Abstract
Vaccinia virus (VACV) is the current live virus vaccine used to protect humans against smallpox and monkeypox, but its use is contraindicated in several populations because of its virulence. It is therefore important to elucidate the immune evasion mechanisms of VACV. We found that VACV infection of antigen-presenting cells (APCs) significantly decreased major histocompatibility complex (MHC) II antigen presentation and decreased synthesis of 13 chemokines and cytokines, suggesting a potent viral mechanism for immune evasion. In these model systems, responding T cells were not directly affected by virus, indicating that VACV directly affects the APC. VACV significantly decreased nitric oxide production by peritoneal exudate cells and the RAW macrophage cell line in response to lipopolysaccharide (LPS) and interferon (IFN)-γ, decreased class II MHC expression on APCs, and induced apoptosis in macrophages and dendritic cells. However, VACV decreased antigen presentation by 1153 B cells without apparent apoptosis induction, indicating that VACV differentially affects B lymphocytes and other APCs. We show that the key mechanism of VACV inhibition of antigen presentation may be its reduction of antigenic peptide loaded into the cleft of MHC class II molecules. These data indicate that VACV evades the host immune response by impairing critical functions of the APC.
Keywords: antigen presentation, apoptosis, cytokine, macrophage, major histocompatibility complex, poxvirus, vaccinia
Introduction
Poxviruses are large, double-stranded DNA viruses (∼200 kb) that replicate in the cytoplasm of host cells.1 Poxviruses have a world-wide distribution and are highly successful pathogens, infecting a tremendous variety of animals including insects, reptiles, birds and mammals. The most infamous member of Family Poxviridae is smallpox, which is estimated to have caused 500 million human deaths in the 1900s before its eradication from nature.2 The eradication of smallpox was achieved by the most successful vaccination campaign in history using a related virus, vaccinia virus (VACV). Today there remain multiple poxvirus threats to humans, including the use of smallpox as a bio-terrorism weapon in a now largely unvaccinated population. New poxviruses are identified each year in animal populations, and several zoonotic poxviruses appear to be emerging world-wide: Cantagalo in South America,3 Tanapox in Africa, Europe and the USA,4,5 and buffalopox in India.6 Molluscum contagiosum virus causes wart-like lesions, occurs commonly,7 is now emerging as a sexually transmitted disease, and accounts for approximately 300 000 doctor visits each year in the USA.8 The most dangerous poxvirus extant today is monkeypox virus, which causes a smallpox-like illness in humans, is endemic to Africa, and caused an outbreak in humans in the USA in 2003.9 Studying the immune evasion tactics of this virus family will shed light on how these viruses evolved into such successful pathogens.
While VACV is used as a live virus vaccine, it can also be pathogenic. In a report on almost 39 000 volunteers vaccinated as first responders, it was found that 1 of every 450 vaccinees had to be hospitalized because of adverse vaccine reactions and that 1 death occurred per 13 000 vaccinations.10 Vaccination is contraindicated for individuals with any history of eczema or immunodeficiency disorders because fatalities may occur. Otherwise-healthy individuals can acquire a disseminated poxvirus rash, myopericarditis, and rarely fatal encephalitis from vaccination.10–13 Understanding viral evasion mechanisms will aid in developing safer vaccines.
For healthy individuals, an effective immune response limits viral replication of the less pathogenic poxviruses. However, poxviruses encode a plethora of immunomodulatory proteins, including proteins that block apoptosis, chemokine and cytokine binding and synthesis, and cell signalling.14–16 In addition, poxviruses directly infect human and rodent immune cells both in vivo and in vitro, including lymphocytes, natural killer (NK) cells, and monocytes/macrophages17,18 and have been shown to decrease antigen presentation in several types of antigen-presenting cell (APC).19,20 We wanted to further explore the effects of VACV on antigen presentation interactions between APCs and the responding T cells and were especially interested to study primary APCs responding to an inflammatory stimulus, as this has not been previously studied. We chose to use rat peritoneal macrophages elicited by injection of killed Corynebacterium parvum to study how virus interacts with primary APCs that would be recruited to a site of infection/inflammation.21,22 Also, we were interested to explore how VACV interacts with the rat immune system, as rats are a natural reservoir for orthopoxviruses and can transmit them to primates.9,23 While the natural host of VACV is unknown, several orthopoxviruses spread naturally in rodents, including rats.9,23 Here we describe the effects of VACV on a major histocompatibility complex (MHC) class II restricted antigen presentation system in which rat macrophages present a peptide of myelin basic protein to cognate antigen-specific CD4+ T lymphocytes.24,25 CD4+ T cells are crucial for viral clearance in poxvirus infection.26,27 In addition, we explore viral effects on T and B lymphocytes, dendritic cells and macrophage lines. Our data indicate that VACV infection rapidly disables various APCs by affecting relevant cell surface proteins, and in some cases inducing apoptosis of APCs, thus blocking downstream T-cell activation events and cytokine production. Further, our data indicate that the mechanism of immune interference is not necessarily apoptosis-dependent as has been previously suggested,28 but rather VACV blocks presentation of antigenic peptides in MHC class II molecules.
Materials and methods
Cells and virus
VACV Western Reserve (WR) stocks were propagated using BS-C-1 cells as previously described.29 For titrations of VACV, BS-C-1 monolayers were fixed and stained with 0·1% crystal violet in 20% ethanol. All cells were grown in media containing 10% fetal bovine serum. CD4+ RsL.11 T-cell clones were derived and maintained as previously described.24 CTLL-2 cells [ATCC # TIB-214; American Type Culture Collection (ATCC), Rockville, MD] were maintained in RPMI supplemented with 0·4% interleukin (IL)-2.24 RAW 264·7 (ATCC # TIB-71), a mouse macrophage cell line, was maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM). The mouse B-cell line 1153 and the T-cell hybridoma B04 (HEL74–88 specific) (kind gifts from Janice Blum, University of Indiana School of Medicine, Indianapolis, IN) were maintained in RPMI.19
Peritoneal macrophage isolation and antigen presentation assays
Lewis rats (bred and maintained at the Association for Assessment and Accreditation of Laboratory Animal Care-certified Brody School of Medicine animal care facility at East Carolina University) were injected intraperitoneally with 200 μg of inactivated C. parvum in 5 ml of Hanks’ balanced salt solution (HBSS). Two to three days later, the rats were killed and peritoneal exudate cells (PECs) were harvested by washing the peritoneum in cold HBSS. PECs were washed, infected for 5 hr, and placed into 96-well plates. PECs were incubated in quadruplicate with 50–500 nm guinea pig myelin basic protein (GPMBP) for 30 min, followed by addition of 25 000 Lewis rat CD4+ RsL.11 clones specific for GPMBP. For experiments involving 1153 B cells, 25 000 B cells (I-Ab) were infected for 6 hr. Cells were then plated in a 96-well format and incubated with varying concentrations of the antigen HEL74–88 (a gift from Janice Blum) for 1 hr.19 Then 25 000 B04 T cells specific for the HEL74–88 peptide in the context of I-Ab were added to each well. The antigen presentation plates were incubated for 15–48 hr at 37° and 5·0% CO2. After incubation, 50 μl of supernatants was transferred into an empty 96-well plate and frozen for the following assays. All animal experiments were in compliance with East Carolina University Animal Care and Use committee (K139) and National Institutes of Health (NIH) guidelines.
