we examined whether higher ratios were associated with disease activity among a cohort of 20 SLE patients, as assessed using the SLEDAI score.31 As shown in Fig. 4, analysis of mDCs showed a significant correlation between the SLEDAI score and the CD32a/CD32b ratio (Fig. 4), determined using the Spearman two-tailed test, with a Spearman r value of 0·71 (P value of 0·0006). By contrast, there was no significant correlation between the SLEDAI score and the CD32a/CD32b ratio for iDCs (Fig. 4), suggesting that mDCs could be more important in the progression of the disease.
Figure 4.
Alterations in the ratio of activating/inhibitory Fcγ receptors (FcγRs) correlate with the severity of systemic lupus erythematosus (SLE). The SLE Disease Activity Index (SLEDAI) was plotted for each of 20 patients against each respective CD32a/CD32b ratio for immature dendritic cells (iDCs) and for mature dendritic cells (mDCs). Statistical analysis shows a Spearman r value of 0·44 for iDCs, and a Spearman r value of 0·71 for iDCs (P values of 0·06 and 0·0006, respectively).
Discussion
It is well accepted that maturation status is linked with the ability of DCs to induce either immunity or tolerance.2,3,26,27 Because classical costimulatory molecules and also low-affinity FcγRs have recently been reported to be strongly involved in the maturation status of DCs,2–4,9,22 we explored, in this study, the expression of these key molecules in patients with SLE.
Our results allowed us to conclude that the balance of activating/inhibitory low-affinity FcγRs is augmented in DCs from SLE patients, which, in turn, suggests that these DCs can be more immunogenic or less tolerogenic than DCs from healthy donors. To our knowledge, this is the first report describing an imbalance of the expression of low-affinity FcγRs on DCs derived from SLE patients.
While a genetic polymorphism on the promoter sequence of the CD32b gene was shown previously to cause a reduced expression of this receptor on B cells from SLE patients,32 no such decrease was observed on iDCs from the same individuals. Our data extend this knowledge by showing significant differences on the expression of CD32a and CD32b on mDCs from SLE patients as compared with mDCs from healthy controls. It remains to be evaluated whether the differences in receptor expression seen in our patients are caused by polymorphisms in the promoter regions of CD32a and CD32b. This notion is supported by the observation that polymorphism in the promoter of the FCGR2B gene can increase the susceptibility to SLE.33–35
In addition, our findings show a clear up-regulation of CD86 and a clear down-modulation of CD80 and CD83 on the surface of DCs from SLE patients, compared with healthy controls. It is noteworthy that the related costimulatory molecules CD80 and CD86 are deregulated in an opposite way in DCs from SLE patients, in light of the recent report that CD80 promotes costimulation for Tregs, whereas CD86 impairs activation of these cells.28 Therefore, by assessing the CD86/CD80 ratio, it is possible to infer whether DCs have the potential to promote or to inhibit the suppressive function of Tregs. As DCs derived from our cohort of SLE patients showed an elevated CD86/CD80 ratio, our data suggest that, in conjunction with the activated state of DCs conferred by the high CD32a/CD32b ratio, these antigen-presenting cells may also be capable of inhibiting Treg function and further amplifying autoimmunity. Most strikingly, we found that higher ratios of the activating/inhibitory FcγRs expressed on mature DCs were associated with higher disease activity, as measured by the SLEDAI score. This represents a potential biomarker for disease activity, and also suggests a target for therapeutic intervention. DC maturation therefore represents a plausible pathway for intervention, given that this cell type bears the most potential in both inducing and amplifying autoimmune pathogenesis, through its ability to license autoreactive naïve T cells.
In conclusion, our data demonstrate that DCs from SLE patients display an altered expression of key receptors on their surface, consisting of an increased ratio of activating/inhibitory low-affinity FcγRs and an altered expression of costimulatory molecules, which could contribute to the loss of peripheral tolerance. These findings may help to elucidate, in greater detail, the emerging role of DCs in autoimmune diseases and could also help in the development of novel immunotherapeutic strategies for the treatment of IC-related diseases.
Acknowledgments
We would like to thank Dr M. Wiesendanger for critically reading the manuscript and Dr Rosa Perez for giving us blood samples from liver-transplanted patients. We are also grateful to Carla Henríquez and Paula Zamora for their help on patient monitoring and DC preparation, respectively. This work was supported by grants from FONDECYT 1085281, 1070352, 3070018 and Millennium Nucleus on Immunology and Immunotherapy P04/030-F. LC is a CONICYT fellow.
