Abstract
The cell surface association between CD26 and adenosine deaminase (ADA) has a costimulatory function during T-cell activation. Several studies have revealed correlations among CD4+ CD26+ T-cell depletion, increased serum levels of ADA, and the evolution of human immunodeficiency virus (HIV) infection, implicating CD26 and ADA in HIV disease progression. In this context, we aimed to determine whether ADA costimulation could be altered during HIV infection. ADA costimulation was investigated in cells from HIV-infected patients (n = 36) in terms of proliferation and cytokine secretion. An effect of ADA on T-cell proliferation was found in HIV-1-infected patients and correlated positively with the CD4+ percentage and the nadir CD4 count and negatively with viral load, demonstrating that the response depends on the immunological status of the patient. The robust ADA-induced increase in cytokine production [interferon (IFN)-γ, interleukin (IL)-6 and IL-10] was markedly reduced in T cells from HIV-1-infected subjects. To eliminate some of the variables associated with immunological defects in HIV-1-infected patients, anti-CD3 plus ADA assays with T cells from healthy volunteers were performed in the presence of recombinant glycoprotein 120 (gp120). It was found that gp120 was responsible for the impairment of the ADA–CD26 interaction and consequently of the ADA-induced effect on both costimulation and cytokine production. The gp120-mediated disruption of the CD26–ADA interaction is a novel mechanism that might explain, at least in part, the altered immunological features observed in HIV-1-infected patients and may have significant relevance in AIDS pathogenesis.
Keywords: activation, costimulation, cytokines, human immunodeficiency virus, T cells
Introduction
The mechanisms of human immunodeficiency virus (HIV) infection and the host response following infection are complex and have only partially been characterized. HIV infection causes a progressive decline in the number and function of CD4+ T cells, leaving the host vulnerable to opportunistic infections.1,2 Other cells are also directly or indirectly affected by the virus, including CD8+ T cells,3,4 monocytes,5 macrophages,6 B lymphocytes,7 neutrophils8 and dendritic cells.9 Dysfunction of these cells plays a major role in particular aspects of HIV pathogenesis. As a consequence, HIV-infected individuals have various perturbations of their immunological status, such as an inability to respond to recall antigens,10 inappropriate responses to vaccinations,11,12 permanent hyperactivation states13–17 with paradoxical hyporesponsiveness to stimuli,18–22 cell cycle dysregulation23,24 and imbalances in the production of cytokines.25,26
At the molecular level, new insights concerning HIV proteins causing specific irregularities have recently been obtained.27,28 For example, signalling and apoptotic pathways, transcriptional activation and intracellular protein trafficking in HIV-infected cells are altered by negative factor (Nef) and trans-activator of transcription (Tat) proteins.29–31 Other HIV proteins implicated in causing anomalies during infection are viral protein R (Vpr),32–38 virion infectivity factor (Vif)39,40 and glycoprotein 120 (gp120).41,42 Therefore, the viral mechanisms used to attack the host are quite diverse and have been extensively studied. Understanding the damage HIV causes has implications for both clinical care and basic research. In this context, in vitro assays have shown that HIV-1 infectious particles and also the soluble envelope glycoprotein gp120 are able to inhibit adenosine deaminase (ADA) binding to CD26 on the cell surface of a variety of cells, from peripheral lymphocytes to T-cell lines,43,44 but no evidence concerning the possible physiological consequences of this inhibition has been reported. In the present work, evidence is presented demonstrating that ADA enhances the CD3-mediated proliferation of the T cells of HIV-1-infected individuals, directly correlating with the CD4 percentage and inversely with the viral load. It is also shown that ADA enhances T helper type 1 (Th1) and pro-inflammatory cytokine secretion in CD3-triggered T cells, and that the HIV envelope glycoprotein gp120 markedly reduces this effect. These results could help to explain, at least in part, the progressive impairment of the immune system in HIV-infected patients and shed new light on our understanding of AIDS pathophysiology.
Materials and methods
Sampling and study population
All blood samples were non-fasting and obtained by venipuncture from the antecubital vein. Ethylenediaminetetraacetic acid (EDTA)-treated vacutainers (Becton Dickinson, San Diego, CA) were used as collecting tubes and every sample was processed immediately after extraction. A sample of HIV-1-infected patients (n = 36) and healthy control volunteers (n = 10) were chosen for the study. All individuals gave informed consent. The characteristics of the HIV-infected patients are shown in Table 1. The median age of the HIV-1-infected individuals was 38 years (range 26–74 years), and 86% were male. Thirty-six per cent of patients were successfully treated with highly active antiretroviral therapy (HAART). Of these, six out of 13 (46%) were on a protease-inhibitor-containing HAART regimen. In patients with detectable plasma viraemia, the mean [± standard deviation (SD)] viral load was 4·29 ± 0·71 log10 HIV-1 RNA copies/ml (range 2·97–5·30 log10 copies/ml). The mean (± SD) absolute and percentage CD4 T-cell counts were 704 ± 236 (range 297–1274) cells/μl and 30·1 ± 7·4 (range 12·5–45·1), respectively. The mean (± SD) nadir CD4 count was 457 ± 134 (range 210–920) cells/μl. The mean (± SD) absolute and percentage CD8 T-cell counts were 1107 ± 492 (range 381–2338) cells/μl and 45·3 ± 11·3 (range 23·2–74·0), respectively. Two out of 36 patients were coinfected with hepatitis C virus and one with hepatitis B virus. Just one patient was coinfected with both hepatitis B and C viruses. Sexual contact was the most frequently reported risk factor for acquiring HIV-1 (31 out of 36 patients).
Table 1.
Clinical information for patients with human immunodeficiency virus 1 (HIV-1) infection
| CD4+ T cells |
CD4+ T-cell nadir |
CD8+ T cells |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Sex (% male) | Age (years) | Viral load (log copies/ml) | Count (cells/μl) | Percentage | Count (cells/μl) | Percentage | Count (cells/μl) | Percentage |
| HAART-treated [mean ± SD (range)] | 100 | 41 ± 13 (27–74) | ≤ DL | 841 ± 77 (497–1274) | 34·7 ± 6·2 (22·5–45·1) | 431 ± 119 (241–645) | 23·6 ± 6·4 (17·9–38·0) | 962 ± 418 (400–1710) | 39·1 ± 11·0 (23·2–62·5) |
| Untreated [mean ± SD (range)] | 78 | 37 ± 9 (26–62) | 4·29 ± 0·71 (2·97–5·30) | 626 ± 172 (297–996) | 27·4 ± 6·8 (12·5–38·2) | 471 ± 143 (210–920) | 25·5 ± 8·3 (12·5–40·1) | 1,189 ± 519 (381–2338) | 48·8 ± 10·1 (31·1–74·0) |
Thirty-six per cent of patients were successfully treated with highly active antiretroviral therapy (HAART). Of these, six of 13 patients (46%) were on a protease-inhibitor-containing HAART regimen. Two of 36 patients were coinfected with hepatitis C virus and one with hepatitis B virus. Just one patient was coinfected with both hepatitis B and C viruses. Sexual contact was the most frequently reported risk factor for acquiring HIV-1 infection (31 of 36 patients).
DL, detection limit; SD, standard deviation.