Bone marrow-derived dendritic cells
Rat bone marrow was harvested from the femur and tibia. The cells were placed in RPMI supplemented with 10% fetal bovine serum, 1%l-glutamine, 1% penicillin–streptomycin, and 0·1% granulocyte–macrophage colony-stimulating factor (GM-CSF)-containing baculovirus supernatant. After 3 days of culture, the culture medium was removed and replaced with fresh culture medium. On day 7, the dendritic cells were collected and used for the antigen presentation assay and fluorescence-activated cell sorter (FACS) analysis.
CTLL IL-2 bioassay
IL-2 was measured using previously described methods.24 Briefly, 10 000 CTLL clones (quadruplicate) were washed, re-suspended in RPMI, and added to the collected supernatants. The plates were incubated for 48 hr at 37° and 5·0% CO2, followed by the addition of 10 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt/phenazine methosulphate (MTS/PMS) [2·0 mg/ml MTS; Promega (Madison, WI) and 0·1 mg/ml PMS; Sigma, St Louis, MO] to measure cell proliferation.30 The absorbance was read at 24, 48 and 72 hr post MTS at 492 nm filtered, and 690 nm reference. Medium only was used to define the background control level and known IL-2-containing supernatants were used as a positive control.
Nitric oxide (NO) measurement
Griess reagent (50 μl; 1% sulphanilamide +0·1% N-[1-naphthy]ethylenediamine in 2·5% phosphoric acid) was added to 50 μl of the quadruplicate harvested supernatants. The absorbance was read at 540 nm after a 5-min incubation at room temperature.31
Measurement of cytokines
Fifty microlitres of the harvested supernatants (triplicate) was analysed using the LincoPlex 24 rat cytokine/chemokine luminex bead immunoassay kit according to the manufacturer’s instructions (Linco Research, Billerica, MA). The supernatants were incubated with a panel of anti-cytokine antibodies (Abs) immobilized on Luminex beads (Bio-Rad Laboratories, Hercules, CA). The following cytokines were analysed: IL-1α, IL-1β, IL-2, IL-6, IL-18, macrophage inflammatory protein (MIP)-1α, GM-CSF, interferon (IFN)-α, growth regulated oncogene alpha/keratinocyte attractant (GRO/KC), RANTES, tumour necrosis factor (TNF)-α, monocyte chemotactic protein (MCP)-1, eotaxin, granulocyte colony-stimulating factor (G-CSF), IL-4, IL-9, IL-13, IL-5 and IL-10. Samples were run according to the manufacturer’s instructions (Bio-Rad) and analysed on the BioPlex protein array reader (Bio-Rad) in the Duke University Human Vaccine Institute Immune Reconstitution Core Facility (Durham, NC).
RsL.11 stimulation assay
CD4+ RsL.11 T lymphocytes were washed and re-suspended in RPMI. The cells were infected with VACV WR for 3–4 hr, plated in tripliciate in a 96-well format, and stimulated with PEC/50 nm GPMBP, 25 μg/ml Con A (Sigma), or 100 nm phorbol 12-myristate 13-acetate (PMA)/2 μm ionomycin (Sigma). The plates were incubated at 37° and 5% CO2. A volume of 50 μl of the harvested supernatants was collected at 20 and 40 hr and assayed for IL-2 using the CTLL bioassay already described.
Metabolism assays
PEC and 1153 B cells were infected with VACV [multiplicity of infection (MOI) of 2 and 5, respectively]. Total cellular metabolism was measured by the addition of 10 μl of MTS/PMS30 4–24 hours post infection (hpi), and analysed similarly to the method described above for the CTLL IL-2 Bioassay. Absorbance was read at 492 nm at various times post infection.
Flow cytometry
Cells were infected with VACV WR for 4 hr and then washed in FACS buffer [phosphate-buffered saline (PBS) containing 0·1% heat-inactivated fetal bovine serum (FBS) and 0·1% sodium azide]. Then 3 × 105 cells were stained with an anti-MHC II concentrated supernatant (Y3P, AF6120 and MKS4) or OX1 anti-CD45 for 1 hr on ice, washed once, and then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) (Southern Biotech, Birmingham, AL). MHC II expression was measured using a FACScan (Becton Dickinson, Franklin Lakes, NJ) equipped with the cell quest software (Becton Dickinson). For apoptosis measurement, cells were infected and then stained with annexinV-FITC/propidium iodide (PI) (BD Pharmingen, Franklin Lakes, NJ) per the manufacturer’s instructions.
One-step growth curve
A one-step growth curve was obtained to measure viral replication in permissive cells and PEC as previously described.29
Statistical analysis
Experiments were repeated three times and representative data with standard deviation bars are shown. A two-tailed Student’s t-test was used to compare uninfected with VACV-infected groups. P-values < 0·05 were considered significant.
Peptide MHC association
The Y-Ae antibody specifically detects a complex of peptide 52–68 from I-Ed MHC class II bound in the cleft of MHC class II I-Ab.32,33 This complex is formed in mice that express both alleles. Spleens were harvested from B10.A-H2^i5 H2-T18^a/(5R)SgSnJ mice (Jackson Laboratories, Bar Harbor, ME); C57Bl/6 mice were used as a negative control. Unfractionated splenocytes were infected with purified virus for 3 hr (MOI = 10) and then incubated with biotin-conjugated Y-Ae (eBioscience, San Diego, CA). Cells were washed and incubated with streptavidin-R-phycoerythrin (Southern Biotech). Samples were analysed using a FACScan equipped with cell quest software.
