Abstract
We previously showed that introduction of transporter associated with antigen processing (TAP) 1 into TAP-negative CMT.64, a major histocompatibility complex class I (MHC-I) down-regulated mouse lung carcinoma cell line, enhanced T-cell immunity against TAP-deficient tumour cells. Here, we have addressed two questions: (1) whether such immunity can be further augmented by co-expression of TAP1 with B7.1 or H-2Kb genes, and (2) which T-cell priming mechanism (tumour direct priming or dendritic cell cross-priming) plays the major role in inducing an immune response against TAP-deficient tumours. We introduced the B7.1 or H-2Kb gene into TAP1-expressing CMT.64 cells and determined which gene co-expressed with TAP1 was able to provide greater protective immunity against TAP-deficient tumour cells. Our results show that immunization of mice with B7.1 and TAP1 co-expressing but not H-2Kb and TAP1 co-expressing CMT.64 cells dramatically augments T-cell-mediated immunity, as shown by an increase in survival of mice inoculated with live CMT.64 cells. In addition, our results suggest that induction of T-cell-mediated immunity against TAP-deficient tumour cells could be mainly through tumour direct priming rather than dendritic cell cross-priming as they show that T cells generated by tumour cell-lysate-loaded dendritic cells recognized TAP-deficient tumour cells much less than TAP-proficient tumour cells. These data suggest that direct priming by TAP1 and B7.1 co-expressing tumour cells is potentially a major mechanism to facilitate immune responses against TAP-deficient tumour cells.
Keywords: B7.1, transporter associated with antigen processing, T cells, tumour immunity
Introduction
Transporter associated with antigen processing (TAP) is a heterodimer consisting of TAP1 and TAP2 subunits. The function of TAP is to transport cytosolic peptides into the lumen of the endoplasmic reticulum (ER) where the peptides are loaded onto major histocompatibility complex class I (MHC-I) molecules and finally displayed on the cell surface for CD8+ T-cell recognition.1 Deficiency in TAP expression is frequently observed in human cancer,2,3 resulting in failure of surface expression of TAP-dependent peptide antigens, thus abolishing CD8+ T-cell recognition.2,3 However, accumulating data from different research groups indicate that TAP-deficient tumour cells can present TAP-independent antigens and that these antigens can induce a tumour antigen-specific T-cell response.4–7 For example, TAP-deficient T lymphoma RMA-S cells (which express TAP1 only) can present TAP-independent weak MHC-I binding peptides.5 Introduction of the costimulatory molecule B7.1 into RMA-S cells can induce a T-cell response against RMA-S cells but not against TAP-proficient RMA-S cells (cells transfected with the TAP2 gene).4 One TAP-independent antigen that has been identified from an ER-lumen LAG1 homolog, ceramide synthase 5 (Lass5) protein is presented only in TAP-deficient and not in TAP-proficient cells.7 In addition, many reports have shown that introduction of the TAP1 gene into TAP-negative murine tumour cells increases tumour antigenicity and immunogenicity,6,8,9 suggesting that antigens presented on TAP1-expressing tumour cells can provide immune responses.
Dendritic cell (DC) cross-priming and tumour direct priming are two mechanisms involved in the induction of T-cell-mediated tumour antigen-specific immune responses.10–13 DC cross-priming requires uptake of antigens from apoptotic cells followed by processing and presentation of antigens on the cell surface for T-cell induction.10,12 Many reports indicate that antigens captured by DCs for CD8+ T-cell priming can be processed through the conventional MHC-I antigen presentation pathway14,15 as well as through trogocytosis of a donor MHC-I–peptide complex.16,17 In addition to cross-priming, tumour cells are able to directly prime T cells.11,13 As the costimulatory molecule B7.1 plays an important role in augmenting CD8+ T-cell-mediated tumour antigen-specific immune responses,18–20 direct priming of CD8+ T cells by B7.1-modified tumour cells seems highly probable, as indicated by Cayeux et al.21 However, another study comparing the two priming mechanisms showed that cross-priming is a more effective mechanism than direct priming.22 That study used antigen presentation-proficient cells as the detection system and thus it is questionable if its conclusions are applicable to antigen presentation-deficient cells. Transduction of B7.1 into TAP1-expressing RMA-S cells facilitates the response of T cells to TAP1-expressing or TAP1−/− cells but not to TAP-proficient cells, indicating that the generated T cells recognize TAP-independent antigens.4,7 This raises the question of whether normal DCs efficiently cross-present such antigens for T-cell priming.
In previous studies, we introduced a TAP1 gene into the TAP-negative murine lung carcinoma cell line CMT.64 and found that TAP1 expression increased tumour antigenicity and immunogenicity6 and decreased the numbers of CD3+/interleukin (IL)-10-positive tumour-infiltrating lymphocytes.9 In the present study, we introduced B7.1 or H-2Kb into TAP1-expressing CMT.64 tumour cells and determined if co-expression of TAP1 and B7.1 or TAP1 and H-2Kb in the CMT.64 cells further augmented the T-cell-mediated immune response against TAP-negative CMT.64 cells. In addition, we explored whether tumour direct priming played a dominant role in induction of T cells that recognize TAP-deficient tumour cells.
