Abstract
Signalling pathways mediated by MyD88 are important for sensing Toll-like receptor (TLR) ligands and directing an immune response. However, the influence of MyD88-derived cytokines and interferon (IFN)-α/β, the latter being made by both MyD88-dependent and -independent pathways, in phenotypic and functional dendritic cell (DC) maturation during infection is poorly understood. Here we investigate the contribution of MyD88-dependent and -independent pathways to DC maturation, CD8 T-cell activation and the generation of protective memory against Listeria monocytogenes. We show that neither MyD88 deficiency alone nor MyD88/IFN-αβR double deficiency alters Listeria-induced costimulatory molecule up-regulation on DCs in vivo. In contrast, DCs from infected IFN-αβR−/− mice had higher CD80 and CD86 expression than wild-type DCs. We then examined the function of DCs matured in infected knockout mice. We found that DCs from Listeria-infected MyD88−/− and MyD88−/− IFN-αβR−/− mice induced little or no IFN-γ by CD8 T cells, respectively. In contrast, DCs from infected IFN-αβR−/− mice had a greater capacity to induce IFN-γ compared with DCs from infected wild-type mice. When the CD8 T-cell memory response was analysed, infected MyD88−/− and MyD88−/−IFN-αβR−/− mice were found to have fewer bacteria-specific memory CD8 T cells than wild-type mice. However, the fraction of bacteria-specific CD8 T cells making IFN-γ was similar in all mouse strains, and MyD88−/− and MyD88−/−IFN-αβR−/− mice survived lethal challenge. Together the data suggest an inhibitory effect of IFN-α/β on functional DC maturation during Listeria infection and reveal overlapping roles of MyD88-induced cytokines and IFN-α/β in DC maturation and protective anti-Listeria immunity.
Keywords: bacterial infection, costimulatory molecules, innate immunity, T-cell activation, Toll-like receptors
Introduction
Developing immunity to a pathogen is a complex process in which dendritic cells (DCs) play a fundamental role as a result of their capacity to stimulate naïve T cells.1 The central role of DCs in eliciting immunity is underscored by data showing that in vivo DC depletion abrogates naïve T-cell activation.2,3 Indeed, the first report using DC ablation demonstrated that DCs were critical to generating the protective CD8 T-cell immune response required to eliminate the intracellular bacterium Listeria monocytogenes.3 Depletion of DCs also diminishes the generation of anti-Listeria memory CD8 T cells,4 reiterating the pivotal role of DCs in primary and memory immune responses to Listeria and other pathogens.
Despite their central role in immunity to infection, however, steady-state DCs must undergo a process called maturation to prime naïve T cells.5 Cytokines that induce DC maturation include tumour necrosis factor (TNF)-α, interleukin (IL)-1β, interferon (IFN)-γ and IFN-α/β.6,7 These DC-activating cytokines are produced during infection when DCs and other cells sense the microbe. Microbial recognition by host cells occurs through receptors that recognize conserved structures, and the Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors are critical to sensing bacterial infection and setting in motion the immune response. TLR engagement by their ligands signals through adaptor proteins to activate cytokine transcription, and MyD88 is a central adaptor in TLR-mediated signalling.8 Indeed, infection of MyD88−/− mice with Listeria showed that this adaptor is necessary to ensure survival to primary infection.9–12 However, MyD88 is dispensable for a protective memory T-cell response to Listeria.13,14
As mentioned above, MyD88-dependent signalling results in production of several cytokines that can induce DC maturation (TNF-α, IFN-γ, IL-1β and IFN-α/β).6,7 However, MyD88-independent signalling can also trigger production of factors that influence DC maturation, particularly IFN-α/β.8,15 Nothing is known, however, about the relative contribution of MyD88-dependent versus – independent cytokines in Listeria-induced DC maturation in vivo, which is investigated here.
L. monocytogenes can escape the phagosome of a host cell and colonize the cytosol. In this way Listeria triggers a MyD88-, Nod2-independent intracellular pathway that activates the production of IFN-α/β.16,17 Although well known for its antiviral properties, IFN-α/β also influences the innate and adaptive immune response to non-viral pathogens, including Listeria, in complex ways.18 For example, IFN-α/β can influence primary and memory T-cell responses.15 In addition, IFN-αβR−/− mice have enhanced survival to Listeria infection.19–21 However, nothing is known about the impact of IFN-α/β on DCs during infection with Listeria. In addition, the role of IFN-α/β in the development of CD8 memory T cells remains largely unknown, despite the critical role of CD8 T cells in clearing Listeria infection.22
Thus, this study examines the roles of two important signalling pathways, MyD88 and IFN-α/β, in DC maturation during Listeria infection and reveals distinct effects of these pathways. We also investigate the influence of MyD88 and IFN-α/β on development of protective anti-Listeria CD8 T-cell memory and demonstrate that they are dispensable for the generation of INF-γ-producing memory CD8 T cells. This study reveals complementary roles of MyD88-induced cytokines and IFN-α/β in DC maturation and development of protective immunity to Listeria.
Materials and methods
Mice
C57BL/6 mice were purchased from Charles River Laboratories (Sulzfeld, Germany). MyD88−/−23 mice, IFN-αβR−/−24 and ovalbumin (OVA)257–264 peptide (SIINFEKL)-specific transgenic T-cell receptor (TCR) mice (OT-I) were all ≥ 8 generations on the C57BL/6 background. The genotype of MyD88−/− and IFN-αβR−/− mice was further confirmed by polymerase chain reaction (PCR). MyD88−/− and IFN-αβR−/− mice were crossed to generate MyD88−/−IFN-αβR−/− double knockout mice (called DKO mice). All mice were bred and maintained at the Experimental Biomedicine animal facility of Göteborg University. Mice were used between 8 and 12 weeks of age and provided with food and water ad libitum. All experiments were performed following protocols approved by the government animal ethical committee and institutional animal use and care guidelines.