Results
VACV decreases IL-2 production
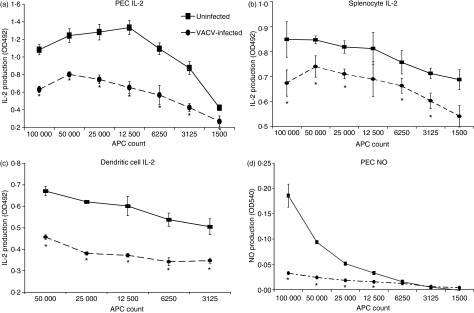
As antigen presentation is the seminal event triggering an adaptive immune response, we wanted to assess the effects of VACV infection on this pivotal point of the immune response using primary cells recruited to a site of inflammation. PECs were harvested from rats, infected with VACV, and pulsed with a model antigen (MBP). Various numbers of infected cells were then incubated with cognate CD4+ T cells. In order to measure antigen presentation responses, supernatant IL-2 was measured. VACV infection significantly (Student’s t-test P < 0·05) reduced IL-2 production by T cells 15–48 hpi (Fig. 1a). VACV also significantly decreased IL-2 production using rat splenocytes (Fig. 1b), dendritic cells (Fig. 1c), and PECs that had been rested overnight prior to infection (not shown) as APCs. In the absence of antigen or either cell type, no IL-2 was produced. Together, these data indicated that VACV significantly decreased the amount of IL-2 that is produced by CD4+ T lymphocytes.
Figure 1.
Vaccinia virus (VACV) infection of primary antigen-presenting cells (APCs) inhibits the amount of interleukin (IL)-2 produced by cognate CD4+ T cells and the amount of nitric oxide (NO) produced by the APCs. Freshly isolated peritoneal exudate cells (PECs) (a), splenocytes (b) or dendritic cells (c) were isolated from a rat, infected with VACV Western Reserve [multiplicity of infection (MOI) = 2] for 5 hr, pulsed for 30 min with 50 nm guinea pig myelin basic protein (GPMBP), and then incubated with the cognate CD4+ RsL.11 T-cell line. Uninfected PECs served as a control. At 15 to 48 hpi, supernatants (50 μl) were collected and assayed for either IL-2 using the IL-2-dependent cell line CTLL (a–c) or for NO using Greiss reagent (d). For the CTLL bioassay, medium only was used to define the background control level and known IL-2-containing supernatants were used as a positive control. Proliferation was read as absorbance at 492 nm. IL-2 production was determined as the mean optical density (OD) value of the experimental group minus the background control level. *P < 0·05.
VACV decreases NO response
During antigen presentation, both the T lymphocyte and the APC become activated, and macrophages are stimulated to produce NO,34 an important antiviral defence. Using the same antigen presentation system described above, supernatants were assayed for the production of NO. Uninfected PECs produced increasing amounts of NO with increasing numbers of APCs (Fig. 1d), while VACV-infected PECs were markedly impaired in their ability to secrete NO. NO was not produced in the absence of antigen or either cell type (data not shown), indicating that this is a specific antigen presentation response. These results indicated that both T lymphocyte and macrophage responses were suppressed by VACV.
RsL.11 T cells are refractory to VACV
We next sought to determine which cell type was being directly affected by VACV in this antigen presentation system. While we intentionally incubated VACV with APCs (macrophages, splenocytes and dendritic cells) prior to addition of T cells, the RsL.11 T cells could have become infected during co-incubation with the infected APCs. To assess the effects of virus infection on IL-2 production by the RsL.11, RsL.11 T cells were incubated in triplicate with VACV for 3 hr, washed, and then incubated with a titration of uninfected PECs pulsed with antigen, similar to the above-described experiments. As shown in Fig. 2a, VACV did not significantly decrease IL-2 production when preincubated with T cells. We also assessed the effect of VACV infection on RsL.11 responses to mitogenic and chemical stimulation. RsL.11 T cells were incubated with VACV for 4 hr, and then stimulated for 24 to 48 hr with Con A, PMA and ionomycin, or uninfected antigen (Ag)-pulsed PECs. VACV did not significantly decrease IL-2 production by RsL.11 T cells in response to any of these stimuli (Fig. 2b). Control groups included the stimulants in medium with no RsL.11 T cells and did not give readings above background in the IL-2 bioassay. These data suggest that RsL.11 IL-2 synthesis and secretion are not affected by VACV.
Figure 2.
Vaccinia virus (VACV) effects on antigen presentation are not attributable to a decreased capacity of the RsL.11 T cell to produce interleukin (IL)-2 or the CTLL to respond to IL-2. (a) RsL.11 T cells were infected with WR [multiplicity of infection (MOI) = 4] for 4 hr and then incubated with decreasing numbers of antigen (Ag)-pulsed peritoneal exudate cells (PECs). After 24–48 hr, supernatants (50 μl) were collected and assayed for IL-2 production using the CTLL IL-2 bioassay. (b) RsL.11 T lymphocytes were uninfected or infected for 3–4 hr (dark bars) and then stimulated with PECs/50 nm GPMBP, 25 μg/ml Con A, or 100 nm PMA/2 μm ionomycin. Supernatants (50 μl) were collected at 20 and 40 hr and assayed for IL-2. (c) RsL.11 T cells were infected with VACV for 10 hr, and growth metabolism was measured by reduction of 10 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt/phenazine methosulphate (MTS/PMS); absorbance at 492 nm. (d) CTLL cells were infected with VACV (MOI = 5) for 4 hr and then placed in media with varying amounts of an IL-2-containing supernatant. Proliferation was measured by adding 10 μl of MTS/PMS.
To determine whether RsL.11 T cells could be affected by VACV, we also measured cellular proliferation after incubation with virus. RsL.11 T cells were incubated with VACV for 10 hr, and then MTS was added to measure metabolism. VACV did not significantly affect the RsL.11 T cell metabolism up to 64 hpi (Fig. 2c). These data suggest that RsL.11 T cells are completely refractory to VACV. We therefore incubated RsL.11 T cells with infectious virus and compared progeny virus production with that in a known permissive cell line, BS-C-1. After 72 hr, a 100-fold increase in virus was measurable in infected BS-C-1 cells, but no increase in virus could be detected in cells or supernatants of RsL.11 T cells, indicating that they are non-permissive for VACV infection (data not shown). Thus, there is no evidence that these cells even become infected under these conditions.