Materials and methods
Animals
The C57BL/6 (H-2b) mouse strain and T-cell-deficient nude mouse (H-2b) strain were obtained from the National Cancer Institute (NCI) and housed in the animal facility at the Louisiana State University (LSU) Health Sciences Center. All mice used for the experiments were 6–10-week-old females and were maintained in pathogen-free facilities and treated in accordance with the guidelines of the Animal Use Committee at the LSU Health Sciences Center.
Vectors
Recombinant vaccinia virus (VV) carrying an entire TAP1 gene (VV-TAP1) and recombinant VV carrying an H-2Kb gene (VV-Kb)23 were kindly provided by Dr J. Yewdell (Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD). VV carrying a B7.1 gene24 (VV-B7.1) was kindly provided by Dr J. Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, Bethesda, MD). VV carrying a GFP gene (VV-GFP) was kindly provided by Dr K. Kirkegaard (Microbiology and Immunology, Stanford University, School of Medicine, Stanford, CA). The vector inserted with the TAP1 gene is pcDNA3.1/His (Invitrogen Corporation, Carlsbad, CA). The vector inserted with the Kb gene is pEF4/Myc-His (Invitrogen). A B7.1 vector25 was provided by Dr S. Li (Louisiana State University, Baton Rouge, LA). B7.1 cDNA was isolated and inserted into a pUB6/V5-His vector (Invitrogen). A mouse TAP2 gene was inserted into a pUB6/V5-His vector.
Cell lines and cell culture
The mouse lung carcinoma cell line CMT.64-7 (H-2b), designated as CMT.64,26 was kindly provided by Dr Gunnel Hallden (Imperial College of Science, London, UK). Five CMT.64 transfectants were generated by transfection with empty vector, mouse TAP1, and/or the B7.1 gene(s). CMT.64/pp (two empty vectors, pcDNA3.1/His and pUB6/V5-His), CMT.64/pp1 (two empty vectors, pcDNA3.1/His and pEF4/Myc-His), CMT.TAP1/pEF4,17 CMT.TAP1/Kb,17 CMT.TAP1/B7.1 and CMT.TAP1,2 cl.21 (i.e. clone 21 of CMT.64 cells transfected with TAP1 and TAP2 genes were inserted into pcDNA3.1/His and pUB6/V5-His vectors, respectively) cell lines were generated for this study. Transfectants were maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) containing 10% fetal bovine serum (FBS), 1000 μg/ml neomycin (for the pcDNA3.1/His vector) and 20 μg/ml blasticidin (for the pUB6/V5-His vector) or 1000 μg/ml neomycin and 40 μg/ml Zeocin selection medium (Invitrogen, Frederick, MD). CMT.64 and mouse mammary tumour Mid-T2 (H-2q)27 cells were cultured in DMEM.
MHC-I and B7.1 expression
Surface MHC-I and B7.1 expression was detected by a FACScan analyser (Becton Dickinson, Mountain View, CA) using direct and indirect immunofluorescence. For B7.1 expression, a fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD80 (clone 16-10A1) monoclonal antibody (mAb) (Biolegend, San Diego, CA) was used, and phosphate-buffered saline (PBS) was used as a negative control. For H-2Kb expression, an H-2Kb-specific Y-3 primary mAb [American Type Culture Collection (ATCC), Manassas, VA] and an FITC-conjugated goat anti-mouse immunoglobulin (IgG) secondary antibody (Ab) (Jackson ImmunoResearch, West Grove, PA) were used, and an irrelevant primary mAb, 15-1-5P, specific for H-2Kk and H-2Dk (ATCC) was used as a negative control.
Detection of TAP
Expression of the mouse TAP1 protein was determined by western blot.9 For TAP1 expression, blots were incubated with a goat anti-mouse TAP1 polyclonal antibody (Ab) at 1 : 1000 dilution (according to the manufacturer’s instructions) followed by a horseradish peroxidase (HRP)-labelled bovine anti-goat Ab (1 : 2000 dilution) (both from Santa Cruz Biotechnology, Santa Cruz, CA). For TAP2 expression, blots were incubated in mouse antiserum at 1 : 1000 dilution followed by an HRP-labelled goat anti-mouse IgG Ab (1 : 3000 dilution) (Santa Cruz Biotechnology). Mouse antiserum against the mouse TAP2 protein was created for this study by immunizing BALB/c mice with a peptide sequence (mapping at the C-terminus of mTAP2), DGQDVYAHLVQQRLEA-C, with a cysteine at the C-terminus, linked to a keyhole limpet hemocyanin (KLH) carrier protein (Pierce Biotechnology, Rockford, IL). This antiserum can detect the mouse TAP2 protein in lysates of RMA cells but not in TAP2-deficient RMA-S or CMT.64 cells. For a loading control, the enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as described previously.6,9
Preparation of bone marrow-derived DCs
Bone marrow-derived DCs were generated from 6- to 8-week-old C57BL/6 female mice. DCs were cultured as described previously28 with some modification. DCs were cultured in RPMI-1640 complete medium containing 10 ng/ml each of recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 (PeproTech Inc., Rock Hill, NJ). On day 3 of culture, fresh medium was added to the DC cultures. On day 6 of culture, DCs were exposed for 6 hr to a lysate of γ-irradiated CMT.TAP1/B7.1 tumour cells at a concentration corresponding to a DC:cell ratio of 1 : 1 and were subsequently subjected to maturation with 100 ng/ml lipopolysaccharide (LPS; Sigma, St Louis, MO) overnight. The matured DCs were washed twice with PBS and used for mouse injection.