Viral load measurements
Plasma HIV-RNA copy levels were evaluated using the commercially available Versant HIV-1 RNA 3·0 assay (Bayer Diagnostics, Tarrytown, NY) according to the manufacturer’s protocol. The procedure targets highly conserved regions of the HIV-1 polymerase (pol) gene and uses proprietary branched DNA (bDNA) signal amplification technology. The lower limit of quantification for this assay is 50 (1·7 log10) copies/ml.
Isolation of lymphocytes
Human peripheral blood mononuclear cells (PBMC) were isolated from fresh EDTA-anticoagulated blood from HIV-1-infected patients or healthy donors using the standard Ficoll gradient method.45,46 PBMC were depleted of monocytes by adherence to tissue culture flask incubating 2 hr in XVIVO-15 medium (Bio-Whittaker, Walkersville, MD) supplemented with 1% autologous serum, 50 μg/ml gentamycin (Braun, Melsungen, Germany) and 2·5 μg/ml fungizone (Bristol-Myers Squibb, Munchen, Germany). Non-adherent cells were collected and washed three times with pre-warmed, serum-free XVIVO-10 medium (Bio-Whittaker), counted and re-suspended in the appropriate volume of XVIVO-10 medium.
ADA preparation and recombinant proteins
ADA from calf intestine (Roche Diagnostic Inc., Mannheim, Germany) was desalted by passage through a PD-10 column (Amersham Pharmacia Biotech, Uppsala, Sweden) and its enzymatic activity was evaluated for consumption of adenosine measured as the kinetic decrease in absorbance at 265 nm.47
Full-length glycosylated and baculovirus-expressed recombinant HIV-1 IIIB gp120 from ImmunoDiagnostics Inc. was kindly provided by the Centralised Facility for AIDS Reagents (CFAR). HIV-1 IIIB p55 group-specific antigen (Gag), also recombinant, full-length glycosylated and baculovirus-expressed, was helpfully provided by the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID).
Proliferation assays
In anti-CD3-induced proliferation experiments, T cells (2 × 105 cells/well) were incubated in XVIVO-10 medium with the indicated effectors and then with 1 ng/ml of the soluble mouse monoclonal antibody (mAb) anti-CD3 (OKT3)48,49 for the indicated times (see figure legends) in 96 round-well plates at 37° in a humid atmosphere of 5% CO2. T-cell proliferation was measured as [3H]thymidine incorporation in a 6–7-day assay at 37° in a humid atmosphere of 5% CO2. Cells were pulsed for the last 18 hr with 1 μCi thymidine/well ([3H]methyl, 2 Ci/mmol; Moravek Bioquemicals, Brea, CA) and fixed in 3·7% formaldehyde for 30 min, harvested onto filters and extensively washed using a cell harvester (LKB 1295-001; Wallac, Gaithersburg, MD). Tritium incorporation was determined using a liquid scintillation counter (1205 betaplate; Wallac).
Cytokine determination
IFN-γ, tumour necrosis factor (TNF)-α, IL-10, IL-6, IL-4 and IL-2 levels were determined by using a BD Cytometric Bead Array Human Th1/Th2 cytokine kit (Becton Dickinson Bioscience Pharmingen, San Diego, CA) according to the manufacturer’s protocol, using appropriately diluted culture supernatants.
Immunostaining and flow cytometry
Using three-colour flow cytometry, subpopulations of CD3, CD4 and CD8 cells were determined for the patients. Samples containing 105 cells were used for direct staining with the appropriate mAb conjugated with peridinin-chlorophyll-protein complex (PerCP), phycoerythrin (PE) or fluorescein isothiocyanate (FITC): CD3-PerCP, CD4-PE, CD8-FITC, CD4-PerCP, CD8-PerCP, CD45RO-PE and CD45RA-FITC (all of them obtained from Becton-Dickinson, Mountain View, CA). Mouse immunoglobulin isotypes conjugated with PerCP, PE or FITC were always used as negative controls for non-specific binding. The stained cells were analysed on a FACSCalibur flow cytometer (Becton Dickinson, San José, CA). A minimum of 5000 cells were examined on the cytometer. Lymphocytes were gated on the basis of forward- and side-scatter parameters. A gating region was referred to an FL3/SS histogram where a FL3 (CD3, CD4 or CD8) region was defined. This region was further analysed for the expression of FL1 and FL2. Data were analysed using cellquest software (Becton Dickinson, Erembodegem-Aalst, Belgium).
Statistical analysis
Statistical analyses of the data were based on the calculation of the mean, standard deviation and range, according to the nature of the variables. Differences between means were assessed using Student’s t-test for paired or unpaired data. In all tests, differences were considered significant when P-values were < 0·05.
Results
Effect of ADA on proliferation of T cells from HIV-1-infected patients
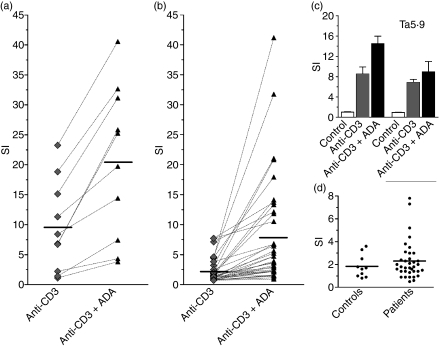
In order to test whether ADA costimulation occurs in HIV-1-infected T cells, fresh blood samples were collected from a cohort of patients (n = 36) and from healthy control volunteers (n = 10). Clinical details of the HIV-1-infected patients are summarized in Table 1. Blood samples were processed as described in the Materials and methods and assays of soluble anti-CD3-induced proliferation in the presence or absence of exogenously added ADA were performed. ADA increased T-cell proliferation expressed as the stimulation index (SI; calculated as indicated in Fig. 1) in every tested donor (Fig. 1a,b). The mean SI (± SD) for anti-CD3-induced proliferation achieved in healthy donors in the absence of exogenously added ADA was 9·6 ± 7·6 (range 1·2 to 23·3), while in the presence of ADA the SI value increased to 20·6 ± 12·7 (range 3·9 to 40·6) (Fig. 1a). Therefore, an average increase of 2·7-fold was observed in the presence of exogenously added ADA (P< 0·001 for ADA-treated versus non-treated; paired Student’s t-test). To check whether the ADA effect is attributable to ADA-mediated T-cell surface CD26 engagement,50–52 proliferation was assayed in conditions under which the ADA–CD26 interaction was blocked by the presence of the anti-CD26 antibody Ta5·9, which is directed against the ADA-binding domain of CD26.53 In these conditions the ADA effect was abolished (Fig. 1c).
Figure 1.
Adenosine deaminase (ADA) potentiates human T-lymphocyte proliferation triggered by anti-CD3. T cells from healthy donors (a, c, d) or human immunodeficiency virus 1 (HIV-1)-infected patients (b, d) were freshly isolated and stimulated with 1 ng/ml anti-CD3 [except in (d), where anti-CD3 was absent] in a 7-day assay in the absence or presence of 4 μm ADA. Proliferation was determined as [3H]thymidine incorporation. Values are expressed as the stimulation index (SI), calculated as the ratio of [3H]thymidine incorporated in stimulated T cells versus non-stimulated T cells. (a, b) T cells were stimulated in the absence (rhombuses) or presence (triangles) of ADA. Data are the mean of triplicates and each pair of linked symbols represents a different donor. (c) T cells were preincubated without or with 33 μg/ml of monoclonal antibody anti-CD26 Ta5·9 as indicated in the Materials and methods for 15 min before 4 μm ADA (black bars) or medium (grey bars) was added. T cells were triggered with 1 ng/ml anti-CD3 (black and grey bars) or not triggered (white bars). Representative data (mean ± standard deviation of triplicates) for one of 10 independent experiments are shown. (d) T cells from healthy donors (controls) or HIV-1-infected individuals (patients) were treated with 4 μm ADA in the absence of anti-CD3, and the SI was determined as the ratio of [3H]thymidine incorporated in ADA-treated versus non-treated T cells. Each dot represents the mean of triplicate measurements for the same donor and a solid line indicates the mean SI of each group.