Bacteria and animal infections
Three bacterial strains were used: wild-type L. monocytogenes 10403s, 10403s expressing full-length OVA (called OVA-LM) and the ActA− derivative of 10403s expressing full-length OVA (called ActA-OVA-LM).25 Bacteria were grown from glycerol stocks in brain and heart infusion (BHI) medium overnight with shaking at 37°. The bacterial concentration was determined by reading the optical density at 600 nm and was confirmed by viable plate counts.
Mice were injected intravenously (i.v.) with 100 μl of an overnight culture of bacteria diluted in phosphate-buffered saline (PBS) in the lateral tail vein as indicated in the figure legends. Doses administered were 2 × 103 to 3 × 104 for C57BL/6 mice, 3 × 104 for IFNαβR−/− mice and 2–3 × 102 for both MyD88−/− and MyD88−/−IFN-αβR−/− mice. These doses were chosen to achieve equivalent bacterial burdens for the different mouse strains at the time at which the mice were killed. For experiments shown inFigs 4 and 5, mice were infected with 5 × 106 ActA-deficient OVA-expressing Listeria(ActA-OVA-LM) followed by a challenge 4 weeks later with 2 × 105 to 1 × 106 OVA-LM. In all experiments, the bacterial dose administered was confirmed by viable plating on BHI agar plates. At the time at which mice were killed, the bacterial burden in the mesenteric lymph nodes (MLN) and the spleen was determined by plating serial dilutions of organ suspensions on BHI agar plates.
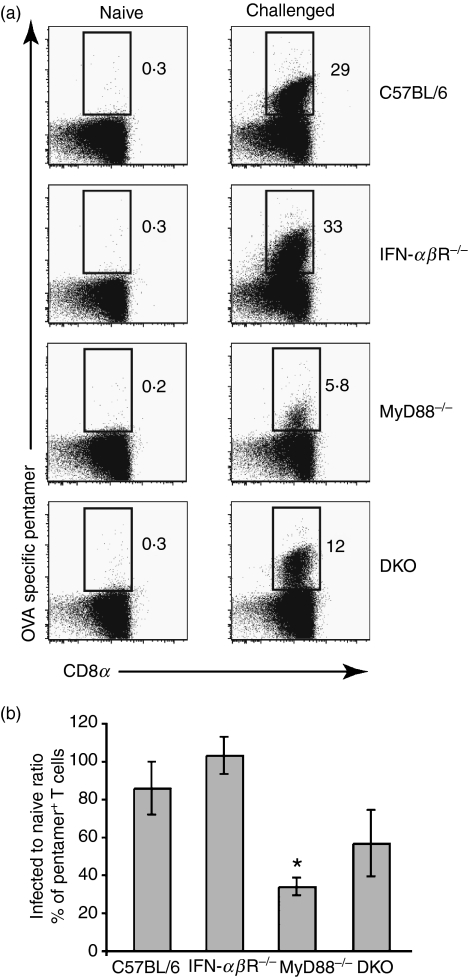
Figure 4.
Antigen-specific memory T cells are reduced in the absence of MyD88 and partially restored by the simultaneous lack of MyD88 and interferon (IFN)-αβR. Mice were infected with the ActA− derivative of Listeria monocytogenes 10403s expressing full-length ovalbumin (ActA-OVA-LM) and challenged 4 weeks later with Listeria monocytogenes 10403s expressing full-length ovalbumin (OVA-LM). (a) 7-Aminoactinomycin D (7AAD)− T-cell receptor (TCR) αβ+ CD4− cells were gated and analysed for CD8 and OVA-pentamer reactivity. Dot plots represent OVA-specific CD8 T cells detected by pentamer staining 5 days after challenge. (b) The bar graph represents data pooled from three experiments analysing a total of six to 11 mice. The bars indicate the ratio of the percentage of OVA-specific T cells in infected to naïve mice gated as in (a). Error bars are the SEM. *P < 0·05.
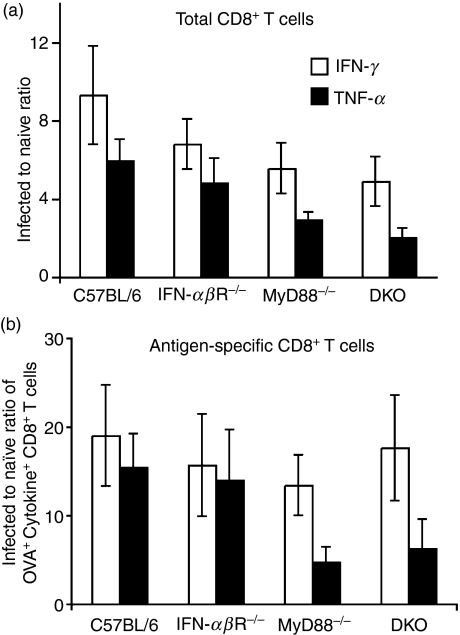
Figure 5.