Generation of cytolytic T lymphocytes (CTLs) and cytotoxicity tests
The CMT.TAP1/B7.1 cell-lysate-loaded mature DCs (1 × 106 cells per mouse) or γ-irradiated (10 000 rads) CMT.TAP1/B7.1 cells (5 × 106 cells per mouse) were injected intraperitoneally (i.p.) into C57BL/6 mice. After 7 days, the immunized splenocytes were collected and re-stimulated with relevant cells used for immunization. For re-stimulation with tumour cell-lysate-loaded DCs, DCs were γ-irradiated (10 000 rads) and added to the splenocyte culture at a 1 : 30 ratio of DC:splenocyte. For re-stimulation with CMT.TAP1/B7.1 cells, cells were γ-irradiated and treated with 30 μg/ml mitomycin-c for 2 hr and added to the T-cell culture at a 1 : 10 ratio of cell:splenocyte. The immunized splenocytes were cultured for 5 days and used as effectors in standard 51Cr-release assays, and the targets were CMT.64/pp and CMT.TAP1,2 cl.21 cells.
Enzyme-linked immunosorbent assay (ELISA) analysis of antigen-specific interferon (IFN)-γ-secreting splenocytes
C57BL/6 mice (n = 3) were injected i.p. with γ-irradiated tumour cells (5 × 106 cells per mouse). Seven days after immunization, the splenocytes were re-stimulated with γ-irradiated CMT.64 cells (treated with 30 μg/ml mitomycin-c for 2 hr) at a ratio of 1 : 7 (tumour cell:splenocyte). Supernatants of the culture were collected at day 5 after in vitro re-stimulation. The levels of secreted IFN-γ were determined using a Mouse IFN-γ Quantikine ELISA assay (R&D Systems Inc., Minneapolis, MN). Analysis of variance (anova) was performed and differences were considered significant at P < 0·05.
Detection of tumour challenge experiments (memory immune response)
C57BL/6 mice or nude mice were immunized i.p. with γ-irradiated transfectants (2 × 106 cells per mouse) (Table 2) or with γ-irradiated and mitomycin-c (30 μg/ml)-treated CMT.64 cells (5 × 106 cells/mouse) infected with either VV-GFP + VV-GFP, VV-GFP + VV-TAP1 or VV-TAP1 + VV-B7.1. After a 20-day immunization period, mice were challenged i.p. with CMT.64 tumour cells (2·5 × 105 cells per mouse), and the time of morbidity was recorded. Each group contained 10 mice. Statistics for mouse survival were obtained using the Kaplan–Meier log rank survival test and differences were considered significant atP<0·05.
Table 2.
Differences in immune protection of mice from transporter associated with antigen processing (TAP)-negative tumour challenge by transfectants
| Cell line for immunization | C57BL/6 survival | Time (days)1 | P-value2 | Nude mouse survival | Time (days)1 |
|---|---|---|---|---|---|
| MID-T2 | 0/10 | 26 | 0/10 | 24 | |
| CMT.64/pp | 0/10 | 59 | > 0·053 | 0/10 | 38 |
| CMT.64/pp1 | 0/10 | 62 | > 0·053 | Not tested | – |
| CMT.TAP1/pEF4 | 4/10 | – | > 0·054, < 0·055 | 0/10 | 39 |
| CMT.TAP1/Kb | 5/10 | – | > 0·054, < 0·055 | Not tested | – |
| CMT.TAP1/B7.1 | 9/10 | – | < 0·056 | 0/10 | 40 |
C57BL/6 mice and nude mice (n = 10 in each group) were immunized intraperitoneally (i.p.) with γ-irradiated tumour cells (2 × 106 cells per mouse). After a 20-day immunization, the mice were challenged i.p. with live CMT.64 cells (2·5 × 105 cells per mouse), and the time of morbidity was recorded for another 120 days.
All mice were dead in a mouse group (0/10) at the day after live tumour cell challenge.
Statistical analysis was performed in tumour-bearing C57BL/6 mouse groups.
Comparison between two mouse groups immunized with CMT.64/pp and CMT.64/pp1 cells.
Comparison between two groups immunized with CMT.TAP1/pEF4 and CMT.TAP1/Kb cells.
Mice immunized with either CMT.TAP1/pEF4 or CMT.TAP1/Kb cells compared with mice immunized with either CMT.64/pp or CMT.64/pp1 cells.
Mice immunized with CMT.TAP1/B7.1 cells compared with mice immunized with CMT.TAP1/pEF4 or CMT.TAP1/Kb cells.
All nude mouse groups showed no statistical difference.
Detection of primary immune response to tumour in vivo
C57BL/6 mice were inoculated with live CMT.64 cells (1 × 105 cells per mouse). Three and six days after live tumour inoculation, mice (n = 10 in each group) were treated i.p with γ-irradiated CMT.TAP1/pEF4 tumour cells (1 × 107 cells per mouse) infected with 1 : 1 [multiplicity of infection (MOI)] VV-B7.1, VV-Kb or VV-GFP, and the time of morbidity was recorded. Mice treated i.p. with γ-irradiated CMT.64/pp1 cells infected with 1 : 1 (MOI) VV-GFP were used as a negative control.