In HIV-1-infected patients, the mean SI for anti-CD3-induced T-cell proliferation was 2·3 ± 2·0 (range 0·8–7·8; Fig. 1b). This proliferation was markedly lower than that observed in controls (P< 0·0001; unpaired Student’s t-test). In the presence of exogenously added ADA, the mean proliferation increased to 8·1 ± 9·1 (range 1·0–41·2), which was significantly lower (P <0·0001; unpaired Student’s t-test) than that observed in HIV-uninfected individuals. Despite the low proliferation index found in cells of HIV-infected individuals, ADA significantly improved T-cell activation. In fact, ADA induced a 3·5-fold increase in SI (P =0·0001; ADA-treated versus non-treated, by paired Student’s t-test). No relevant ADA-induced proliferation (mean SI < 3) was detected in the absence of anti-CD3 in both healthy and HIV-infected donors (Fig. 1d), confirming the costimulatory role of ADA in T-cell proliferation.
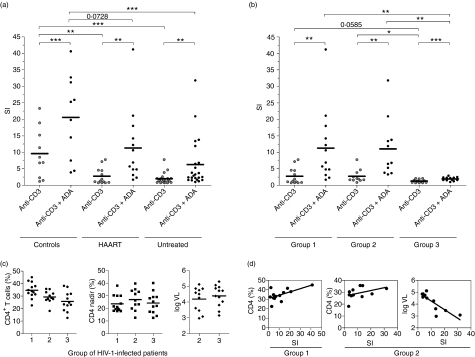
When ADA costimulation was further analysed in HIV-1-infected individuals, those patients receiving HAART were found to be the best ADA responders (Fig. 2a). In HAART-treated patients the mean SI for anti-CD3-induced T-cell proliferation was 2·8 ± 2·5 (range 0·8–7·8). This proliferation was markedly lower than that observed in controls (P <0·01; unpaired Student’s t-test). In the presence of ADA, the mean proliferation increased to 11·3 ± 10·9 (range 1·7–41·2), which was lower than that observed in HIV-uninfected individuals (Fig. 2a), but this difference did not reach statistical significance (P =0·07; unpaired Student’s t-test). Regarding untreated (viraemic) patients, the mean anti-CD3-induced proliferation achieved in the absence of exogenously added ADA was 2·0 ± 1·6 (range 0·8 to 7·8), while in the presence of ADA the mean SI value increased to 6·3 ± 7·6 (range 1·0 to 31·8). Thus, proliferation in untreated individuals resulted in indexes lower than those of controls, in either the presence or the absence of ADA (P <0·001; unpaired Student’s t-test, in both cases). When anti-CD3-mediated proliferation in the absence of ADA was compared in HAART-treated and untreated patients, no significant differences were found. In the presence of ADA, the mean proliferation in untreated patients was almost 2-fold lower than that in HAART-treated patients; nevertheless, statistical significance was not reached because of the high variability in the ADA response and the limited number of individuals available for the assays.
Figure 2.
Adenosine deaminase (ADA)-induced costimulatory effect in different groups of human immunodeficiency virus 1 (HIV-1)-infected donors. (a, b) T cells from HIV-1-infected patients (n = 36) were stimulated with 1 ng/ml anti-CD3 for 7 days in the absence (anti-CD3) or presence of 4 μm ADA (anti-CD3 + ADA) and proliferation was determined as [3H]thymidine incorporation. Values are expressed as the stimulation index (SI), calculated as the ratio of [3H]thymidine incorporated in stimulated T cells versus non-stimulated T cells. (a) Comparison among healthy controls, highly active antiretroviral therapy (HAART)-treated patients and untreated patients. (b) Comparison among HAART-treated patients (group 1), untreated patients who were still responders (group 2) and untreated patients with a low response (group 3). (c) The main characteristics of the three groups of HIV-1-infected patients. (d) Main correlations found in the response to anti-CD3 plus ADA stimulation in groups 1 and 2. *P< 0·05; **P< 0·01; *** P< 0·001, by paired or unpaired Student’s t-test.
Correlation of the ADA effect with viral load and CD4 counts
According to the above-mentioned data, the cohort of patients could be divided into various different groups. The first group was composed of patients receiving HAART (Fig. 2b; group 1, representing 36% of the patients included in the analysis and corresponding to the HAART group in Fig. 2a). Untreated patients were divided into two groups according to the T-cell proliferation achieved in the presence of ADA. One of these groups (group 2) consisted of patients who were untreated but nevertheless responders (31% of the total; Fig. 2b) and the other (group 3) consisted of untreated patients with very low SI (< 4) (33% of the total; Fig. 2b). The first group had undetectable viral load (Fig. 2c) and a relatively high percentage of CD4+ T cells (mean 34·7 ± 6·2; range 23–45). The second and third groups had detectable viral load (mean 4·19 ± 0·77; range 2·97–5·12 log10 copies/ml; and mean 4·39 ± 0·68; range 3·15–5·30 log10 copies/ml, respectively) and a lower percentage of CD4+ cells (mean 29·3 ± 5·0; range 18·1–35·7; and mean 25·7 ± 7·9; range 12·5–38·2, respectively) than the treated group (Fig. 2c; P< 0·05 for the comparison of the first and second groups and P <0·005 for the comparison of the first and third groups, by Student’s t-test). No statistically significant differences were found between the second and third groups either in the percentage of CD4+ cells or in the viral load. In all three groups, irrespective of the magnitude of the anti-CD3-mediated response, ADA exerted a costimulatory effect (P <0·01 by paired Student’s t-test in the first and second groups and P <0·001 by paired Student’s t-test in the third group; Fig. 2b). In groups 1, 2 and 3 the mean anti-CD3-induced T-cell proliferation in the absence of ADA was 2·8 ± 2·5 (range 0·8–7·8), 2·7 ± 2·0 (range 0·9–7·8) and 1·3 ± 0·5 (range 0·8–2·1), respectively, while in the presence of ADA it was 11·3 ± 10·9 (range 1·7–41·2), 11·0 ± 8·8 (range 3·3–31·8) and 1·9 ± 0·58 (range 1·0–2·9), respectively. In the presence of ADA, statistically significant differences were found among groups: group 3 displayed lower proliferation than groups 1 and 2 (P< 0·01 by unpaired Student’s t-test). In the absence of ADA, group 3 also showed lower proliferation than group 2 (P< 0·05 by unpaired Student’s t-test).