CTLLs and VACV infection
As CTLL cells were used to measure IL-2 in supernatants, it was also possible that VACV might be affecting the ability of the CTLL to proliferate in response to IL-2. To assess virus effects on CTLL, we incubated the CTLL cells with VACV for 5 hr and then measured proliferation in response to IL-2-containing supernatants.25 The addition of IL-2 to the medium increased the metabolism/proliferation of the uninfected CTLLs over the proliferation of CTLLs incubated with no IL-2 (Fig. 2d), and VACV did not significantly reduce this response. As the amount of virus used in this experiment was four times higher than levels contained in supernatants from antigen presentation assays, it is unlikely that VACV affected CTLL responses to IL-2 in the antigen presentation assay. Together, these data suggest that VACV acts directly on the APCs, not the T cells used in these assays.
Antigen concentration
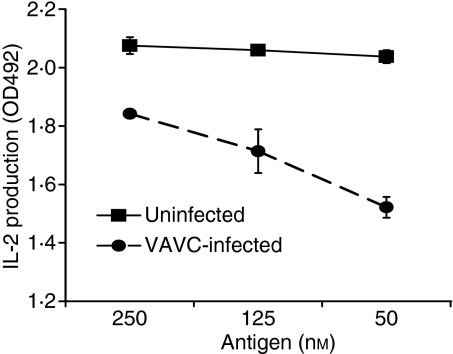
As the T lymphocytes in these assays were minimally affected by VACV infection, we theorized that the APCs were being affected by VACV and that increasing antigen concentrations might overcome the VACV-induced decrease in antigen presentation capacity. In infected PECs presenting antigen to RsL.11 T cells (similar to experiments depicted in Fig. 1), a titration of GPMBP antigen (50–250 nm) showed that higher antigen concentrations augmented the weak antigen-presenting capacity of infected APCs (Fig. 3). In contrast, increasing antigen in this concentration range did not significantly enhance the efficiency of uninfected APCs to stimulate IL-2 production.
Figure 3.
Increased antigen concentration augments the antigen presentation capacity of infected antigen-presenting cells (APCs). Peritoneal exudate cells (PECs) were infected with vaccinia virus (VACV) [multiplicity of infection (MOI) = 2] for 4 hr and then pulsed with a titration of guinea pig myelin basic protein (GPMBP; 50–250 nm) before being co-cultured with antigen-specific T cells. Supernatants (50 μl) were collected at 24 to 48 hours post infection (hpi) and assayed for interleukin (IL)-2 using the CTLL IL-2 bioassay.
B-cell antigen presentation
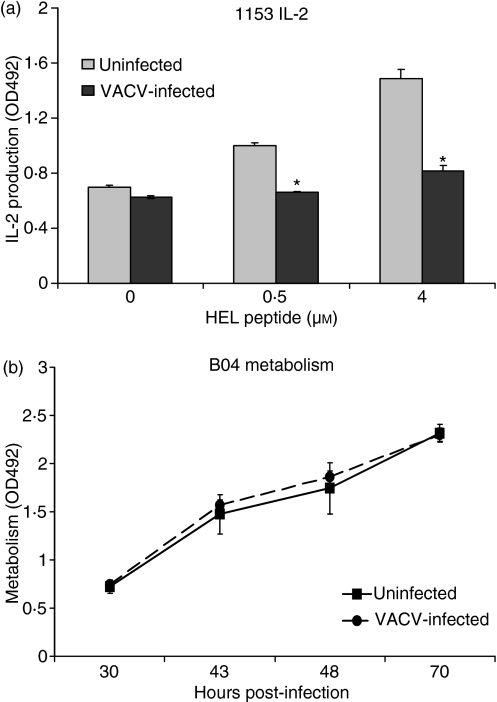
We next determined if VACV affects antigen presentation by a murine B-cell line. The mouse 1153 B-cell line presents the HEL74–88 peptide to the CD4+ B04 T-cell line.19 1153 B cells were infected for 6 hr, incubated with varying concentrations of the HEL74–88 peptide, and then incubated with the cognate B04 T-cell line. The presence of VACV significantly suppressed B-cell antigen presentation (IL-2 production; Fig. 4a) (Student’s t-test, P < 0·01). To assess viral effects on the B04 T cells, T cells were incubated with virus for 24 hr, at which time MTS was added in order to measure metabolism. VACV did not affect the proliferation/metabolism of the B04 T cells up to 70 hpi (Fig. 4b), suggesting that the T cells were also not directly affected by VACV in this antigen presentation system. These data indicate that VACV blocks antigen presentation of multiple antigens presented by various APCs from both rats and mice.
Figure 4.
Vaccinia virus (VACV)-infected mouse B cells are impaired in their ability to stimulate antigen-specific CD4+ T cells to produce interleukin (IL)-2. (a) 25 000 1153 B cells were infected for 6 hr, and then pulsed with the HEL74-88 antigen for 1 hr, followed by the addition of 25 000 B04 T cells. After a 24- to 48-hr incubation, supernatants (50 μl) were collected and assayed for IL-2 using the CTLL IL-2 bioassay. (b) 25 000 B04 T cells were infected for 24 hr. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt/phenazine methosulphate (MTS/PMS) was then added to measure metabolism. *P < 0.01.
VACV inhibits NO production from PECs and RAW macrophages
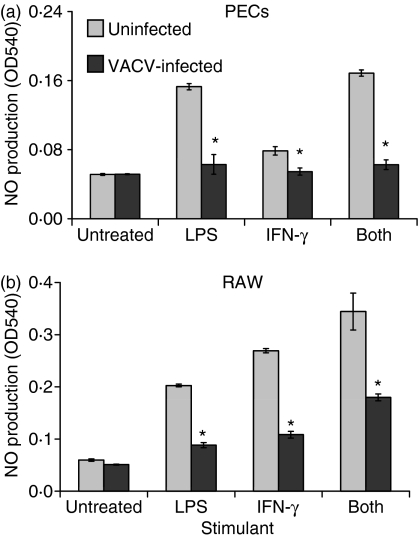
In order to study VACV regulation of isolated APCs, we used lipopolysaccharide (LPS) and interferon (IFN)-γ to stimulate PECs and the RAW 264·7 murine macrophage cell line.35 PECs and RAW cells were infected for 4 hr and stimulated with LPS, IFN-γ, or both, and supernatants were assayed for NO. All stimuli induced NO production in uninfected PECs and RAW cells; however, this induction was significantly (Student’s t-test, P < 0·05), and in some cases completely, blocked by VACV infection (Fig. 5). These data suggest that macrophages are highly sensitive to VACV infection.
Figure 5.
Vaccinia virus (VACV) decreases the amount of nitric oxide (NO) that is produced by stimulated macrophages. Peritoneal exudate cells (PECs) (a) or RAW 264.7 macrophages (b) were infected at a multiplicity of infection (MOI) of 1 for 4 hr, and were then stimulated to produce NO by the addition of lipopolysaccharide (LPS), interferon (IFN)-γ, or a combination of both. Supernatants (50 μl) were collected at 24–48 hr and then assayed for NO production by the addition of Greiss reagent. Absorbance was read at 540 nm. *P < 0·01.