Results and discussion
We previously showed that immunization of C57BL/6 mice with γ-irradiated TAP1-transfected CMT.64 cells provided approximately 33% protection in mice challenged with TAP-deficient tumour cells.6 These results suggested that TAP1 expression in the tumour cells played a critical role in augmenting a tumour antigen-specific immune response. To test whether such immune responses can be further augmented by co-expression of TAP1 with a Kb or B7.1 gene, we generated single and double gene-expressing cell lines (Table 1). In western blot analysis, all TAP1-expressing cell lines expressed similar levels of TAP1. The Kb-transfected CMT.TAP1/Kb line showed higher expression of Kb than the other cell lines. The B7.1-transfected CMT.TAP1/B7.1 line expressed a high level of the B7.1 molecule, similar to expression in mature DCs. The CMT.TAP1,2 cl.21 cell line expressed both TAP1 and TAP2 and a relatively high level of the Kb molecule. CMT.64/pp and CMT.64/pp1, two empty vector transfectants, expressed relevant molecules similar to those seen in wild-type CMT.64 cells.
Table 1.
Expression of transporter associated with antigen processing 1 (TAP1), TAP2, Kb and B7.1 molecules in CMT.64 transfectants
| MFI |
||||
|---|---|---|---|---|
| Cell line | TAP1 expression | TAP2 expression | H-2Kb expression | B7.1 expression |
| CMT.64 | − | − | 6·5 | 0·1 |
| CMT.64/pp | − | − | 5·7 | 0 |
| CMT.64/pp1 | − | − | 6·2 | 0·1 |
| 1CMT.TAP1/pEF4 | + | − | 6·6 | 0 |
| 1CMT.TAP1/Kb | + | − | 28·4 | 0 |
| CMT.TAP1/B7.1 | + | − | 6·2 | 830 |
| CMT.TAP1,2 cl.2 | + | + | 23·5 | 0·1 |
| Mature DCs | Not tested | Not tested | Not tested | 870 |
Fluorescence-activated cell sorter (FACS) assays were performed to detect surface Kb and B7.1 molecules. The results were normalized by subtracting the mean fluorescence intensity (MFI) of the negative control from each result (the MFI of negative controls ranged between 4 and 7). Expression of TAP1 and TAP2 was determined by western blot.
Cell lines were reported previously.17
DC, dendritic cell.
To determine whether Kb or B7.1 co-expression with TAP1 increased memory immune response, we immunized C57BL/6 mice with γ-irradiated transfectants and evaluated the ability of the cells to induce immunity that protected mice from TAP-negative CMT.64 cell challenge. Table 2 summarizes these experiments. Immunization with TAP1-expressing CMT.TAP1/pEF4 cells significantly increased the level of mouse protection, compared with immunization with experimental control cells, CMT.64/pp or CMT.64/pp1 (P < 0·05; the experimental control cells provided minimal immune responses). The increased protection by CMT.TAP1/pEF4 cell immunization was not further augmented by TAP1 and Kb co-expressing CMT.TAP1/Kb cell immunization (P > 0·05), while significantly increased protection was observed after TAP1 and B7.1 co-expressing CMT.TAP1/B7.1 cell immunization (P < 0·05). Our results suggest that B7.1 but not Kb co-expression with TAP1 in CMT.64 cells dramatically augments memory immune responses against TAP-negative tumours. Because the immunized mice were challenged with TAP-negative tumour cells, we asked whether immunization provided strong natural killer (NK) activity to protect mice from TAP-negative tumour cell attack. To answer this question, T-cell-deficient nude mice were immunized with tumour cells, followed by challenge with CMT.64 cells, and survival was monitored. The results indicated that survival of nude mice immunized with either CMT.TAP1/B7.1 cells or CMT.TAP1/pEF4 cells was not statistically different from the survival of a negative control (breast cell line MID-T2) and an experimental control (CMT.64/pp cells). Thus, NK activity does not play a major role in protection of mice from tumour attack.
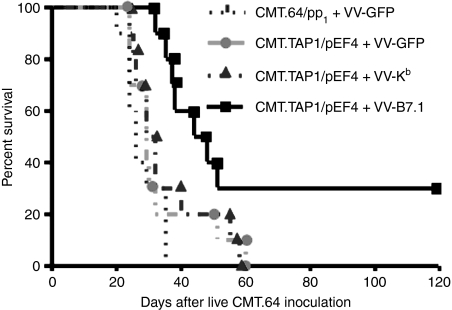
In the next experiment, we addressed whether B7.1 and TAP1 co-expression could elicit a primary immune response capable of providing immune protection in TAP-negative tumour-bearing mice. In these experiments, mice were inoculated i.p. with CMT.64 cells, followed by immunization twice with γ-irradiated VV-B7.1-, VV-Kb- or VV-GFP-infected CMT.TAP1/pEF4 cells at days 3 and 6 after live tumour inoculation, and survival was monitored. Immunization with γ-irradiated VV-GFP-infected CMT.64/pp1 cells was used as a control. The results of this experiment showed that immunization with VV-B7.1-infected CMT.TAP1/pEF4 cells significantly prolonged survival compared with immunization with VV-GFP-infected or VV-Kb-infected CMT.TAP1/pEF4 cells (P < 0·05) (Fig. 1). The latter two were statistically significant in survival time compared with the control (P < 0·05), but they had no biological significance because all mice died during the experimental period. These data suggest that TAP1 and B7.1 co-expression elicits a primary immune response that improves survival in CMT.64-bearing mice.