A strong positive correlation between the ADA effects on proliferation and CD4+ cells (see Fig. 2d) or nadir CD4 T-cell count was detected in a statistical analysis using Pearson’s correlation coefficient (r) and the probability of type I error (P). A correlation was found in group 1 (r = 0·604, P = 0·049 and r = 0·677, P = 0·022, respectively, for CD4 cells and nadir CD4 count) and in group 2 (r = 0·604, P = 0·049 and r = 0·677, P = 0·022, respectively). In group 2, a negative correlation between the ADA effects on proliferation and (i) current viral load (see Fig. 2d), (ii) maximal viral load or (iii) average viral load in the previous 12 months was also found (i: r = −0·832, P = 0·001; ii: r = −0·622, P = 0·031 and iii: r = −0·680, P = 0·021). The response to ADA also correlated with the percentage of naïve-phenotype CD8 T cells (measured as the CD8+ CD45RA+ CD45RO− T-cell percentage) in group 1 (r = 0·952, P = 0·013). However, for stimulation with anti-CD3 alone, the response was associated positively with the percentage of CD4+ T cells in groups 1 and 2 (r = 0·632, P = 0·022 for group 1 and r = 0·647, P = 0·021 for group 2) while in group 2 it correlated negatively with viral load (r = −0·660, P = 0·027). Furthermore, the response to anti-CD3 correlated positively with CD8+ CD45RA+ CD45RO− and CD4+ CD45RA+ CD45RO− cell counts (r = 0·853, P = 0·043 and r = 0·815, P = 0·025, respectively, for groups 1 and 2). All these data indicate that the patient’s immune status strongly influences the anti-CD3 response and the costimulatory role of ADA in anti-CD3-induced T-cell proliferation.
ADA enhances anti-CD3-induced cytokine production in patients
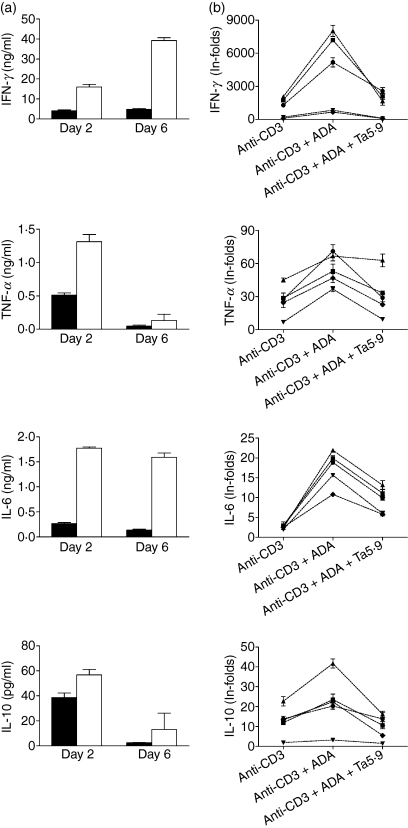
Th1/Th2 and pro-inflammatory cytokines were measured in anti-CD3-stimulated T-cell culture supernatants from five healthy and five HIV-infected donors (two of these patients, from group 1, were successfully treated with HAART, had no detectable viral load and had relatively high percentages of CD4+ T cells: 34·8% and 27·5%, respectively; while the other three, from group 2, were untreated viraemic patients with viral loads of 5·12, 3·61 and 4·59 log10 copies/ml and CD4 percentages of 32·0%, 26·3% and 27·8%, respectively) in the absence or presence of exogenously added ADA. In the healthy control group, at days 2 and 6 of treatment, ADA strongly enhanced the anti-CD3-mediated secretion of IFN-γ, TNF-α and IL-6 and less strongly enhanced that of IL-10 (Fig. 3a). IL-4 and IL-2 were also tested but their level was very low (< 12 pg/ml in the presence or absence of ADA or even undetectable). The observed increase in cytokine production was consistently found in all tested donors (Fig. 3b; P <0·001, P <0·05, P <0·0001 and P< 0·01 for IFN-γ, TNF-α, IL-6 and IL-10, respectively, for ADA-treated versus non-treated; paired Student’s t-test). The effect on cytokine production was attributable to the ADA–CD26 interaction, as the monoclonal antibody Ta5·9, which is directed against the ADA-binding site of CD26, markedly reduced the ADA-enhanced secretion of cytokines (Fig. 3b; P =0·001, P <0·05, P <0·0001 and P <0·005 for IFN-γ, TNF-α, IL-6 and IL-10, respectively; ADA + Ta5·9-treated versus ADA-treated, by paired Student’s t-test). In cells from HIV-1-infected donors, ADA enhanced the anti-CD3-mediated secretion of IFN-γ, TNF-α, IL-6 and IL-10 at days 2 and 6 of treatment (Fig. 4a). IL-4 and IL-2 were also tested but their levels were very low (< 20 pg/ml for IL-4 and < 10 pg/ml for IL-2 in the presence or absence of ADA) or even undetectable. The observed increase in cytokine production was consistently found in all the HIV-infected donors tested (Fig. 4b; P <0·05 in all cases; ADA-treated versus non-treated, by paired Student’s t-test). These results are qualitatively similar to those obtained with healthy controls, but cytokine secretion in controls was markedly higher than in HIV-infected patients. In fact, when cells were stimulated with anti-CD3, the secretion of IFN-γ, TNF-α and IL-10 in T cells from healthy controls was, respectively, 8·9, 9·1 and 2·7 times greater than that produced by T cells from infected individuals (P <0·001, P <0·005 and P =0·001 for IFN-γ, TNF-α and IL-10, respectively, by unpaired Student’s t-test). When cells were stimulated with anti-CD3 plus ADA, cytokine secretion of T cells from healthy controls for the same set of cytokines was 5·6, 4·5 and 2·9 times greater than that of T cells from HIV-infected individuals (P < 0·005 in all cases by unpaired Student’s t-test).
Figure 3.
Adenosine deaminase (ADA) increases cytokine secretion in cultures of anti-CD3-triggered T cells from healthy donors. T cells from healthy donors were stimulated with 1 ng/ml anti-CD3 in the absence or presence of 4 μm ADA and cytokine levels were determined at days 2 and 6 (a) or day 2 (b) in culture supernatants as indicated in the Materials and methods. (a) Cytokine production when cells were stimulated with anti-CD3 in the absence (black columns) or presence of 4 μm ADA (white columns); data from one representative experiment of five are shown. Data are the mean ± standard deviation of triplicates. (b) Cytokine production in culture supernatants of anti-CD3-triggered T cells from five healthy donors in the absence of ADA (anti-CD3), in the presence of 4 μm ADA (anti-CD3 + ADA) or in the presence of 4 μm ADA but with preincubation of T cells for 15 min with 33 μg/ml of the monoclonal antibody anti-CD26 Ta5·9 (anti-CD3 + ADA + Ta5·9). Values are expressed as in-folds, calculated as the ratio of the cytokine concentration (pg/ml) produced by stimulated T cells versus that produced by non-stimulated T cells. Each symbol represents a healthy donor. Data are the mean ± standard deviation of triplicates. For each donor, IL-2 and IL-4 production was also analysed, and was < 12 pg/ml or even undetectable (data not shown).
Figure 4.