Listeria-infected knockout mice retain wild-type levels of bacteria-specific CD8 T cells making interferon (IFN)-γ. Mice were infected and challenged as described in Fig. 4. (a) The bar graph shows the ratio of total IFN-γ-producing (open bars) or tumour necrosis factor (TNF)-α-producing (black bars) CD8 T cells in infected mice to those in naïve mice of the different strains, as indicated. Cells were gated as 7-aminoactinomycin D (7AAD)−, T-cell receptor (TCR) αβ+, CD4−, CD8α+, cytokine+ cells. (b) The bar graph indicates the ratio of ovalbumin (OVA)-specific IFN-γ-producing (open bars) and TNF-α-producing (black bars) CD8 T cells in infected mice to those in naïve mice. Values for both naïve and infected mice were obtained by multiplying the fraction of cytokine+ OVA+ CD8+ T cells by the percentage of IFN-γ+ or TNF-α+ CD8+ T cells. No significant differences between any knockout strain and wild-type mice were detected in the statistical analysis in the data shown in (a) or (b).
Preparation of cell suspensions
At the indicated time-points, the spleens were collected and single-cell suspensions were prepared by digestion with 0·45 mg/ml Liberase (Roche, Basel, Switzerland) for 30 min at 37°. Tissue was disaggregated by repetitive pippeting and erythrocytes were lysed with a hypotonic solution of NH4Cl. The cells were washed and re-suspended in RPMI (Invitrogen, Paisley, UK) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Sigma-Aldrich, St Louis, MO). A fraction was stained with trypan blue (Gibco Life Technologies) to determine the number of viable cells.
Antibodies
The following antibodies were purchased from BD PharMingen (San Diego, CA) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-Cy7 or allophycocyanin (APC): CD8α (53–6·7), CD11b (M1/70), CD11c (HL3), CD19 (1D3), NK1·1 (PK136), CD80 (16-10A1), CD86 (GL1), TCR-αβ (H57-597), IFN-γ (XMG1·2) and TNF-α (MP6-XT22). Armenian hamster immunoglobulin G2 (IgG2)-PE (B81-3), rat IgG1-FITC (R3-34), rat IgG2a-PE (R35-95) and rat IgG2b-PE (A95-1) isotype controls were also purchased from BD PharMingen. Anti-CD4 (GK1·5) was conjugated with QDot 605 using a conjugation kit following the manufacturer’s instructions (BD PharMingen). MHC-I pentamers loaded with OVA peptide SIINFEKL were from ProImmune (Oxford, UK). Pentamers were used according to the manufacturer’s recommendations.
Flow cytometry
Single-cell suspensions were stained in Hanks’ balanced salt solution (HBSS) containing 3% FCS, 5 mm ethylenediaminetetraacetic acid (EDTA) and 20 mm HEPES (Invitrogen). Samples were first blocked with anti-FcγRII/III monoclonal antibody (clone 2·4G2) for 15 min at 4°. Cells were washed, antibody cocktails were added and the cells were incubated for 20 min at 4°. When MHC-I pentamers were used, the cells were incubated for 40 min at 4°. 7-Aminoactinomycin D (7AAD; Sigma-Aldrich) was always used to exclude non-viable cells.
Intracellular IFN-γ and TNF-α were detected directly ex vivo. Cell suspensions (106 cells/ml) in RPMI supplemented with 10% heat-inactivated FCS, 2 mm sodium pyruvate, 20 mm HEPES and 0·05 mm 2-mercaptoethanol (all from Invitrogen) were incubated for 4 hr at 37° in 24-well tissue culture plates (CoStar-Corning, Cambridge, MA) in the presence of 5 μg/ml Brefeldin A (Sigma-Aldrich). Cells were stained for surface molecules, fixed with 2% formaldehyde (HistoLab Products AB, Göteborg, Sweden) and re-suspended in permeabilization buffer [HBSS containing 0·5% bovine serum albumin (BSA), 0·5% saponin and 0·05% azide]. FITC-conjugated anti-IFN-γ and TNF-α diluted in permeabilization buffer were added. Cells were detected using an LSRII flow cytometer (BD Biosciences, San Diego, CA) with diva software (BD Biosciences). Data were analysed using flowjo software (Tree Star Inc, Ashland, OR).
Ex vivo T-cell stimulation
Mice were infected i.v. as described above and, after 48 hr, spleens were pooled and single-cell suspensions were prepared. CD11c-expressing cells were magnetically enriched using anti-CD11c magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and an AutoMACS (Miltenyi Biotec). Cells were then stained as above and CD11chigh cells were sorted at low pressure using a FACSAria cell sorter fitted with a 100-μm nozzle and diva software (BD Bioscience). Purity was > 98·5%.
CD8 T cells from OT-I mice were isolated using the CD8α+ T-cell isolation kit from Miltenyi Biotec following the manufacturer’s protocol. The procedure always rendered > 85% purity. OT-I cells were labelled with carboxyfluorescein succinimidyl ester (CFSE) by incubating 107 cells in 1 ml of 1 μm CFSE diluted in PBS for 8 min. The reaction was stopped by the addition of 1 ml of FCS. The cells were washed twice and re-suspended in culture medium. DCs and CFSE-labelled OT-I cells were incubated in RPMI containing gentamicin in 96-well round-bottom plates at the indicated ratios. After 3·5 days, the co-culture supernatant was collected and stored at −20° until assayed for IFN-γ content. The cells were harvested and stained with anti-TCR-APC, a cocktail of anti-CD11c-, CD11b-, CD19- and NK1·1, all labelled with PE, and anti-CD8α-PE-Cy7. Cells were acquired in an LSRII flow cytometer using diva software.