Figure 1.
Increased survival in tumour-bearing mice treated with transporter associated with antigen processing 1 (TAP1)- and B7.1-expressing tumour cells. C57BL/6 mice were inoculated with live CMT.64 cells (1 × 105 cells per mouse). Three and six days after live tumour cell inoculation, mice (n = 10 in each group) were treated intraperitoneally (i.p.) with γ-irradiated CMT.TAP1/pEF4 tumour cells (1 × 107 cells per mouse) that had been infected either with 1 : 1 [multiplicity of infection (MOI)] vaccinia virus (VV)-B7.1 and VV-Kb or with 1 : 1 (MOI) VV-GFP, and the time of morbidity was recorded. Mice treated with γ-irradiated CMT.64/pp1 cells infected with 1 : 1 (MOI) VV-GFP were used as a negative control (P < 0·05 for mice immunized with CMT.TAP1/pEF4 cells infected with VV-B7.1 compared with mice immunized with CMT.TAP1/pEF4 cells infected with VV-GFP; P < 0·05 for mice immunized with CMT.TAP1/pEF4 cells infected with VV-GFP compared with mice immunized with CMT.64/pp1 cells infected with VV-GFP).
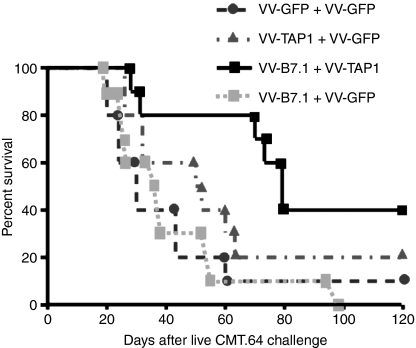
For cancer immunotherapy, viral gene-delivery vehicles are often used. To investigate whether virally infected TAP-negative tumour cells can elicit a tumour antigen-specific immune response, mice were immunized with γ-irradiated and mitomycin-c-treated CMT.64 cells that were infected with VV-TAP1 + VV-B7.1, VV-TAP1 + VV-GFP, VV-B7.1 + VV-GFP or VV-GFP + VV-GFP (used as a control) overnight. After day 20, the immunized mice were challenged with CMT.64 tumour cells. Survival results showed that mice immunized with VV-TAP1 + VV-B7.1-infected CMT.64 cells had a higher survival rate than the control mice (P < 0·05), while survival of mice immunized with VV-TAP1 + VV-GFP- or VV-B7.1 + VV-GFP-infected CMT.64 cells did not show a statistically significant improvement compared with the control mouse group (P > 0·05) (Fig. 2). These results indicate that viral gene-delivery vehicles can be used for cancer immunotherapy and that such therapy requires both TAP1 and B7.1 genes to be delivered into tumour cells.
Figure 2.
Immunization with tumour cells infected with vaccinia virus (VV)-B7.1 + VV-transporter associated with antigen processing 1 (TAP1) increases survival in mice challenged with CMT.64 cells. C57BL/6 mice (n = 10 in each group) were inoculated intraperitoneally (i.p.) with 5 × 106 cells/mouse γ-irradiated CMT.64 cells that had been infected with 1 : 3 [multiplicity of infection (MOI)] VV-GFP + 1 : 3 (MOI) VV-GFP, 1 : 3 (MOI) VV-TAP1 + 1 : 3 (MOI) VV-GFP or 1 : 3 (MOI) VV-B7.1 + 1 : 3 (MOI) VV-TAP1. After a 20-day immunization, mice were challenged i.p. with 2·5 × 105 CMT.64 cells and survival was recorded (P < 0·05 for mice immunized with CMT.64 cells infected with VV-B7.1 + VV-TAP1 compared with mice immunized with CMT.64 cells infected with VV-GFP + VV-GFP; P > 0·05 for mice immunized with CMT.64 cells infected with VV-TAP1 + VV-GFP compared with mice immunized with CMT.64 cells infected with VV-GFP + VV-GFP).
T cells play a major role in the response to tumours.29–31 We have confirmed that NK activity does not play a major role in the immune response in our TAP-deficient tumour model (Table 2). Thus, we believe that immunization with TAP1 and B7.1 co-expressing CMT.64 cells augments a T-cell-mediated immune response. As CD8+ T cells can produce IFN-γ upon activation by specific antigens, we performed an IFN-γ-based ELISA assay to compare CMT.TAP1/pEF4- and CMT.TAP1/B7.1-generated T-cell populations for IFN-γ secretion when activated by stimulation with CMT.64 cells. The CMT.64-generated T-cell population was used as a control. The results of this experiment showed that the amount of IFN-γ secreted by the CMT.TAP1/B7.1-generated T-cell population was significantly greater than that for the CMT.TAP1/pEF4-generated T-cell population, although the latter was significantly greater than that for the control T-cell population (Fig. 3). Thus, our data indicate that T cells generated by immunization with B7.1 and TAP1 co-expressing tumour cells can be efficiently activated by CMT.64 cells.