Adenosine deaminase (ADA) increases cytokine secretion in cultures of anti-CD3-triggered T cells from human immunodeficiency virus 1 (HIV-1)-infected patients. T cells from HIV-1-infected patients were stimulated with 1 ng/ml anti-CD3 in the absence or presence of 4 μm ADA and cytokine levels were determined at days 2 and 6 (a) or day 2 (b) in culture supernatants as indicated in the Materials and methods. (a) Cytokine production when cells were stimulated with anti-CD3 in the absence (black columns) or presence of 4 μm ADA (white columns); data from one representative experiment of five are shown. Data are the mean ± standard deviation of triplicates. (b) Cytokine production in culture supernatants of anti-CD3-triggered T cells from five HIV-1-infected individuals in the absence of ADA (anti-CD3) or in the presence of 4 μm ADA (anti-CD3 + ADA). Values are expressed as in-folds, calculated as the ratio of the cytokine concentration (pg/ml) produced by stimulated T cells versus that produced by non-stimulated T cells. Each symbol represents an HIV-1-infected individual. Data are the mean ± standard deviation of triplicates. For each donor, IL-2 and IL-4 production was also analysed, and was < 20 pg/ml in the case of IL-4 and < 10 pg/ml for IL-2.
The impairment of ADA-induced costimulation of T-cell activation is caused by the HIV-1 protein gp120
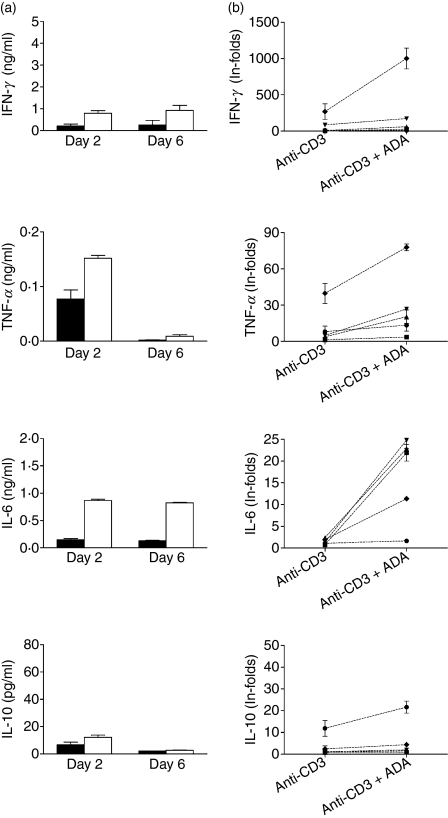
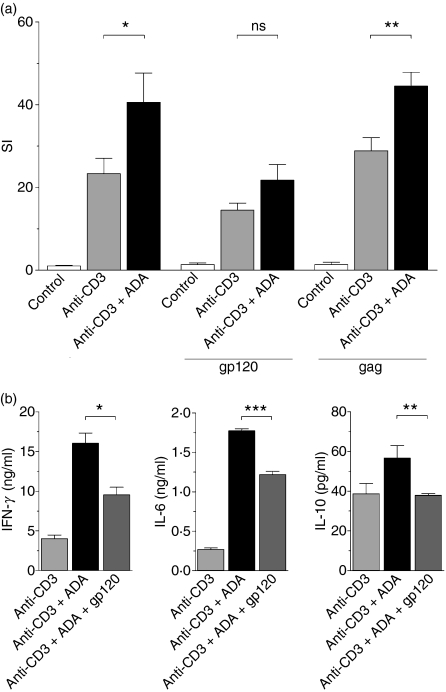
To investigate the effect of the HIV-1 envelope glycoprotein gp120 on ADA-mediated costimulation, experiments with T cells from healthy donors were performed in the absence or presence of the soluble form of this protein or HIV-1 p55 gag (used as a control). As shown in Fig. 5a, 100 nm gp120 inhibited the ADA-induced costimulatory effect on anti-CD3-mediated T-cell proliferation. A small decrease was also observed for anti-CD3 in the presence of gp120, but this could probably be explained by the displacement of the endogenous ADA released to the culture medium. The specificity of the gp120 inhibition was demonstrated by the lack of an effect of the HIV-1 protein gag, which does not interact with the ADA–CD26 complex. It should be noted that gp120 markedly diminished the anti-CD3 plus ADA-mediated secretion of the main Th1 cytokine IFN-γ and also of IL-6 and IL-10 (Fig. 5b). These results indicate that gp120 blocks the interaction of ADA with CD26 on the T-cell surface, thus preventing ADA function and contributing to the impairment of stimulus-mediated responses observed in cells from patients.
Figure 5.
The human immunodeficiency virus 1 (HIV-1) envelope glycoprotein gp120 inhibits the adenosine deaminase (ADA)-induced costimulatory effect. (a) Proliferation of unstimulated (white columns), 1 ng/ml anti-CD3-stimulated (grey columns) or 1 ng/ml anti-CD3 plus 4 μm ADA-stimulated (black columns) T cells obtained from a healthy donor was determined in the absence or presence of 100 nm HIV-1 gp120 (gp120) or 100 nm HIV-1 Gag (gag) as a control. [3H]Thymidine incorporation was measured after 6 days of culture and expressed as the stimulation index (SI), calculated as the ratio of [3H] thymidine incorporated in stimulated T cells versus non-stimulated T cells. One representative experiment of nine with different donors is shown and data are the mean ± standard deviation of triplicates. (b) Cytokine secretion of T cells stimulated with 1 ng/ml anti-CD3 (light grey columns), or with 1 ng/ml anti-CD3 plus 4 μm ADA in the absence of gp120 (black columns) or in the presence of 100 nm gp120 (dark grey columns) was determined as indicated in the Materials and methods. One representative experiment of five with different donors is shown and data are the mean ± standard deviation of triplicates. *P < 0·05; **P< 0·01; ***P < 0·001; ns, not significant, by paired Student’s t-test.
Discussion
The role of CD26 in immune regulation has been extensively characterized, with recent findings elucidating its linkage with signalling pathways and structures involved in T-lymphocyte activation as well as antigen-presenting cell (APC)–T-cell interaction (see Ohnuma et al.54 for a review). As ADA costimulation is attributable to its interaction with CD26 on the T-cell surface,50,51 and taking into account the fact that HIV-1 infectious particles and also the soluble envelope glycoprotein gp120 are able to inhibit this interaction,43,44 we hypothesized that gp120-mediated disruption of the CD26–ADA interaction might be a novel mechanism of pathology explaining some of the immunological alterations observed in HIV-1-infected patients. According to this hypothesis, the ADA costimulatory effect would be impaired during the progression of the infection, leading to a lack of T-cell activation or response to stimuli. To test the hypothesis, we designed anti-CD3-mediated proliferation assays for T-cells with suboptimal doses of antibody (in order to be able to detect costimulatory effects) and samples from a diverse cohort of patients were tested. In all these assays an ADA effect occurred but was highly variable among individuals. In spite of this, T-cell proliferation was found to be significantly lower in patients than controls. When stimulated with anti-CD3 alone, T cells from patients showed very low SIs compared with controls; this is consistent with the unresponsiveness to stimuli described in the literature.18–22 When exogenous ADA was added to cultures, the responses were strongly enhanced in samples from both controls and patients. The proliferation with ADA was consistently lower in T cells from HIV-1-infected patients than in cells from healthy individuals. Taking into account the fact that HIV-1 infectious particles and also gp120 are able to inhibit the binding of ADA to CD26 on the cell surface,43,44 the results suggest that the CD26–ADA interaction is impaired in T cells of infected individuals.