VACV infection globally alters cytokine responses in antigen presentation
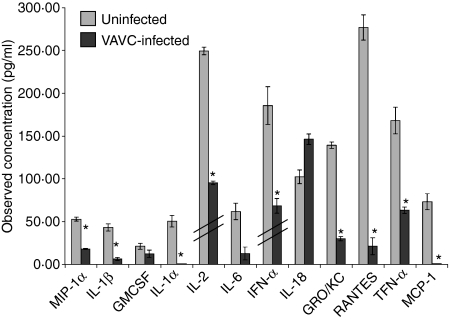
As the result of antigen presentation, both the APC and the responding T cell produce numerous cytokines initiating the specific immune response. As VACV inhibited the amount of both IL-2 and NO produced in this rat model of antigen presentation, we assessed the effect of VACV on synthesis of other cytokines and chemokines. Using a multiplex bead-based assay, we determined that VACV reproducibly and significantly (P < 0·05) inhibited the production of MIP-1α, IL-1β, IL-1α, IL-2, IFN-α, GRO/KC, RANTES, TNF-α and MCP-1 (Fig. 6) in response to PEC antigen presentation to RsL.11 T cells. These data confirm the IL-2 bioassay results in Fig. 1. In addition, VACV decreased the secretion of IL-6 and GM-CSF; however, the reduction was not always significant. Interestingly, VACV infection did not inhibit IL-18 production.
Figure 6.
Vaccinia virus (VACV) globally reduces cytokine synthesis by antigen-presenting cells (APCs) and responding T cells. Peritoneal exudate cells (PECs) were harvested from Lewis rats, infected with VACV Western Reserve [multiplicity of infection (MOI) = 2] for 5 hr, pulsed with 50 nm guinea pig myelin basic protein (GPMBP), and then co-cultured with the cognate CD4+ RsL.11 T-cell line. Supernatants (50 μl) were collected at 24 hours post infection (hpi) and assayed for the production of cytokines using the LincoPlex 24 rat cytokine/chemokine Luminex bead immunoassay kit. Interleukin (IL)-2 and interferon (IFN)-α values were divided by 20 and 100, respectively (hatch marks) to fit to scale. *P < 0·05.
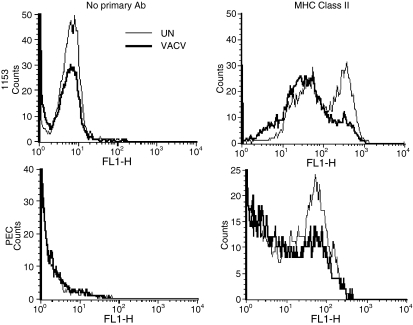
VACV decreases MHC class II expression
Because VACV infection decreased MHC class II-mediated antigen presentation, we assessed the effects of VACV on the expression of MHC II molecules on the surface of PECs. PECs were infected for 4 hr, and MHC II expression was measured by flow cytometry. In three experiments, VACV mildly decreased MHC class II surface expression. For example, compared with uninfected PECs, VACV decreased the percentage of high-expressing MHC II-positive cells from 47% to 37% [Fig. 7; mean fluorescence intensity (MFI) 26 ± 0·3 versus 19·7 ± 1·8]. We also assessed the ability of VACV to inhibit MHC II expression in 1153 B cells. 1153 B cells were infected and MHC II expression was measured as above. In uninfected 1153 B cells, 38% expressed high levels of MHC II, while only 15% of VACV-infected B cells were class II bright (MFI 144 ± 13·4, versus 76 ± 2·8). These data indicated that VACV decreases MHC II expression on APCs soon after infection. In the same experiments, VACV infection did not decrease CD45RO expression, indicating that the reduction observed was specific and not just a consequence of general membrane protein perturbation caused by viral infection.
Figure 7.
Vaccinia virus (VACV) decreases major histocompatibility complex (MHC) class II expression. Peritoneal exudate cells (PECs) or 1153 cells were infected with VACV for 5 hr, washed and incubated with no primary antibody [mean fluorescence intensity (MFI) for 1153: uninfected cells, 8·4 ± 0·59; VACV-infected cells, 5·4 ± 0·44; PECs uninfected 2·35 ± 0·06; VACV-infected 2·38 ± 0·48], anti-class II MHC (MFI for uninfected cells, 145 ± 13·4; VACV-infected cells, 76 ± 2·8), or anti-CD45 and then fluorescein isothiocyanate (FITC)-conjugated secondary antibody and analysed by flow cytometry.
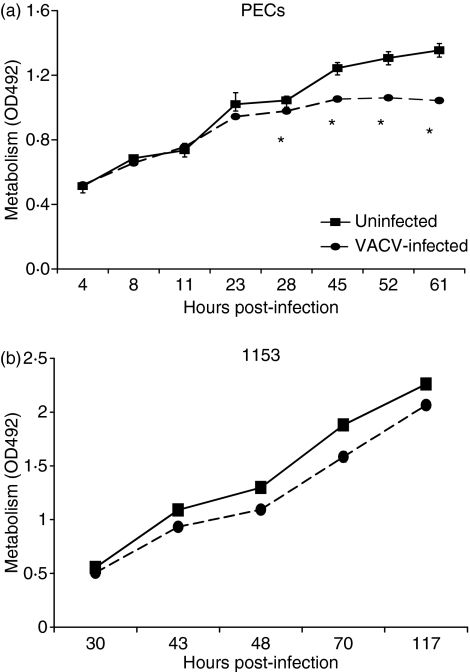
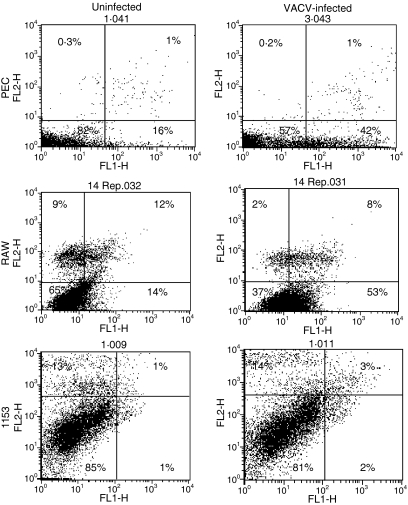
VACV induces apoptosis in PECs and RAW macrophages
Because VACV infection blocked PEC responses, we assessed the effects of VACV on the viability of the PECs. PECs were infected with VACV in triplicate, and total cellular metabolism was measured through the reduction of MTS. Differences in metabolism between the groups became apparent 24 hpi (Fig. 8a), suggesting that the virus is killing cells. However, VACV infection had only a minor effect on 1153 B cells (Fig. 8b), even 100 hpi. We therefore measured the ability of VACV to induce apoptosis in these cells using PI and annexin. It was previously reported that VACV induces apoptosis of the murine macrophage J774 cell line.36 VACV infection increased early apoptotic cell percentages in PECs from 16% to 42% and in the RAW 264·7 macrophage line from 14% to 53% (Fig. 9) at 4 hpi. VACV induced apoptosis similarly in rat bone marrow-derived dendritic cells and mouse splenic dendritic cells (data not shown). However, using similar assays we could not detect VACV induction of apoptosis in three experiments in the 1153 B-cell line (Fig. 9) from 6 to 24 hpi, even though VACV also suppressed antigen presentation by this line.