Figure 3.
Splenocytes immunized with transporter associated with antigen processing 1 (TAP1) and B7.1 co-expressing tumour cells increased interferon (IFN)-γ production. C57BL/6 mice (n = 3) were injected intraperitoneally (i.p.) with γ-irradiated tumour cells (5 × 106 cells per mouse). Seven days after immunization, the splenocytes were stimulated with γ-irradiated CMT.64 cells (which were treated with 30 μg/ml mitomycin-c) at a ratio of 1 : 7 (tumour cells:spleenocytes). Supernatants of the culture were collected at day 5 after in vitro re-stimulation. The levels of secreted IFN-γ were quantified. The mean value of the results for three mice is shown. Statistical significance: **P < 0·0001; *P < 0·005.
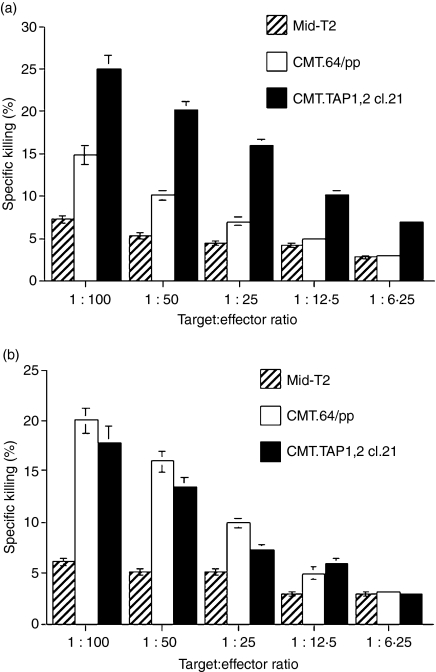
Many reports have shown that induction of T-cell-mediated tumour antigen-specific immunity requires DC cross-priming10,12,14,15,17 and/or tumour direct priming.11,13 If DC cross-priming is a major mechanism in our tumour model, the generated T cells should efficiently recognize TAP-negative and low MHC-I expressing CMT.64 cells. If DCs cannot prime such T cells, or prime them less efficiently, this may suggest that tumour direct priming is a major mechanism for T-cell generation. Two types of experiment were performed to address this question. In the first experiment, a T-cell population generated by immunization of mice with CMT.TAP1/B7.1 cell-lysate-loaded DCs was used to quantify activity against CMT.64/pp and CMT.TAP1,2 cl.21 cells (both cell lines were transfected with vectors carrying identical vector backbones) and Mid-T2 cells (negative control cells). Results of this experiment showed that the generated T cells recognized TAP-proficient CMT.TAP1,2 cl.21 cells more efficiently than TAP-negative CMT.64/pp cells and recognized the latter only slightly better than control Mid-T2 cells (Fig. 4a). These results confirm that DC-generated T cells recognize TAP-negative tumours less efficiently. However, these results do not indicate whether less efficient recognition of CMT.64/pp cells is caused by reduced killing activity of T cells or by low MHC-I expression on the tumour cells (see Table 1).
Figure 4.
Different recognition of transporter associated with antigen processing (TAP)-deficient CMT.64 cells by T cells generated by CMT.TAP1/B7.1 cells and by CMT.TAP1/B7.1 cell-lysate-loaded dendritic cells (DCs). Standard 51Cr-release assays were performed using major histocompatibility complex class I (MHC-I) mismatched Mid-T2 (negative control), CMT.64/pp and CMT.TAP1,2 cl.21 cells as targets. (a) Splenocyte-derived T cells were generated as follows. Bone marrow-derived DCs were loaded with lysates of γ-irradiated CMT.TAP1/B7.1 cells for 6 hr, followed by addition of lipopolysaccharide (LPS) for DC maturation. After washing, DCs were injected intraperitoneally (i.p.) into C57BL/6 mice (1 × 106 cells per mouse) for immunization. After a 7-day immunization, the splenocytes were collected and re-stimulated with CMT.TAP1/B7.1 cell-lysate-loaded DCs for 5 days. (b) Splenocyte-derived T cells were generated by immunization of mice with γ-irradiated CMT.TAP1/B7.1 cells (5 × 106 cells/mouse). After a 7-day immunization, the splenocytes were re-stimulated in vitro with γ-irradiated and mitomycin-c-treated CMT.TAP1/B7.1 cells for 4–5 days. One of three experiments is shown.