According to the hypothesis of an impaired CD26–ADA interaction occurring in vivo in T cells of HIV-infected individuals, it would be reasonable to assume that the patients with more advanced disease should be those with higher viral loads. In support of this assumption, the highest ADA costimulation was achieved in patients with controlled viral loads. Three qualitatively different outputs for ADA costimulation were detected. So, according to the size of the ADA effect, patients could be classified as good responders (corresponding to the HAART-treated group), untreated but still responders and untreated with a poor response.
ADA costimulation was positively correlated with the percentage of CD4+ cells and inversely with the viral load. Good responders had well-controlled (undetectable) viral loads as a result of HAART. Given the fact that CD4 T-cell counts provide an indicator of immunocompetence in HIV disease,55 the results presented here reveal that the immunological status of patients strongly influences the response to ADA costimuli. The fact that some patients (essentially in the group of untreated patients with a poor response) did not respond properly (SI < 4) indicates that at least a minimal immunological competence is needed to find clear responses to ADA costimuli. This observation is supported by the fact that anti-CD3 and anti-CD3 plus ADA responses correlated positively with the percentage of naïve T cells (measured as CD4+ or CD8+ CD45RA+ CD45RO− T cells). Thymic volume56 and the number of T cells with the naïve phenotype57,58 are reliable markers of immune reconstitution in HIV-positive patients, thus indicating that individuals with a conserved capacity to generate cells de novo in the thymus59 are those with the best clinical status. In addition, the T-cell proliferation achieved when cells were stimulated with anti-CD3 plus ADA correlated with the nadir CD4 T-cell count in the group of HAART-treated patients, thus indicating that, despite the relative normalization of CD4 T-cell counts achieved following HAART, functional immune restoration in terms of ADA response is incomplete and attenuated by prior depletion of the CD4 T-cell pool. Nadir CD4 counts have been demonstrated to be strongly related to other factors, such as the achievement of good vaccine responses,60,61 and to predict the decline of CD4+ T cell counts after interruption of HAART62 and also proliferative responses.61,63,64 The molecular mechanisms involved in the correlation between functional immune competence and nadir CD4 T-cell count are not well understood.65 However, cellular proliferation is a critical factor in immunization responses.66 Therefore, as ADA has a relevant role in T-cell proliferation, it would be of interest to investigate in the future whether the effect of gp120 on the ADA–CD26 complex may lead to other perturbations found in HIV-infected individuals.
Once the key influence of the clinical immune status of the patients had been revealed, we carried out anti-CD3 plus ADA-induced proliferation of T cells of healthy volunteers in the presence of soluble gp120. This eliminates some of the variables leading to immunological defects found in HIV-1-infected patients. In these conditions, gp120 not only abolished the ADA effect as measured by proliferation but also led to a marked reduction in the secretion of the pro-inflammatory cytokines IL-6 and IL-10 and of the main Th1 cytokine IFN-γ. If the envelope glycoprotein gp120 is responsible for the impairment of the ADA–CD26 interaction in these circumstances, it can be assumed that such an impairment would also take place in the circumstances of an ADA-induced effect in cells from HIV-infected patients. Indeed, the inhibition of ADA binding to CD26 by viral particles is likely to occur in vivo, as it has been shown that the 50% inhibitory concentration (IC50) for inhibition of ADA binding to the Jurkat T-cell line is similar to the dissociation constant (Kd or affinity) values reported for binding of gp120 to CD4.43,67 However, ADA is found at high levels in the serum of HIV-infected patients,68–73 and this could be, at least in part, attributable to the displacement of ADA from its anchor on the T-cell surface. As previously reported, a CD4–gp120 interaction is required for efficient inhibition of ADA binding to CD26.44
As the gp120-mediated blockade of the CD26–ADA interaction is relevant enough to be reflected as a diminished cytokine secretion promoted by ADA (a fact that is also shown in a reduced T-cell proliferation) and as ADA has a key role in T-cell activation events50,51 and in the maturation of the immune system,74,75 it can be speculated that continuous disruption of ADA–CD26 complexes in HIV-1-infected patients could result in a chronic state of impairment of responses to stimuli. Taken together, our results indicate that gp120-mediated disruption of the CD26–ADA interaction is a novel mechanism of pathology which partially explains some of the immunological alterations observed in HIV-1-infected patients. It should be noted that the size of the effect in terms of ADA-mediated responses depends not only on the quantities of virus and CD4 T cells, but also on the previous immunological status of the patient, particularly nadir CD4 counts.
Acknowledgments
The authors are grateful to all blood donors and to Mª Carmen Pardo, Montserrat Compte and MªÁngeles López for technical assistance with blood extraction. Recombinant HIV-1 gp120 from ImmunoDiagnostics Inc. was kindly provided by the Centralised Facility for AIDS Reagents (CFAR) supported by the EU Programme European Vaccine against AIDS (EVA)/Medical Research Council (MRC), contract QLKZ-CT-1999-00609, the National Institute for Biological Standards and Control (NIBSC), AIDS Vaccine Integrated Project (AVIP), contract LSHP-CT-2004-503487, and the UK Medical Research Council. HIV-1 Gag was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID). This study was supported in part by Fundació Marató of Catalonian Telethon Grant 02/021010 and FIPSE 36750/08 to RF, the Center for Research and Development of HIV Vaccines in Catalonia (IDIBAPS-HIVACAT), Red Temática Cooperativa de Grupos de Investigación en Sida del Fondo de Investigación Sanitaria (Fondo de Investigación Sanitaria de la Seguridad Social) (ISCIII-RETIC RD06/006), FIPSE 36536/05, FIS06-1259, SAF 05/05566, FIS PI050058, FIT 090100-2005-9 and FIS 04/0503 (FIPSE is a non-profit foundation including: the Spanish Ministry of Health, Abbott Laboratories, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp and Dohme and Roche). MP was supported by contract FIS 03/00072 from the Fundació Privada Clínic per la Recerca Biomèdica in collaboration with the Spanish Health Department.
Disclosures
The authors have no known conflict of interest.