Figure 8.
Vaccinia virus (VACV) inhibits the metabolism of macrophages. Peritoneal exudate cells (PECs) (a) were infected with VACV [multiplicity of infection (MOI) = 2], and 1153 B cells (b) at an MOI of 5 and metabolism was measured by the reduction of 10 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt/phenazine methosulphate (MTS/PMS). Standard deviation error bars are shown on all figures but are too small to be visible on some graphs. *P < 0·05.
Figure 9.
Vaccinia virus (VACV) induces apoptosis in macrophages. Peritoneal exudate cells (PECs) (a) or RAW 264.7 macrophages (b) were infected with VACV for 4 hr [MOI = 2]. 1153 B cells (c) were infected at an MOI = 5 for 6 hr. Apoptosis was assessed by staining cells with annexinV-fluorescein isothiocyanate (FITC) and propidium iodide (PI) and analysed by flow cytometry. Variation was not higher than 2% between duplicate samples.
Viral replication
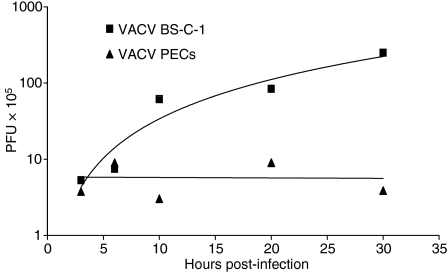
Induction of apoptosis in virally infected cells is one host defence against viral replication, and VACV has been reported to induce apoptosis in macrophages and dendritic cells.36,37 We therefore determined the ability of VACV to replicate in PECs compared with BS-C-1 cells, which are permissive for viral replication. Figure 10 shows that VACV WR was able to increase infectious virus particles 2-logs by 30 hpi in BS-C-1 cells, but no replication was apparent in PECs at 10, 20 or 30 hpi. Thus VACV may induce apoptosis in professional APCs and block their function, but the virus also sacrifices the ability to replicate in the cells.
Figure 10.
Vaccinia virus (VACV) does not replicate to high titres in peritoneal exudate cells (PECs). BS-C-1 cells or PECs were infected with VACV [multiplicity of infection (MOI) = 10]. Cells were collected at designated times post-infection. Cell-associated virus was titrated on BS-C-1 monolayers. PFU, plaque-forming units.
Peptide association with MHC class II
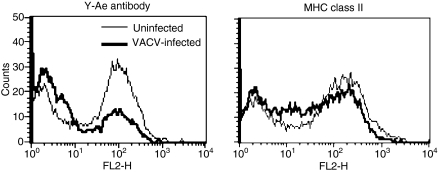
One level of immune regulation, both in homeostasis and infection, lies in controlling the association of peptides with the MHC molecules that present them.38 As VACV inhibits antigen presentation, but only modestly affects class II MHC expression, we tested the effect of VACV on peptide loading in the MHC class II molecule employing the Y-Ae antibody. The Y-Ae antibody specifically detects a complex of peptide 52–68 from I-Ed MHC class II bound in the cleft of MHC class II I-Ab.32,33 MHC class II molecules frequently present self peptides from surface proteins that recirculate through endosomes and are degraded, similarly to exogenous antigens that are internalized. The Y-Ae antibody recognizes a major determinant (approximately 12% of MHC molecules) in mice expressing both of these class II molecules.33,39 We measured the amount of peptide bound in the cleft of the MHC class II molecule on the surface of uninfected and VACV-infected spleen cells. As Fig. 11 shows, 63% of uninfected cells expressed MHC class II molecules complexed with this I-E peptide, while only 30% of VACV-infected cells were positive at 3 hpi (MFI 78·2 ± 9·6 for uninfected cells compared with 38 ± 2·8 for VACV-infected cells). In contrast, surface MHC class II I-Ab levels were only modestly reduced (Fig. 11). These data indicate that VACV interferes intracellularly with the expression of surface proteins required for antigen presentation and specifically blocks expression of peptide-loaded MHC class II.
Figure 11.
Vaccinia virus (VACV) decreases peptide–major histocompatibility complex (MHC) complexes. Spleens were harvested from B10.A-H2^i5 H2-T18^a/(5R)SgSnJ mice and uninfected or VACV-infected with purified virus for 3 hr [multiplicity of infection (MOI) = 10] and then incubated with biotin-conjugated Y-Ae and streptavidin-R-phycoerythrin. C57Bl/6 mice and were used as a negative control and showed no staining above background.
Discussion
We have shown here that VACV blocks class II MHC antigen presentation by primary rodent cells, including unfractionated splenocytes, PECs and dendritic cells, and by a B-cell line. This probably represents an evolved immune evasion mechanism that is advantageous to the virus even though the virus sacrifices the ability to replicate progeny virions in APCs. Responses to antigen presentation of both CD4+ T lymphocytes and the APCs are inhibited (Fig. 1) and this has the overall effect of limiting downstream cytokine and chemokine production (Fig. 6) and presumably multiple aspects of the antiviral immune response. Notably, many of the cytokines that are regulated by VACV are chemotactic factors (MIP-1a, IL-1, TNF-a, GRO/KC, RANTES and MCP-1), which aid in the migration of immune response cells to sites of inflammation and infection.