To evaluate these two possibilities, a T-cell population was generated by immunization with γ-irradiated CMT.TAP1/B7.1 cells, and its ability to recognize CMT.64/pp cells was determined. If the generated T cells can efficiently recognize CMT.64/pp cells, this suggests that the T cells contain a subpopulation that recognizes TAP-negative and low MHC-I expressing cells. Thus, T-cell activity, not low MHC-I expression, appears to influence T-cell recognition. Our results confirm this and show that T cells killed CMT.64/pp and CMT.TAP1,2 cl.21 cells equally well (Fig. 4b). Killing activities cannot be attributed to NK cells because depletion of NK cells in a CMT.TAP1/B7.1-generated T-cell population by NK-specific antibody and complement did not reduce killing activity (data not shown). Collectively, our results demonstrate that the CMT.TAP1/B7.1-generated T-cell population recognizes TAP-negative tumour cells more efficiently than the DC-generated T-cell population. This difference may reflect the fact that the CMT.TAP1/B7.1-generated T cells include subpopulations that recognize MHC-I-restricted TAP-independent antigens and/or that have NK-like activity.32–35
TAP-deficient tumour cells present TAP-independent antigenic peptides4,5,7,36,37 that can induce T-cell responses. A Lass5 epitope derived from an ER lumen protein7 is one of the epitopes that can be presented by many TAP-deficient cells7 as well as CMT.64 cells (data not shown; Xiao-Lin Li et al., manuscript in preparation). Although TAP-deficient tumour cells present TAP-independent antigens, normal DCs loaded with the TAP-deficient tumour lysates do not efficiently induce a T-cell-mediated immune response against TAP-negative tumour cells. This suggests that the cross-presentation by DCs cannot provide a suitable level of TAP-independent antigens for cytolytic T-cell priming. In contrast, B7.1 and TAP1 co-expressing tumour cells can induce such T cells. This suggests that tumour direct priming may be a major mechanism for TAP-independent antigen-specific T-cell generation.
T-cell-mediated immune responses induced by B7.1-positive TAP1-expressing tumour cells provide more potent protection than that induced by B7.1-negative TAP1-expressing tumour cells. This is probably a consequence of the B7.1-positive tumour cells providing more efficient tumour-direct priming because B7.1 expression decreases the threshold for antigen-specific T-cell generation and activation.38–40
In addition to TAP-independent antigen-specific T-cell generation, the possibility cannot be excluded that the CMT.TAP1/B7.1-generated T cells contain a subpopulation with NK-like activity, because reports indicate that some T cells can specifically recognize low MHC-I expressing tumour cells.33,34 However, the lack of such T cells in the DC-generated T-cell population suggests that tumour direct priming may have occurred.
In summary, generation of a T-cell-mediated immune response against TAP-deficient tumour cells may preferentially require tumour direct priming. In consideration of the fact that many types of tumour are deficient in TAP expression,2,3 providing strategies to reinforce tumour direct priming or improve DC cross-priming of T cells to recognize TAP-deficient tumour cells will benefit cancer immunotherapy in the future.
Acknowledgments
We thank Dr Jonathan Yewdell, Dr Jeffrey Schlom, and Dr Sulin Li for their generosity in providing reagents for this work. This work was supported by the Louisiana Board of Regents, the Feist-Weiller Cancer Center, the Louisiana State Gene Therapy Program and the Department of Cellular Biology & Anatomy at LSU Health Sciences Center-Shreveport. D-QZ is supported by the National Natural Science Foundation of China (grants 30471593 and 30670939), the Shanghai Commission of Science and Technology (grant 07JC14033) and the Shanghai Leading Academic Discipline Project (grant T0206).
Disclosures
None
References
- 1.Abele R, Tampe R. The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology (Bethesda) 2004;19:216–24. doi: 10.1152/physiol.00002.2004. [DOI] [PubMed] [Google Scholar]
- 2.Seliger B, Maeurer MJ, Ferrone S. TAP off – tumors on. Immunol Today. 1997;18:292–9. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 3.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–64. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 4.Wolpert EZ, Petersson M, Chambers BJ, Sandberg JK, Kiessling R, Ljunggren HG, Karre K. Generation of CD8+ T cells specific for transporter associated with antigen processing deficient cells. Proc Natl Acad Sci USA. 1997;94:11496–501. doi: 10.1073/pnas.94.21.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Silva AD, Boesteanu A, Song R, Nagy N, Harhaj E, Harding CV, Joyce S. Thermolabile H-2Kb molecules expressed by transporter associated with antigen processing-deficient RMA-S cells are occupied by low-affinity peptides. J Immunol. 1999;163:4413–20. [PubMed] [Google Scholar]
- 6.Alimonti J, Zhang QJ, Gabathuler R, Reid G, Chen SS, Jefferies WA. TAP expression provides a general method for improving the recognition of malignant cells in vivo. Nat Biotechnol. 2000;18:515–20. doi: 10.1038/75373. [DOI] [PubMed] [Google Scholar]