Detection of cytokines in organ lysates and culture supernatants
Spleen lysates from naïve and infected mice were obtained as previously described26 and stored at −20° until cytokines were measured. IFN-α was assessed using an enzyme-linked immunosorbent assay (ELISA) kit from PBL Biomedical Laboratories (Piscataway, NJ). TNF-α, IL-1β and IL-6 were quantified using ELISA sets (BD Bioscience). IFN-γ in DC-OT-I culture supernatants and organ lysates was measured using an IFN-γ ELISA set (BD Bioscience).
Statistics
Statistical analysis was performed using the spss software (SPSS, Chicago, IL). Means were compared using a Mann–Whitney U test. All comparisons were made against the C57BL/6 mice control group. The following applies to the symbols in all figures: ***P < 0·001, **P < 0·01 and *P < 0·05.
Results
Up-regulation of CD80 and CD86 is enhanced in the absence of IFN-αβR but not MyD88
Previous results from our group indicated that the expression of CD80 and CD86 is tissue specific27 and perhaps influenced by the route of infection (oral versus i.v.; data not shown). Thus, to eliminate these confounding factors in the study of the impact of MyD88 and IFN-αβ on costimulatory molecule expression on DCs, MyD88−/−, IFN-αβR−/− and C57BL/6 mice were infected i.v. and splenic DCs were analysed. DCs in the spleen of i.v. infected MyD88−/− mice expressed CD80 and CD86 to a similar extent as DCs of infected wild-type mice (Fig. 1b). In contrast, DCs of infected IFN-αβR−/− mice showed a higher level of CD80 and CD86 expression than DCs of infected wild-type mice.
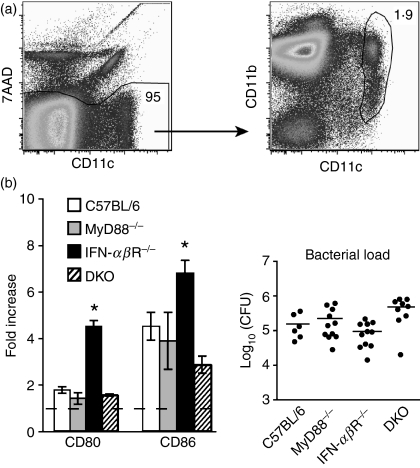
Figure 1.
CD80 and CD86 up-regulation occurs independently of MyD88 and interferon (IFN)-αβR. C57BL/6, IFN-αβR−/−, MyD88−/− and MyD88−/−IFN-αβR−/− double knockout (DKO) mice were infected intravenously (i.v.) with Listeria 10403s and 48 hr later splenocytes were stained with anti- CD11c, CD80 or CD86 and 7-aminoactinomycin D (7AAD) and analysed by flow cytometry. (a) The dot plots show gating of live (7AAD−) CD11chi conventional dendritic cells (DCs) from a naïve C57BL/6 mouse as an example. The numbers indicate the percentage of the gated population. (b) The bars indicate the ratio of the median fluorescence intensity (MFI) of CD80 and CD86 on total DCs, as gated in (a) (7AAD− CD11chi), for infected mice to that for naïve mice. Error bars indicate the standard error of the mean. The bacterial load of the mice analysed is shown to the right. Data are pooled from two to four independent experiments using a total of six to 11 mice for each strain. *P < 0·05.
Thus, neither MyD88 nor IFN-αβR deficiency had a negative impact on costimulatory molecule up-regulation on splenic DCs during systemic Listeria infection. This led us to directly address the effect of the simultaneous absence of these two factors. We therefore made MyD88−/− IFN-αβR−/− double knockout (DKO) mice and infected them with Listeria. Surprisingly, splenic DCs from these mice up-regulated CD80 and CD86 to a similar extent as DCs from infected wild-type mice (Fig. 1b). Thus, CD80 and CD86 can be up-regulated on splenic DCs during systemic Listeria infection even in the absence of MyD88-dependent cytokine production and responsiveness to IFN-α/β produced independently of MyD88.
Altered cytokine profile of infected knockout mice
To understand the mechanism of costimulatory molecule up-regulation during Listeria infection, we next analysed the cytokine profiles of infected MyD88−/−, IFN-αβR−/− and DKO mice. We reasoned that the content of cytokines with the potential to influence DC maturation, such as IL-1β, TNF-α and IFN-α,15,26,28 as well as other potent cytokines such as IL-6 and IFN-γ,6,29 may differ in the infected knockout mice. As shown in Fig. 2, infected MyD88−/− mice showed decreased production of TNF-α and IFN-γ compared with wild-type mice, in agreement with previous reports.9,10 They also made less IL-6 and IL-1β. However, infected MyD88−/− mice produced more IFN-α compared with infected wild-type mice (Fig. 2b). Thus, in the absence of MyD88 alone, TNF-α as well as IFN-α/β could enhance CD80 and CD86 expression while IL-1β, although produced, can not contribute (because of defective IL-1β signalling in MyD88−/− mice).23
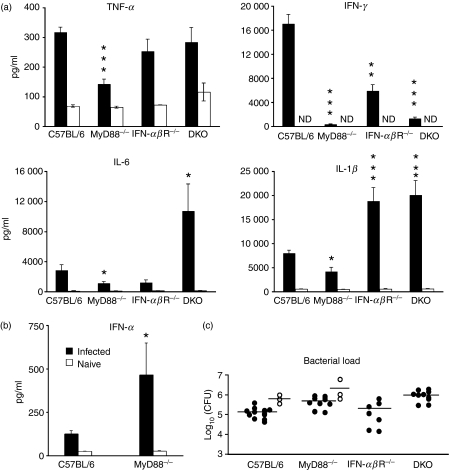
Figure 2.