We have found the T lymphocytes used here to be refractory to virus effects, with VACV directly affecting the APCs. It has been reported in humans that VACV preferentially infects subsets of CD14+ cells, B cells to a lesser extent, and only activated primary T cells.40 Our results are similar to work recently published showing that VACV decreases MHC class II antigen presentation in B-cell, fibroblast and macrophage cell lines, as well as primary immature dendritic cells.19 Antigen-presenting capacity could be partly restored in our studies by increasing the amount of antigen, suggesting that some process of antigen uptake or processing or presentation is blocked. Data from the Blum laboratory indicate that intracellular antigen is not the limiting factor.19 We found that VACV alters class II MHC expression in APCs within a few hours after infection. This may simply be a consequence of apoptosis induction as we and others have shown that VACV induces apoptosis in human and rodent macrophages and dendritic cells.36,37 Other groups have shown a decrease in CD86 and MHC class II on human dendritic cells correlating with apoptosis induction and reduced ability to present antigen.28,41 VACV modulation of class II MHC has been controversial. Other groups have shown VACV-induced increases in MHC class II, but with inhibition of maturation-induced MHC class II, class I, CD86 and CD80.42 Some differences may be attributable to the type of preparation of virus used to infect APCs, as the ‘crude’ virus preparation normally used for infections is a cell lysate, which can act as an inflammatory stimulus. Another group recently reported that VACV decreased MHC II-restricted antigen presentation by specifically inhibiting the expression of surface MHC class II on splenic dendritic cells isolated from VACV-infected mice.20 We did not measure the amount or stability of MHC class II proteins in our studies because other laboratories have shown that the amount of MHC protein is not directly related to its functional surface expression. For example, peptide–MHC complexes can be inhibited with no decrease in the amount of MHC class II proteins in cells,19 and MHC class II mRNA levels can be decreased with no decrease in MHC class II surface expression.20 In summary, there is growing evidence to indicate that VACV modulates MHC class II-restricted antigen presentation by specifically affecting functions of the APC.
Interestingly, the inhibition of antigen presentation appears distinct for professional APCs and the 1153 B-cell line. Class II MHC expression was reduced in this B-cell line, but, unlike in other APCs, apoptosis was not detected. This suggests that VACV may decrease class II even in the absence of apoptosis induction. Importantly, we have shown that VACV strongly reduced the amount of peptide in the cleft of MHC class II molecules on infected primary APCs by flow cytometry. These data are supported by a study in a human B-cell line where VACV infection decreased peptide association with MHC class II measured by western blot.19 As this effect is demonstrable with different antigens in different cells, it is likely that this is a key mechanism used by VACV to suppress immune responses. How this occurs is currently under investigation.
We do not believe that VACV simply reduces antigen presentation because it kills infected cells. First, we show in Fig. 3 that increasing antigen concentrations restore antigen-presenting capacity in VACV-infected cells, suggesting that the cells are not dead, but rather disabled in terms of antigen presentation. In Fig. 8, we show that differences in the metabolism of APCs are not seen until almost 24 hpi, but that VACV reduces the surface expression of MHC class II and the amount of peptide in its cleft by 5 hpi, and the antigen presentation-induced T-cell responses have been diminished by 15 hpi of APC. In addition, VACV does not induce apoptosis in 1153 B cells, but still blocks their antigen-presenting capacity and MHC class II expression (Figs 4 and 7). Together these data suggest a specific VACV-induced inhibition of antigen presentation.
We studied antigen presentation in rodent cells. As most extant poxviruses have evolved a broad host range, the immunomodulatory mechanisms appear to be conserved and functional in a number of host species. For example, rats are a natural host for cowpox virus and can transmit it to primates, as demonstrated by a recent outbreak in a primate colony in Europe with widespread fatalities.23,43 Most VACV APC work has been undertaken in the mouse model; our results show that VACV blocks antigen presentation and T-cell priming in a rodent known to transmit orthopoxviruses to primates.
VACV caused an apparent broad decrease in post-antigen presentation cytokine production. The notable exception to VACV inhibition was IL-18. IL-18 is stored intracellularly as pro-IL-18 and rapidly released upon stimulation.44 As IL-18 is an important factor in the induction of antiviral IFN-γ production, its storage for rapid release may be an evolved host mechanism to contravene immunomodulation by viral gene products. The importance of this cytokine in antiviral defence is underscored by the possession of an IL-18-binding protein encoded by poxviruses.45 VACV produces several soluble cytokine-binding proteins that may interfere with detection of these cytokines if, and only if, they block binding of the detection antibody. However, because there is a general decrease in cytokine production it seems unlikely that the decrease would be caused by VACV-specific binding proteins.
VACV rapidly decreases antigen-presenting capacity, alters expression of class II MHC and induces apoptosis in multiple APC types, including peritoneal macrophages responding to inflammatory stimuli. These data expand our knowledge of viral interference mechanisms and suppression of immunity that contribute to poxvirus pathogenesis; however, the viral gene products involved in this inhibition and the intracellular mechanisms remain to be elucidated. An increased understanding of poxviral pathogenesis will aid in the development of antiviral drugs and novel tailored vaccine strains, which exclude immunosuppressive poxviral genes, but which retain and efficiently express the protective antigenic target epitopes that stimulate the immune system.
Acknowledgments
The authors wish to thank Dr Greg Sempowski (Duke University) for help with the Luminex data and Dr Janice Blum (Indiana University School of Medicine) for cells, reagents and helpful discussions. This work was supported by grants to R Roper from East Carolina University, The North Carolina Biotechnology Center and NIH grant #U54 AI057157 from Southeastern Regional Center of Excellence for Emerging Infections and Biodefense. Manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Glossary
Abbreviations:
- GPMBP
guinea pig myelin basic protein
- HEL
hen egg lysozyme
- hpi
hours post infection
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- PEC
peritoneal exudate cells
- PMS
phenazine methosulphate
- VACV
vaccinia virus
- WR
Western Reserve
Disclosures
The authors have no conflict of interest.