- 7.van Hall T, Wolpert EZ, van Veelen P, et al. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12:417–24. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal S, Reemtsma K, Bagiella E, Oluwole SF, Braunstein NS. Role of TAP-1 and/or TAP-2 antigen presentation defects in tumorigenicity of mouse melanoma. Cell Immunol. 2004;228:130–7. doi: 10.1016/j.cellimm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang QJ, Seipp RP, Chen SS, Vitalis TZ, Li XL, Choi KB, Jeffries A, Jefferies WA. TAP expression reduces IL-10 expressing tumor infiltrating lymphocytes and restores immunosurveillance against melanoma. Int J Cancer. 2007;120:1935–41. doi: 10.1002/ijc.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–5. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 11.Kundig TM, Bachmann MF, DiPaolo C, et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 1995;268:1343–7. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 12.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–7. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 13.Ochsenbein AF, Sierro S, Odermatt B, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–64. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 14.Norbury CC, Sigal LJ. Cross priming or direct priming: is that really the question? Curr Opin Immunol. 2003;15:82–8. doi: 10.1016/s0952791502000031. [DOI] [PubMed] [Google Scholar]
- 15.Blachere NE, Darnell RB, Albert ML. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 2005;3:e185. doi: 10.1371/journal.pbio.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–24. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 17.Zhang QJ, Li XL, Wang D, et al. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS ONE. 2008;3:e3097. doi: 10.1371/journal.pone.0003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 19.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–70. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 20.Ramarathinam L, Castle M, Wu Y, Liu Y. T cell costimulation by B7/BB1 induces CD8 T cell-dependent tumor rejection: an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells. J Exp Med. 1994;179:1205–14. doi: 10.1084/jem.179.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cayeux S, Richter G, Noffz G, Dorken B, Blankenstein T. Influence of gene-modified (IL-7, IL-4, and B7) tumor cell vaccines on tumor antigen presentation. J Immunol. 1997;158:2834–41. [PubMed] [Google Scholar]
- 22.Huang AY, Bruce AT, Pardoll DM, Levitsky HI. Does B7-1 expression confer antigen-presenting cell capacity to tumors in vivo? J Exp Med. 1996;183:769–76. doi: 10.1084/jem.183.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–7. [PubMed] [Google Scholar]
- 24.Hodge JW, Abrams S, Schlom J, Kantor JA. Induction of antitumor immunity by recombinant vaccinia viruses expressing B7-1 or B7-2 costimulatory molecules. Cancer Res. 1994;54:5552–5. [PubMed] [Google Scholar]
- 25.Liu J, Xia X, Torrero M, Barrett R, Shillitoe EJ, Li S. The mechanism of exogenous B7.1-enhanced IL-12-mediated complete regression of tumors by a single electroporation delivery. Int J Cancer. 2006;119:2113–8. doi: 10.1002/ijc.22100. [DOI] [PubMed] [Google Scholar]
- 26.Hallden G, Hill R, Wang Y, et al. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol Ther. 2003;8:412–24. doi: 10.1016/s1525-0016(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen LL, Gurnani M, Shi B, Terracina G, Johnson RC, Carroll J, Mathis JM, Hajian G. Derivation and initial characterization of a mouse mammary tumor cell line carrying the polyomavirus middle T antigen: utility in the development of novel cancer therapeutics. Cancer Res. 2000;60:7066–74. [PubMed] [Google Scholar]
- 28.Garrigan K, Moroni-Rawson P, McMurray C, Hermans I, Abernethy N, Watson J, Ronchese F. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood. 1996;88:3508–12. [PubMed] [Google Scholar]
- 29.Greenberg PD, Klarnet JP, Kern DE, Cheever MA. Therapy of disseminated tumors by adoptive transfer of specifically immune T cells. Prog Exp Tumor Res. 1988;32:104–27. doi: 10.1159/000414675. [DOI] [PubMed] [Google Scholar]
- 30.Levitsky HI. Tumors derived from antigen presenting cells. Semin Immunol. 1996;8:281–7. doi: 10.1006/smim.1996.0036. [DOI] [PubMed] [Google Scholar]
- 31.Alexander-Miller MA. High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors. Immunol Res. 2005;31:13–24. doi: 10.1385/IR:31:1:13. [DOI] [PubMed] [Google Scholar]
- 32.Chambers CA, Gallinger S, Anderson SK, Giardina S, Ortaldo JR, Hozumi N, Roder J. Expression of the NK-TR gene is required for NK-like activity in human T cells. J Immunol. 1994;152:2669–74. [PubMed] [Google Scholar]
- 33.Mingari MC, Vitale C, Cambiaggi A, Schiavetti F, Melioli G, Ferrini S, Poggi A. Cytolytic T lymphocytes displaying natural killer (NK)-like activity: expression of NK-related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR-mediated target cell lysis or lymphokine production. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda H, Lethe B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 35.von Geldern M, Simm B, Braun M, Weiss EH, Schendel DJ, Falk CS. TCR-independent cytokine stimulation induces non-MHC-restricted T cell activity and is negatively regulated by HLA class I. Eur J Immunol. 2006;36:2347–58. doi: 10.1002/eji.200535387. [DOI] [PubMed] [Google Scholar]
- 36.Henderson RA, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt DF, Engelhard VH. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255:1264–6. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 37.Gabathuler R, Reid G, Kolaitis G, Driscoll J, Jefferies WA. Comparison of cell lines deficient in antigen presentation reveals a functional role for TAP-1 alone in antigen processing. J Exp Med. 1994;180:1415–25. doi: 10.1084/jem.180.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green JM, Noel PJ, Sperling AI, Walunas TL, Gray GS, Bluestone JA, Thompson CB. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–8. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 39.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–9. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 40.Johnston JV, Malacko AR, Mizuno MT, McGowan P, Hellstrom I, Hellstrom KE, Marquardt H, Chen L. B7-CD28 costimulation unveils the hierarchy of tumor epitopes recognized by major histocompatibility complex class I-restricted CD8+ cytolytic T lymphocytes. J Exp Med. 1996;183:791–800. doi: 10.1084/jem.183.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]