Cytokine profile in the spleens of Listeria-infected mice. C57BL/6, interferon (IFN)-αβR−/−, MyD88−/− and MyD88−/−IFN-αβR−/− double knockout (DKO) mice were infected intravenously (i.v.) with Listeria 10403s and 48 hr later the spleens were collected and lysed. (a) Cytokines were measured by enzyme-linked immunosorbent assay (ELISA). Data are pooled values from eight to 12 mice from two or three independent experiments. Error bars indicate the standard error of the mean (SEM). (b) IFN-α was measured by ELISA. Data are for three infected mice per group. Error bars indicate the SEM. Solid and open bars indicate infected and naïve mice, respectively, in both (a) and (b). (c) Bacterial loads of the mice analysed in (a). Solid symbols indicate mice for which interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF)-α and IFN-γ were measured. Open symbols indicate mice for which IFN-α was measured. Comparisons were made against infected C57BL/6 mice. ND, not detected. *P < 0·05; **P < 0·01; ***P < 0·001.
Infected IFN-αβR−/− mice also displayed an altered pattern of inflammatory cytokines, producing less IFN-γ but more IL-1β than infected wild-type mice while maintaining wild-type levels of TNF-α (Fig. 2a). Thus, the enhanced IL-1β production in infected IFN-αβR−/− mice, alone or in combination with TNF-α, may account for the increased costimulatory molecule expression on DCs relative to infected wild-type mice, while IFN-α/β could not have an effect (because of IFN-αβR deficiency).
Similar to infected IFN-αβR−/− mice, DKO mice infected with Listeria produced comparable levels of TNF-α to infected wild-type mice, and more IL-1β than the wild type. They also produced more IL-6 than infected wild-type animals. However, the genetic defects in the DKO mice render them unresponsive to both IL-1β (because of their MyD88 deficiency)23 and IFN-α/β (because of their IFN-αβR deficiency). This suggests two things. First, TNF-α26,29 alone or possibly with IL-629 can support Listeria-induced CD80 and CD86 up-regulation when both MyD88 and IFN-αβR are absent. Secondly, IFN-α/β is not absolutely required to increase CD80 and CD86 in the absence of MyD88.
DCs from infected knockout mice have different abilities to induce T-cell activation
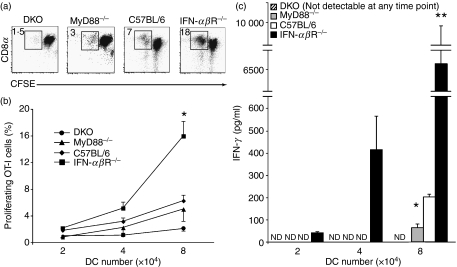
Maturation of DCs into potent antigen-presenting cells involves, among other things, up-regulation of costimulatory molecules.5 However, a high level of costimulatory molecules does not necessarily translate into a full capacity to induce effector T-cell function.5,6,30 We thus asked whether DCs that up-regulate costimulatory molecules during Listeria infection in the absence of MyD88 and IFN-α/βR can elicit fully functional T cells. To this end, DCs from knockout or wild-type mice infected with Listeria expressing OVA (OVA-LM) were purified by cell sorting and co-cultured with CFSE-labelled, OVA-specific CD8 OT-I T cells.
DCs from infected MyD88−/− and DKO mice induced somewhat lower proliferation of OT-I T cells than DCs from wild-type mice, but the difference did not reach statistical significance (Fig. 3a and b). In contrast, DCs from infected IFN-αβR−/− mice induced a significantly stronger response than wild-type DCs (Fig. 3a and b). This is consistent with the higher costimulatory molecule expression on DCs from infected IFN-αβR mice (Fig. 1). DCs from infected IFN-αβR−/− mice also induced stronger IFN-γ production than DCs from infected wild-type mice, whereas DCs from infected MyD88−/− and DKO mice induced little or no IFN-γ, respectively (Fig. 3c).
Figure 3.
Enhanced T-cell stimulatory capacity of dendritic cells (DCs) from mice lacking interferon (IFN)-αβR. C57BL/6, IFN-αβR−/−, MyD88−/− and MyD88−/−IFN-αβR−/− double knockout (DKO) mice (three to six animals per group) were infected with L. monocytogenes 10403s expressing full-length ovalbumin (OVA-LM) and 48 hr later the spleens were collected and pooled. CD11c-expressing cells were magnetically enriched, stained with anti-CD11c-phycoerythrin (PE) and 7-aminoactinomycin D (7AAD) and sorted as live (7AAD−) cells with high expression of CD11c (see Fig. 1a). The cells were co-cultured for 3·5 days with 160 000 carboxyfluorescein succinimidyl ester (CFSE)-labelled OT-I cells, re-stained with anti-CD11c, -T-cell receptor (TCR)-αβ, -NK1·1, -CD11b, -CD4 and -CD8 and analysed by flow cytometry. (a) The numbers in the dot plots indicate the percentage of proliferation from a co-culture well with 80 000 DCs and 160 000 OT-I cells, the highest titration point in the graph in (b). (b) The graph shows the mean proliferation induced by an increasing number of DCs. Error bars are the standard error of the mean (SEM). *P < 0·05. (c) The bars represent the IFN-γ content in the supernatant of the co-culture wells from (b). Errors bar are the SEM. Data are pooled from two to three independent experiments. ND, not detected. *P < 0·05; **P < 0·01.