References
- 1.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–7. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 2.Klatzmann D, Barre-Sinoussi F, Nugeyre MT, et al. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984;225:59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- 3.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–6. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D, Shankar P, Xu Z, et al. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 2003;101:226–35. doi: 10.1182/blood-2002-03-0791. [DOI] [PubMed] [Google Scholar]
- 5.Zhu T, Muthui D, Holte S, et al. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–16. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meltzer MS, Nakamura M, Hansen BD, Turpin JA, Kalter DC, Gendelman HE. Macrophages as susceptible targets for HIV infection, persistent viral reservoirs in tissue, and key immunoregulatory cells that control levels of virus replication and extent of disease. AIDS Res Hum Retroviruses. 1990;6:967–71. doi: 10.1089/aid.1990.6.967. [DOI] [PubMed] [Google Scholar]
- 7.Moir S, Malaspina A, Ogwaro KM, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci USA. 2001;98:10362–7. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Vassilev V, Nosikov VV, Serebrovskaya LV, Ivanova LA, Pokrovsky VV. Clinical significance of HIV DNA in polymorphonuclear neutrophils from patients with HIV infection. J Acquir Immune Defic Syndr. 1993;6:587–91. [PubMed] [Google Scholar]
- 9.Macatonia SE, Lau R, Patterson S, Pinching AJ, Knight SC. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Foley P, Kazazi F, Biti R, Sorrell TC, Cunningham AL. HIV infection of monocytes inhibits the T-lymphocyte proliferative response to recall antigens, via production of eicosanoids. Immunology. 1992;75:391–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Veiga AP, Casseb J, Duarte AJ. Humoral response to hepatitis B vaccination and its relationship with T CD45RA+ (naive) and CD45RO+ (memory) subsets in HIV-1-infected subjects. Vaccine. 2006;24:7124–8. doi: 10.1016/j.vaccine.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 12.Opravil M, Fierz W, Matter L, Blaser J, Luthy R. Poor antibody response after tetanus and pneumococcal vaccination in immunocompromised, HIV-infected patients. Clin Exp Immunol. 1991;84:185–9. doi: 10.1111/j.1365-2249.1991.tb08146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–42. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt PW. Role of immune activation in HIV pathogenesis. Curr HIV/AIDS Rep. 2007;4:42–7. doi: 10.1007/s11904-007-0007-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:332–40. doi: 10.1097/00042560-199808010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kestens L, Vanham G, Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–6. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 18.Gruters RA, Terpstra FG, De Jong R, Van Noesel CJ, Van Lier RA, Miedema F. Selective loss of T cell functions in different stages of HIV infection. Early loss of anti-CD3-induced T cell proliferation followed by decreased anti-CD3-induced cytotoxic T lymphocyte generation in AIDS-related complex and AIDS. Eur J Immunol. 1990;20:1039–44. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- 19.Sieg SF, Harding CV, Lederman MM. HIV-1 infection impairs cell cycle progression of CD4(+) T cells without affecting early activation responses. J Clin Invest. 2001;108:757–64. doi: 10.1172/JCI12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahwa R, McCloskey TW, Aroniadis OC, Strbo N, Krishnan S, Pahwa S. CD8+ T cells in HIV disease exhibit cytokine receptor perturbation and poor T cell receptor activation but are responsive to gamma-chain cytokine-driven proliferation. J Infect Dis. 2006;193:879–87. doi: 10.1086/500471. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen C, Dickmeiss E, Gaub J, et al. T-cell subset alterations and lymphocyte responsiveness to mitogens and antigen during severe primary infection with HIV: a case series of seven consecutive HIV seroconverters. AIDS. 1990;4:523–6. doi: 10.1097/00002030-199006000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Schellekens PT, Roos MT, De Wolf F, Lange JM, Miedema F. Low T-cell responsiveness to activation via CD3/TCR is a prognostic marker for acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus-1 (HIV-1)-infected men. J Clin Immunol. 1990;10:121–7. doi: 10.1007/BF00918194. [DOI] [PubMed] [Google Scholar]
- 23.Galati D, Bocchino M. New insights on the perturbations of T cell cycle during HIV infection. Curr Med Chem. 2007;14:1920–4. doi: 10.2174/092986707781368559. [DOI] [PubMed] [Google Scholar]
- 24.Piedimonte G, Corsi D, Paiardini M, et al. Unscheduled cyclin B expression and p34 cdc2 activation in T lymphocytes from HIV-infected patients. AIDS. 1999;13:1159–64. doi: 10.1097/00002030-199907090-00003. [DOI] [PubMed] [Google Scholar]
- 25.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–9. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barcellini W, Rizzardi GP, Borghi MO, Fain C, Lazzarin A, Meroni PL. TH1 and TH2 cytokine production by peripheral blood mononuclear cells from HIV-infected patients. AIDS. 1994;8:757–62. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Shrivastav S, Kino T, Cunningham T, et al. HIV-1 Vpr suppresses transcriptional activity of PPAR{gamma} and inhibits adipocyte differentiation: Implications for HIV-associated lipodystrophy. Mol Endocrinol. 2008;22:234–47. doi: 10.1210/me.2007-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brugger B, Krautkramer E, Tibroni N, et al. Human Immunodeficiency Virus Type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains. Retrovirology. 2007;4:70. doi: 10.1186/1742-4690-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph AM, Ladha JS, Mojamdar M, Mitra D. Human immunodeficiency virus-1 Nef protein interacts with Tat and enhances HIV-1 gene expression. FEBS Lett. 2003;548:37–42. doi: 10.1016/s0014-5793(03)00725-7. [DOI] [PubMed] [Google Scholar]
- 30.Roeth JF, Collins KL. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol Mol Biol Rev. 2006;70:548–63. doi: 10.1128/MMBR.00042-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peruzzi F. The multiple functions of HIV-1 Tat: proliferation versus apoptosis. Front Biosci. 2006;11:708–17. doi: 10.2741/1829. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizuka N, Yoshizuka-Chadani Y, Krishnan V, Zeichner SL. Human immunodeficiency virus type 1 Vpr-dependent cell cycle arrest through a mitogen-activated protein kinase signal transduction pathway. J Virol. 2005;79:11366–81. doi: 10.1128/JVI.79.17.11366-11381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majumder B, Janket ML, Schafer EA, et al. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005;79:7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumder B, Venkatachari NJ, Schafer EA, Janket ML, Ayyavoo V. Dendritic cells infected with vpr-positive human immunodeficiency virus type 1 induce CD8+ T-cell apoptosis via upregulation of tumor necrosis factor alpha. J Virol. 2007;81:7388–99. doi: 10.1128/JVI.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatachari NJ, Majumder B, Ayyavoo V. Human immunodeficiency virus (HIV) type 1 Vpr induces differential regulation of T cell costimulatory molecules: direct effect of Vpr on T cell activation and immune function. Virol. 2007;358:347–56. doi: 10.1016/j.virol.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Jones GJ, Barsby NL, Cohen EA, et al. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–11. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen JL, Planelles V. The role of Vpr in HIV-1 pathogenesis. Curr HIV Res. 2005;3:43–51. doi: 10.2174/1570162052772988. [DOI] [PubMed] [Google Scholar]
- 38.Tungaturthi PK, Sawaya BE, Singh SP, et al. Role of HIV-1 Vpr in AIDS pathogenesis: relevance and implications of intravirion, intracellular and free Vpr. Biomed Pharmacother. 2003;57:20–4. doi: 10.1016/s0753-3322(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 39.Arriaga ME, Carr J, Li P, Wang B, Saksena NK. Interaction between HIV-1 and APOBEC3 sub-family of proteins. Curr HIV Res. 2006;4:401–9. doi: 10.2174/157016206778560063. [DOI] [PubMed] [Google Scholar]