References
- 1.Moss B. Poxviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2849–84. [Google Scholar]
- 2.Mahalingam S, Damon IK, Lidbury BA. 25 years since the eradication of smallpox: why poxvirus research is still relevant. Trends Immunol. 2004;25:636–9. doi: 10.1016/j.it.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Damaso CR, Esposito JJ, Condit RC, Moussatche N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virol. 2000;277:439–49. doi: 10.1006/viro.2000.0603. [DOI] [PubMed] [Google Scholar]
- 4.Dhar AD, Werchniak AE, Li Y, Brennick JB, Goldsmith CS, Kline R, Damon I, Klaus SN. Tanapox infection in a college student. N Engl J Med. 2004;350:361–6. doi: 10.1056/NEJMoa031467. [DOI] [PubMed] [Google Scholar]
- 5.Stich A, Meyer H, Kohler B, Fleischer K. Tanapox: first report in a European traveller and identification by PCR. Trans R Soc Trop Med Hyg. 2002;96:178–9. doi: 10.1016/s0035-9203(02)90295-6. [DOI] [PubMed] [Google Scholar]
- 6.Kolhapure RM, Deolankar RP, Tupe CD, et al. Investigation of buffalopox outbreaks in Maharashtra State during 1992–1996. Indian J Med Res. 1997;106:441–6. [PubMed] [Google Scholar]
- 7.Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in persons with clinical lesions and seroepidemiology in a representative Australian population. J Infect Dis. 1999;179:701–4. doi: 10.1086/314620. [DOI] [PubMed] [Google Scholar]
- 8.Molino AC, Fleischer AB, Jr, Feldman SR. Patient demographics and utilization of health care services for molluscum contagiosum. Pediatr Dermatol. 2004;21:628–32. doi: 10.1111/j.0736-8046.2004.21602.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virol. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey CG, Iskander JK, Roper MH, et al. Adverse events associated with smallpox vaccination in the United States, January–October 2003. JAMA. 2005;294:2734–43. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 11.Arness MK, Eckart RE, Love SS, et al. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160:642–51. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- 12.Eckart RE, Love SS, Atwood JE, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–5. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Upfal MJ, Cinti S. Smallpox vaccination and adverse cardiac events. Emerg Infect Dis. 2004;10:961–2. doi: 10.3201/eid1005.030967. discussion 2. [DOI] [PubMed] [Google Scholar]
- 14.Haga IR, Bowie AG. Evasion of innate immunity by vaccinia virus. Parasitology. 2005;130(Suppl):S11–25. doi: 10.1017/S0031182005008127. [DOI] [PubMed] [Google Scholar]
- 15.Moss B, Shisler JL. Immunology 101 at poxvirus U: immune evasion genes. Semin Immunol. 2001;13:59–66. doi: 10.1006/smim.2000.0296. [DOI] [PubMed] [Google Scholar]
- 16.Seet BT, Johnston JB, Brunetti CR, et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Puig JM, Sanchez L, Roy G, Blasco R. Susceptibility of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1:10. doi: 10.1186/1743-422X-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–71. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 19.Li P, Wang N, Zhou D, Yee CS, Chang CH, Brutkiewicz RR, Blum JS. Disruption of MHC class II-restricted antigen presentation by vaccinia virus. J Immunol. 2005;175:6481–8. doi: 10.4049/jimmunol.175.10.6481. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Li P, Singh P, et al. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell Immunol. 2007;246:92–102. doi: 10.1016/j.cellimm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–8. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook AD, Braine EL, Hamilton JA. The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response. J Immunol. 2003;171:4816–23. doi: 10.4049/jimmunol.171.9.4816. [DOI] [PubMed] [Google Scholar]
- 23.Martina BE, van Doornum G, Dorrestein GM, Niesters HG, Stittelaar KJ, Wolters MA, van Bolhuis HG, Osterhaus AD. Cowpox virus transmission from rats to monkeys, the Netherlands. Emerg Infect Dis. 2006;12:1005–7. doi: 10.3201/eid1206.051513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannie MD, Norris MS. MHC class-II-restricted antigen presentation by myelin basic protein-specific CD4+ T cells causes prolonged desensitization and outgrowth of CD4- responders. Cell Immunol. 2001;212:51–62. doi: 10.1006/cimm.2001.1843. [DOI] [PubMed] [Google Scholar]
- 25.Patel DM, Dudek RW, Mannie MD. Intercellular exchange of class II MHC complexes: ultrastructural localization and functional presentation of adsorbed I-A/peptide complexes. Cell Immunol. 2001;214:21–34. doi: 10.1006/cimm.2002.1887. [DOI] [PubMed] [Google Scholar]
- 26.Karupiah G, Buller RM, Van Rooijen N, Duarte CJ, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–9. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J Virol. 2006;80:6333–8. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastenmuller W, Drexler I, Ludwig H, Erfle V, Peschel C, Bernhard H, Sutter G. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virol. 2006;350:276–88. doi: 10.1016/j.virol.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Roper RL. Characterization of the vaccinia virus A35R protein and its role in virulence. J Virol. 2006;80:306–13. doi: 10.1128/JVI.80.1.306-313.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 31.Campos-Neto A, Ovendale P, Bement T, Koppi TA, Fanslow WC, Rossi MA, Alderson MR. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–41. [PubMed] [Google Scholar]
- 32.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 33.Rudensky A, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353:660–2. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 34.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 35.Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–7. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 36.Humlova Z, Vokurka M, Esteban M, Melkova Z. Vaccinia virus induces apoptosis of infected macrophages. J Gen Virol. 2002;83(Pt 11):2821–32. doi: 10.1099/0022-1317-83-11-2821. [DOI] [PubMed] [Google Scholar]
- 37.Broder CC, Kennedy PE, Michaels F, Berger EA. Expression of foreign genes in cultured human primary macrophages using recombinant vaccinia virus vectors. Gene. 1994;142:167–74. doi: 10.1016/0378-1119(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 38.Inaba K, Turley S, Iyoda T, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–36. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stimpfling JH, Richardson A. Recombination within the histocompatibility-2 locus of the mouse. Genetics. 1965;51:831–46. doi: 10.1093/genetics/51.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chahroudi A, Chavan R, Koyzr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79:10397–407. doi: 10.1128/JVI.79.16.10397-10407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–8. [PubMed] [Google Scholar]
- 42.Jenne L, Hauser C, Arrighi JF, Saurat JH, Hugin AW. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 2000;7:1575–83. doi: 10.1038/sj.gt.3301287. [DOI] [PubMed] [Google Scholar]
- 43.Matz-Rensing K, Ellerbrok H, Ehlers B, Pauli G, Floto A, Alex M, Czerny CP, Kaup FJ. Fatal poxvirus outbreak in a colony of New World monkeys. Vet Pathol. 2006;43:212–8. doi: 10.1354/vp.43-2-212. [DOI] [PubMed] [Google Scholar]
- 44.Gardella S, Andrei C, Poggi A, Zocchi MR, Rubartelli A. Control of interleukin-18 secretion by dendritic cells: role of calcium influxes. FEBS Lett. 2000;481:245–8. doi: 10.1016/s0014-5793(00)02015-9. [DOI] [PubMed] [Google Scholar]
- 45.Reading PC, Smith GL. Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity. J Virol. 2003;77:9960–8. doi: 10.1128/JVI.77.18.9960-9968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]