Thus, DCs from OVA-LM-infected MyD88-deficient mice have a limited capacity to stimulate IFN-γ production by OT-I T cells, while DCs from infected DKO mice essentially lack this capacity. In contrast, DCs from infected IFN-αβR−/− mice have a greater capacity to induce proliferation and IFN-γ production by naïve OT-I T cells.
Suboptimal memory CD8 T-cell response in the absence of MyD88 is partially restored in the absence of IFN-αβR
Development of a memory T-cell response to Listeria is MyD88-independent.13,14 We hypothesized that this could be mediated by IFN-α/β for two reasons. First, IFN-α/β is produced independently of MyD8816 (Fig. 2). Secondly, during Listeria infection, IFN-α/β has a role in costimulatory molecule expression (Fig. 1) and naïve CD8 T-cell activation (Fig. 3). We thus used a strategy in which MyD88−/−, IFN-αβR−/− and DKO mice were infected with mutant (ActA-deficient) Listeria expressing OVA (ActA-OVA-LM) and challenged 4 weeks later with wild-type Listeria expressing OVA (OVA-LM). We used this strategy because: (i) Listeria strains lacking ActA are less virulent than wild-type bacteria but still induce a strong T-cell response;31 and (ii) MyD88−/− mice are very susceptible to wild-type Listeria9,10 but survive a relatively high dose of ActA-deficient Listeria.13,14 In this way we ensured survival of MyD88−/− mice while preserving the T-cell response.
Figure 4 shows that challenged IFN-αβR−/− mice had a similar frequency of OVA-specific CD8 T cells compared with wild-type mice. However, challenged MyD88−/− mice had a 75% reduction in OVA-specific CD8 T cells. Furthermore, challenged DKO mice had a frequency of antigen-specific CD8 T cells between those of MyD88−/− and wild-type mice, which is consistent with the augmenting effect that IFN-αβR deficiency has on costimulatory molecule expression. No bacterial burden was found in the spleens of any of the challenged mice [100 colony-forming units (CFU) was the limit of detection]. Thus, MyD88-deficient mice developed a memory response that was weaker than that observed in wild-type and IFN-αβR−/− mice in terms of the frequency of antigen-specific CD8 T cells, a defect that was partially restored when both MyD88 and IFN-αβR were absent. Despite this, MyD88−/− hosts were able to clear a lethal challenge with wild-type Listeria.
Similar numbers of IFN-γ-producing antigen-specific memory CD8 T cells are generated in the absence of MyD88 and IFN-αβR signalling despite a reduced total memory response
To understand how mice survive a lethal challenge despite an apparently reduced CD8 T-cell memory response (Fig. 4), we next investigated the effector capacity of the memory pool elicited. Intracellular cytokine staining revealed that production of IFN-γ by total CD8 memory T cells was slightly reduced in the knockout mice compared with the wild type (Fig. 5a), although this was not statistically significant, and IFN-γ production was sequentially lower as the severity of the genetic defect increased (IFN-αβR−/−∼ 1·4 fold, MyD88−/−∼ 1·7 fold and DKO mice ∼ 1·9 fold). A similar trend was observed for production of TNF-α by the total CD8 memory T-cell pool elicited in the infected mice (IFN-αβR−/−∼ 1·2 fold, MyD88−/−∼ 2 fold and DKO mice ∼ 2·9 fold; Fig. 5a).
We then directly assessed how many of these cytokine-producing CD8 T cells were antigen-specific. Although a non-significant trend of fewer antigen-specific TNF-α+ cells was apparent in infected MyD88−/− and DKO mice, the fraction of antigen-specific IFN-γ+ cells was similar in all mouse strains (Fig. 5b). Thus, although the absence of MyD88 has an apparently negative impact on the development of antigen-specific CD8 memory T cells assessed by pentamer staining (Fig. 4a), IFN-γ-producing, antigen-specific memory CD8 T cells were present in similar numbers in wild-type and knockout mice (Fig. 5b), which correlates with effective clearance of the bacterial challenge.
Discussion
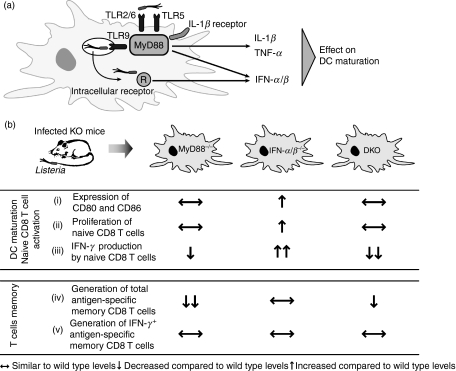
Signalling pathways mediated by MyD88 are important for sensing TLR ligands and directing an immune response.5,8 However, the influence of MyD88-derived cytokines and IFN-α/β, the latter being made by both MyD88-dependent and -independent pathways (Fig. 6a), in phenotypic and functional maturation of DCs in vivo5,7 is not completely understood, especially during bacterial infection. Here we show that MyD88 and IFN-αβ signalling pathways differentially contribute to DC maturation during primary Listeria infection (Fig. 6b). We also show that both are dispensable for the generation of IFN-γ-producing antigen-specific CD8 memory T cells that allow survival of a lethal Listeria challenge (Fig. 6b).
Figure 6.