Disclosure
The authors declare no financial or commercial conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Phenotypic analysis of dendritic cells derived from prednisone and cyclosporine treated liver-transplanted patients.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Gonzalez PA, Carreno LJ, Figueroa CA, Kalergis AM. Modulation of immunological synapse by membrane-bound and soluble ligands. Cytokine Growth Factor Rev. 2007;18:19–31. doi: 10.1016/j.cytogfr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Iruretagoyena MI, Wiesendanger M, Kalergis AM. The dendritic cell-T cell synapse as a determinant of autoimmune pathogenesis. Curr Pharm Des. 2006;12:131–47. doi: 10.2174/138161206775193145. [DOI] [PubMed] [Google Scholar]
- 3.Kalergis AM. Modulation of T cell immunity by TCR/pMHC dwell time and activating/inhibitory receptor pairs on the antigen-presenting cell. Curr Pharm Des. 2003;9:233–44. doi: 10.2174/1381612033392062. [DOI] [PubMed] [Google Scholar]
- 4.Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CA., Jr How the immune system works to protect the host from infection: a personal view. Proc Natl Acad Sci USA. 2001;98:7461–8. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 7.Kleinau S. The impact of Fc receptors on the development of autoimmune diseases. Curr Pharm Des. 2003;9:1861–70. doi: 10.2174/1381612033454414. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz HM, Herrmann M, Kalden JR. The pathogenesis of autoimmune diseases. Scand J Clin Lab Invest Suppl. 2001;235:16–26. doi: 10.1080/003655101753352004. [DOI] [PubMed] [Google Scholar]
- 9.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 10.Wollina U. Immune complexes – pathogenetic factors of autoimmune systemic lupus erythematosus. Allerg Immunol (Leipz) 1984;30:3–13. [PubMed] [Google Scholar]
- 11.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 12.Billadeau DD, Leibson PJ. ITAMs versus ITIMs: striking a balance during cell regulation. J Clin Invest. 2002;109:161–8. doi: 10.1172/JCI14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown EE, Edberg JC, Kimberly RP. Fc receptor genes and the systemic lupus erythematosus diathesis. Autoimmunity. 2007;40:567–81. doi: 10.1080/08916930701763710. [DOI] [PubMed] [Google Scholar]
- 14.Thien M, Renger D, Deicher H, Pichler WJ. Alteration of Fc-receptor phenotype and proliferative capacity of Fc-IgG-receptor positive lymphocytes through interaction with soluble immune complexes of patients with SLE or RA. Rheumatol Int. 1985;5:127–32. doi: 10.1007/BF00541332. [DOI] [PubMed] [Google Scholar]
- 15.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189:179–85. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakov N. ITIMs and ITAMs. The Yin and Yang of antigen and Fc receptor-linked signaling machinery. Immunol Res. 1997;16:85–100. doi: 10.1007/BF02786325. [DOI] [PubMed] [Google Scholar]
- 17.Pan L, Pei P. Signaling transduction by IgG receptors. Chin Med J (Engl) 2003;116:487–94. [PubMed] [Google Scholar]
- 18.Anolik JH. B cell biology and dysfunction in SLE. Bull NYU Hosp Jt Dis. 2007;65:182–6. [PubMed] [Google Scholar]
- 19.Cohen PL. T- and B-cell abnormalities in systemic lupus. J Invest Dermatol. 1993;100:69S–72S. doi: 10.1111/1523-1747.ep12355631. [DOI] [PubMed] [Google Scholar]
- 20.Driver CB, Ishimori M, Weisman MH. The B cell in systemic lupus erythaematosus: a rational target for more effective therapy. Ann Rheum Dis. 2008;67:1374–81. doi: 10.1136/ard.2007.076745. [DOI] [PubMed] [Google Scholar]
- 21.Inman RD. Immune complexes in SLE. Clin Rheum Dis. 1982;8:49–62. [PubMed] [Google Scholar]
- 22.Ivan E, Colovai AI. Human Fc receptors: critical targets in the treatment of autoimmune diseases and transplant rejections. Hum Immunol. 2006;67:479–91. doi: 10.1016/j.humimm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Decker P, Kotter I, Klein R, Berner B, Rammensee HG. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006;45:1087–95. doi: 10.1093/rheumatology/kel061. [DOI] [PubMed] [Google Scholar]
- 24.Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol. 2006;177:5878–89. doi: 10.4049/jimmunol.177.9.5878. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza-Naranjo A, Saez PJ, Johansson CC, et al. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J Immunol. 2007;178:6949–57. doi: 10.4049/jimmunol.178.11.6949. [DOI] [PubMed] [Google Scholar]
- 26.Foell J, Mittler RS. Costimulatory molecules as immunotherapeutic targets in systemic lupus erythematosus. Springer Semin Immunopathol. 2006;28:153–62. doi: 10.1007/s00281-006-0039-y. [DOI] [PubMed] [Google Scholar]
- 27.Inman BA, Frigola X, Dong H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer Drug Targets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–84. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 29.Herrada AA, Contreras FJ, Tobar JA, Pacheco R, Kalergis AM. Immune complex-induced enhancement of bacterial antigen presentation requires Fcgamma receptor III expression on dendritic cells. Proc Natl Acad Sci USA. 2007;104:13402–7. doi: 10.1073/pnas.0700999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002;195:1653–9. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–13. doi: 10.1002/art.1780400506. [DOI] [PubMed] [Google Scholar]
- 32.Su K, Yang H, Li X, Gibson AW, Cafardi JM, Zhou T, Edberg JC, Kimberly RP. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178:3272–80. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blank MC, Stefanescu RN, Masuda E, et al. Decreased transcription of the human FCGR2B gene mediated by the −343 G/C promoter polymorphism and association with systemic lupus erythematosus. Hum Genet. 2005;117:220–7. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 34.Su K, Li X, Edberg JC, Wu J, Ferguson P, Kimberly RP. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. II. Differential binding of GATA4 and Yin-Yang1 transcription factors and correlated receptor expression and function. J Immunol. 2004;172:7192–9. doi: 10.4049/jimmunol.172.11.7192. [DOI] [PubMed] [Google Scholar]
- 35.Su K, Wu J, Edberg JC, Li X, Ferguson P, Cooper GS, Langefeld CD, Kimberly RP. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol. 2004;172:7186–91. doi: 10.4049/jimmunol.172.11.7186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.