- 40.Sakai K, Dimas J, Lenardo MJ. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc Natl Acad Sci USA. 2006;103:3369–74. doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fantuzzi L, Purificato C, Donato K, Belardelli F, Gessani S. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J Virol. 2004;78:9763–72. doi: 10.1128/JVI.78.18.9763-9772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metlas R, Veljkovic V. Does the HIV-1 manipulate immune network via gp120 immunoglobulin-like domain involving V3 loop? Vaccine. 1995;13:355–9. doi: 10.1016/0264-410x(95)98256-a. [DOI] [PubMed] [Google Scholar]
- 43.Valenzuela A, Blanco J, Callebaut C, et al. Adenosine deaminase binding to human CD26 is inhibited by HIV-1 envelope glycoprotein gp120 and viral particles. J Immunol. 1997;158:3721–9. [PubMed] [Google Scholar]
- 44.Blanco J, Valenzuela A, Herrera C, Lluis C, Hovanessian AG, Franco R. The HIV-1 gp120 inhibits the binding of adenosine deaminase to CD26 by a mechanism modulated by CD4 and CXCR4 expression. FEBS Lett. 2000;477:123–8. doi: 10.1016/s0014-5793(00)01751-8. [DOI] [PubMed] [Google Scholar]
- 45.Boyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- 46.Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976;5:9–15. [PubMed] [Google Scholar]
- 47.Franco R, Canela EI, Bozal J. Heterogeneous localization of some purine enzymes in subcellular fractions of rat brain and cerebellum. Neurochem Res. 1986;11:423–35. doi: 10.1007/BF00965016. [DOI] [PubMed] [Google Scholar]
- 48.Kung P, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206:347–9. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 49.Van Wauwe JP, De Mey JR, Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–13. [PubMed] [Google Scholar]
- 50.Martin M, Huguet J, Centelles JJ, Franco R. Expression of ecto-adenosine deaminase and CD26 in human T cells triggered by the TCR-CD3 complex. Possible role of adenosine deaminase as costimulatory molecule. J Immunol. 1995;155:4630–43. [PubMed] [Google Scholar]
- 51.Pacheco R, Martinez-Navio JM, Lejeune M, et al. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc Natl Acad Sci USA. 2005;102:9583–8. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pacheco R, Lluis C, Franco R. Role of CD26-adenosine deaminase interaction in T cell-mediated immunity. Inmunología. 2005;24:235–45. [Google Scholar]
- 53.De Meester I, Vanham G, Kestens L, et al. Binding of adenosine deaminase to the lymphocyte surface via CD26. Eur J Immunol. 1994;24:566–70. doi: 10.1002/eji.1830240311. [DOI] [PubMed] [Google Scholar]
- 54.Ohnuma K, Takahashi N, Yamochi T, Hosono O, Dang NH, Morimoto C. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci. 2008;13:2299–310. doi: 10.2741/2844. [DOI] [PubMed] [Google Scholar]
- 55.Phillips AN, Sabin CA, Elford J, Bofill M, Janossy G, Lee CA. Use of CD4 lymphocyte count to predict long-term survival free of AIDS after HIV infection. BMJ. 1994;309:309–13. doi: 10.1136/bmj.309.6950.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clerici M, Saresella M, Trabattoni D, Ferrante P, Vanzulli A, Vigano A. Thymic volume predicts long-term immune reconstitution in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2002;16:2219–21. doi: 10.1097/00002030-200211080-00015. [DOI] [PubMed] [Google Scholar]
- 57.De PP, Bortolin MT, Zanussi S, Monzoni A, Pratesi C, Giacca M. Changes in thymic function in HIV-positive patients treated with highly active antiretroviral therapy and interleukin-2. Clin Exp Immunol. 2001;125:440–6. doi: 10.1046/j.1365-2249.2001.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandez S, Nolan RC, Price P, et al. Thymic function in severely immunodeficient HIV type 1-infected patients receiving stable and effective antiretroviral therapy. AIDS Res Hum Retroviruses. 2006;22:163–70. doi: 10.1089/aid.2006.22.163. [DOI] [PubMed] [Google Scholar]
- 59.Al-Harthi L, Landay A. Immune recovery in HIV disease: role of the thymus and T cell expansion in immune reconstitution strategies. J Hematother Stem Cell Res. 2002;11:777–86. doi: 10.1089/152581602760404586. [DOI] [PubMed] [Google Scholar]
- 60.Lange CG, Lederman MM. Immune reconstitution with antiretroviral therapies in chronic HIV-1 infection. J Antimicrob Chemother. 2003;51:1–4. doi: 10.1093/jac/dkg071. [DOI] [PubMed] [Google Scholar]
- 61.Lange CG, Valdez H, Medvik K, Asaad R, Lederman MM. CD4+ T-lymphocyte nadir and the effect of highly active antiretroviral therapy on phenotypic and functional immune restoration in HIV-1 infection. Clin Immunol. 2002;102:154–61. doi: 10.1006/clim.2001.5164. [DOI] [PubMed] [Google Scholar]
- 62.Skiest DJ, Morrow P, Allen B, et al. It is safe to stop antiretroviral therapy in patients with preantiretroviral CD4 cell counts > 250 cells/microL. J Acquir Immune Defic Syndr. 2004;37:1351–7. doi: 10.1097/00126334-200411010-00003. [DOI] [PubMed] [Google Scholar]
- 63.Lederman HM, Williams PL, Wu JW, et al. Incomplete immune reconstitution after initiation of highly active antiretroviral therapy in human immunodeficiency virus-infected patients with severe CD4+ cell depletion. J Infect Dis. 2003;188:1794–803. doi: 10.1086/379900. [DOI] [PubMed] [Google Scholar]
- 64.Markowitz N, Bebchuk JD, Abrams DI. Nadir CD4+ T cell count predicts response to subcutaneous recombinant interleukin-2. Clin Infect Dis. 2003;37:e115–20. doi: 10.1086/378293. [DOI] [PubMed] [Google Scholar]
- 65.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–23. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 66.Salerno-Goncalves R, Sztein MB. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 2006;14:536–42. doi: 10.1016/j.tim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Lasky LA, Nakamura G, Smith DH, et al. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987;50:975–85. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- 68.Murray JL, Loftin KC, Munn CG, Reuben JM, Mansell PW, Hersh EM. Elevated adenosine deaminase and purine nucleoside phosphorylase activity in peripheral blood null lymphocytes from patients with acquired immune deficiency syndrome. Blood. 1985;65:1318–24. [PubMed] [Google Scholar]
- 69.Delia S, Mastroianni CM, Massetti AP, et al. Adenosine deaminase activity and acquired immunodeficiency syndrome (AIDS) Clin Chem. 1987;33:1675. [PubMed] [Google Scholar]
- 70.Tsuboi I, Sagawa K, Shichijo S, Yokoyama MM, Ou DW, Wiederhold MD. Adenosine deaminase isoenzyme levels in patients with human T-cell lymphotropic virus type 1 and human immunodeficiency virus type 1 infections. Clin Diagn Lab Immunol. 1995;2:626–30. doi: 10.1128/cdli.2.5.626-630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niedzwicki JG, Mayer KH, Abushanab E, Abernethy DR. Plasma adenosine deaminase2 is a marker for human immunodeficiency virus-1 seroconversion. Am J Hematol. 1991;37:152–5. doi: 10.1002/ajh.2830370303. [DOI] [PubMed] [Google Scholar]
- 72.Cowan MJ, Brady RO, Widder KJ. Elevated erythrocyte adenosine deaminase activity in patients with acquired immunodeficiency syndrome. Proc Natl Acad Sci USA. 1986;83:1089–91. doi: 10.1073/pnas.83.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chittiprol S, Satishchandra P, Bhimasenarao RS, et al. Plasma adenosine deaminase activity among HIV1 Clade C seropositives: relation to CD4 T cell population and antiretroviral therapy. Clin Chim Acta. 2007;377:133–7. doi: 10.1016/j.cca.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Fischer D, Van der Weyden MB, Snyderman R, Kelley WN. A role for adenosine deaminase in human monocyte maturation. J Clin Invest. 1976;58:399–407. doi: 10.1172/JCI108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aldrich MB, Chen W, Blackburn MR, Martinez-Valdez H, Datta SK, Kellems RE. Impaired germinal center maturation in adenosine deaminase deficiency. J Immunol. 2003;171:5562–70. doi: 10.4049/jimmunol.171.10.5562. [DOI] [PubMed] [Google Scholar]