MyD88 and interferon (IFN)-α/β are differentially involved in costimulatory molecule expression and T-cell activation during Listeria infection. (a) Diagram representing the MyD88-dependent and -independent pathways that produce factors influencing dendritic cell (DC) maturation.9,10,16,17 (b) Our data demonstrate that: (i) splenic DCs from infected MyD88−/− mice and mice lacking both MyD88 and IFN-αβR [double knockout (DKO)] express CD80 and CD86 to a similar level as DCs from infected wild-type mice. In contrast, DCs from infected IFN-αβR−/− mice express higher levels of CD80 and CD86 than DCs from infected wild-type mice. A different effect of MyD88 and IFN-α/β on functional DC maturation is apparent, as DCs from infected IFN-αβR−/− mice were more efficient at inducing proliferation (ii) and IFN-γ production (iii) by naïve CD8 T cells than DCs from wild-type mice. In contrast, DCs from MyD88−/− and DKO mice induced similar CD8 T-cell proliferation (ii) and less IFN-γ (iii) than wild-type DCs. Finally, although the lack of MyD88 resulted in a diminished total CD8 T-cell memory population (iv), neither MyD88 nor IFN-α/β was required for the generation of IFN-γ-producing, bacteria-specific memory CD8 T cells (v). IL, interleukin; TLR, Toll-like receptor; TNF, tumour necrosis factor.
We first showed that splenic DCs from infected MyD88−/− mice up-regulated CD80 and CD86 to a similar extent as wild-type mice. As IFN-α/β is produced independently of MyD888,16,17 and influences costimulatory molecule expression on DCs during infection with viruses or Salmonella,32,33 it was a prime candidate to explain MyD88-independent costimulatory molecule up-regulation on DCs during Listeria infection. However, DCs from mice lacking both MyD88 and IFN-α/βR (DKO mice) up-regulated CD80 or CD86 to a similar level as DCs from wild-type mice. This occurred despite the fact that infected MyD88−/− mice had increased IFN-α production relative to infected wild-type mice. Thus, IFN-αβ was not required for Listeria-induced costimulatory molecule up-regulation on DCs in the absence of MyD88.
Analysis of the cytokine profile in the spleens of i.v.-infected MyD88−/− and DKO mice revealed production of several cytokines that can influence DC maturation, such as IL-1β, IL-6, IFN-αβ and TNF-α.15,26,28,29 The lack of functional IL-1β signalling in MyD88−/− mice,23 combined with the overlapping roles of IL-1β and TNF-α in mediating CD80 and CD86 up-regulation in vivo,26 suggests that TNF-α produced independently of MyD88, although reduced in infected MyD88−/− mice (Fig. 2a),12 is involved in costimulatory molecule up-regulation in infected MyD88−/− and DKO mice.
Our data also revealed that IFN-α/β receptor deficiency resulted in enhanced expression of both CD80 and CD86 on splenic DCs in mice given bacteria i.v. This effect on phenotypic DC maturation was reflected in function, as DCs from infected IFN-αβR−/− mice induced greater proliferation and IFN-γ production of naïve CD8 T cells compared with DCs from infected wild-type mice. Previous studies have shown that IFN-αβR−/− animals are more resistant to Listeria infection.19–21 One of these studies proposed that decreased bacterial-induced apoptosis in infected IFN-αβR−/− hosts contributed to the resistance mechanism.20 Our results expand these observations by describing a novel negative role of IFN-α/β in DC maturation and the ability of the DCs to stimulate naïve CD8 T cells during Listeria infection, a function of IFN-α/β not yet described.34 Thus, in the absence of IFN-αβR signalling, DCs seem to have a lower threshold to promote costimulatory molecule expression, which is reflected in more effective development of CD8 T-cell effector functions that mediate bacterial clearance. Whether IFN-α/β directly down-modulates costimulatory molecule expression on DCs, or whether the negative effect of IFN-α/β is mediated through other indirect mechanisms, remains to be explored.
Despite the importance of MyD88 in survival following primary Listeria infection,9,10 memory CD8 T-cell responses develop in the absence of MyD88.13,14 Given our findings concerning the role of IFN-αβR in DC maturation and activation of T cells during primary Listeria infection, we addressed the question of whether IFN-αβ plays a role in generating CD8 memory T cells against Listeria. In agreement with previous studies, we showed that infected MyD88−/− mice are protected against reinfection.13,14 However, we found that challenged MyD88−/− mice had a lower frequency of OVA+ CD8 T cells, while Kursar et al.13 did not. Differences in experimental design, such as a brief infection with wild-type Listeria aborted by antibiotic treatment13 versus our design of infection with the ActA− mutant to ensure host survival, may contribute to this discrepancy.
We showed that defective IFN-αβR signalling alone had little if any effect on generating an antigen-specific memory CD8 T-cell pool. Furthermore, studies in the DKO mice showed that IFN-αβR is not required for development of MyD88-independent CD8 memory. However, despite the fact that MyD88−/− mice (and to a lesser extent DKO mice) had a compromised capacity to generate antigen-specific CD8 memory cells, they survived a lethal challenge as well as wild-type mice. Why? The answer probably lies in the similar frequencies of CD8 T cells making IFN-γ within the antigen-specific memory pool in wild-type and knockout mice. This is further supported by recent data showing the importance of IFN-γ production by bacteria-specific memory CD8 T cells in protective immunity to Listeria.35 Thus, unlike the anti-Listeria primary CD4 T-cell response, which requires IL-12 or IFN-α/β,36,37 the memory CD8 T-cell response to this pathogen is independent of IL-12, IFN-α/β (Fig. 4)36,37 and MyD88 (Fig. 4), although it requires CD4 help at the time of priming.38,39
In summary, we characterize the relative contribution of two important signalling pathways to DC maturation in vivo. We also reveal that MyD88 and IFN-α/β have different effects on DC maturation during Listeria infection (Fig. 6b). Finally, we demonstrate that the development of a memory CD8 T-cell response capable of clearing a lethal challenge is independent of MyD88 and IFN-αβR. These studies help to elucidate the orchestration of a balanced immune response against infection with an intracellular bacterium.
Acknowledgments
The skillful assistance of Emilia Heimann and Anna Rydström is gratefully acknowledged. We thank Dr S. Akira (Research Institute for Microbial Diseases, Osaka University) and Dr J. Demengeot (Instituto Gulbenkian de Ciência, Oeiras, Portugal) for providing MyD88−/− and IFN-αβR−/− mice, respectively. We also thank Dr H. Shen (University of Pennsylvania School of Medicine, Pittsburg, PA) for providing the Listeria strains used. This work was supported by grants from the Swedish Research Council (621-2004-1378; 621-2007-6536) and the Sahlgrenska Academy at Göteborg University and was performed at the Mucosal Immunobiology and Vaccine Center (MIVAC) funded by the Swedish Foundation for Strategic Research.
Glossary
Abbreviations:
- DCs
dendritic cells
- DKO
MyD88−/−IFN-αβR−/− double knockout mice
Disclosures
None.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Brewig N, Kissenpfennig A, Malissen B, Veit A, Bickert T, Fleischer B, Mostbock S, Ritter U. Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J Immunol. 2009;182:774–83. doi: 10.4049/jimmunol.182.2.774. [DOI] [PubMed] [Google Scholar]
- 3.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8 + T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zammit DJ, Cauley LS, Pham Q-M, Lefrançois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–70. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 6.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou B, Reizis B, DeFranco AL. Toll-like Receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–82. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter S, O’Neill LAJ. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 9.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–75. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 10.Seki E, Tsutsui H, Tsuji NM, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–8. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 11.Torres D, Barrier M, Bihl F, Quesniaux VJ, Maillet I, Akira S, Ryffel B, Erard F. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun. 2004;72:2131–9. doi: 10.1128/IAI.72.4.2131-2139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 13.Kursar M, Mittrucker HW, Koch M, Kohler A, Herma M, Kaufmann SH. Protective T cell response against intracellular pathogens in the absence of Toll-like receptor signaling via myeloid differentiation factor 88. Int Immunol. 2004;16:415–21. doi: 10.1093/intimm/dxh047. [DOI] [PubMed] [Google Scholar]
- 14.Way SS, Kollmann TR, Hajjar AM, Wilson CB. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J Immunol. 2003;171:533–7. doi: 10.4049/jimmunol.171.2.533. [DOI] [PubMed] [Google Scholar]
- 15.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 16.McCaffrey RL, Fawcett P, O’Riordan M, Lee KD, Havell EA, Brown PO, Portnoy DA. A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc Natl Acad Sci USA. 2004;101:11386–91. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockinger S, Reutterer B, Schaljo B, et al. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173:7416–25. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 18.Bogdan C, Mattner J, Schleicher U. The role of type I interferons in non-viral infections. Immunol Rev. 2004;202:33–48. doi: 10.1111/j.0105-2896.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 19.Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–33. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–40. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connell RM, Saha SK, Vaidya SA, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–45. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr Opin Microbiol. 2004;7:45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 24.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 25.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–81. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 26.Sundquist M, Wick MJ. TNF-α-dependent and -independent maturation of dendritic cells and recruited CD11cint CD11b+ cells during oral Salmonella infection. J Immunol. 2005;175:3287–98. doi: 10.4049/jimmunol.175.5.3287. [DOI] [PubMed] [Google Scholar]
- 27.Tam MA, Wick MJ. Differential expansion, activation and effector functions of conventional and plasmacytoid dendritic cells in mouse tissues transiently infected with Listeria monocytogenes. Cell Microbiol. 2006;8:1172–87. doi: 10.1111/j.1462-5822.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 28.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–50. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 29.Decker P, Kotter I, Klein R, Berner B, Rammensee HG. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006;45:1087–95. doi: 10.1093/rheumatology/kel061. [DOI] [PubMed] [Google Scholar]
- 30.Spörri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–70. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 31.Goossens PL, Milon G. Induction of protective CD8+ T lymphocytes by an attenuated Listeria monocytogenes actA mutant. Int Immunol. 1992;4:1413–8. doi: 10.1093/intimm/4.12.1413. [DOI] [PubMed] [Google Scholar]
- 32.Montoya M, Edwards MJ, Reid DM, Borrow P. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type 1 IFN. J Immunol. 2005;174:1851–61. doi: 10.4049/jimmunol.174.4.1851. [DOI] [PubMed] [Google Scholar]
- 33.Tam MA, Sundquist M, Wick MJ. MyD88 and IFN-αβ differentially control maturation of bystander but not Salmonella-associated dendritic cells or CD11cint CD11b+ cells during infection. Cell Microbiol. 2008;10:1517–29. doi: 10.1111/j.1462-5822.2008.01144.x. [DOI] [PubMed] [Google Scholar]
- 34.Stockinger S, Decker T. Novel functions of type I interferons revealed by infection studies with Listeria monocytogenes. Immunobiology. 2008;213:889–97. doi: 10.1016/j.imbio.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Messingham KA, Badovinac VP, Jabbari A, Harty JT. A role for IFN- from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes. J Immunol. 2007;179:2457–66. doi: 10.4049/jimmunol.179.4.2457. [DOI] [PubMed] [Google Scholar]
- 36.Orgun NN, Mathis MA, Wilson CB, Way SS. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and Type I IFNs sustains protective CD8 T cells. J Immunol. 2008;180:4109–15. doi: 10.4049/jimmunol.180.6.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178:4498–505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]