Abstract
In previous studies we reported that plasmacytoid dendritic cells (PDC) infiltrating head and neck cancer tissue are functionally impaired, but the molecular basis for the functional deficiency remained unclear. Here we demonstrate that tumour-derived prostaglandin E2 (PGE2) and transforming growth factor-β (TGF-β) increase interleukin-8 (IL-8) but synergistically inhibit interferon-α (IFN-α) and tumour necrosis factor (TNF) production of Toll-like receptor 7 (TLR7)- and Toll-like receptor 9 (TLR9)-stimulated PDC. The inhibitory effect of PGE2 could be mimicked by the induction of cyclic AMP (cAMP) and by inhibitors of cyclooxygenase. The contribution of tumour-derived TGF-β was confirmed by the TGF-β antagonist SB-431542. Suppression of tumour-derived PGE2 and TGF-β restored TLR-induced IFN-α production of PDC. Additionally, PGE2- and TGF-β-treated PDC display a ‘tolerogenic’ phenotype because of a downregulation of CD40 accompanied by an upregulation of CD86. Finally, in TLR-stimulated PDC, PGE2 and TGF-β reduce the CCR7 : CXCR4 ratio, suggesting that PDC are impaired in their ability to migrate to tumour-draining lymph nodes but are retained in stromal cell-derived factor 1 (SDF-1)-expressing tissues. Based on these data, cyclooxygenase inhibitors and TGF-β antagonists may improve TLR7- and TLR9-based tumour immunotherapy.
Keywords: cancer, dendritic cells, Toll receptors/Toll-like receptors
Introduction
Type I interferons (IFN-I) prime innate and adaptive immune cells and are therefore considered to be central players in the immune defence against microbial pathogens and tumour cells. Thus, suppression of IFN-I production represents a very effective means to evade the immune response in different environmental contexts. Previous studies have demonstrated that plasmacytoid dendritic cells (PDC), the major source of Toll-like receptor (TLR)-dependent IFN-I in the human body,1 infiltrate tumour tissue but are functionally impaired.2 It has been postulated that tumour cells secrete factors that downregulate the IFN-I response, thus weakening both innate and adaptive immune recognition of tumour antigens.
Many different strategies for tumour evasion of immune recognition have been described. Among these are the downregulation of costimulatory and adhesion molecules on tumour cells,3,4 tumour-induced synthesis of immunosuppressive cytokines [e.g. interleukin (IL)-10 and transforming growth factor-β (TGF-β)] in immune cells and the secretion of tumour-derived immunomodulatory factors such as vascular endothelial growth factor (VEGF) or prostaglandins,5 also known to support tumour growth, tumour angiogenesis and tumour survival.
The role of IFN-I in anti-tumour responses is to prime immune cells and thereby accelerate and enhance a broad spectrum of immune responses. In innate immunity the effect of IFN-I mainly consists of a positive feedback loop that strengthens pro-inflammatory responses to exogenous and endogenous danger motifs recognized by pattern recognition receptors such as TLRs.6 One major consequence of this is increased IL-12 function and the subsequent shift to a T helper 1-type response that is essential for anti-tumour responses.7,8
Prostaglandins are hormone-like substances synthesized from arachidonic acid and released during inflammatory processes.9 They have multiple regulatory functions, including the regulation of blood pressure levels and modulation of the immune response.10 Similarly to the effects of immunosuppressive cytokines, such as IL-10 and TGF-β, prostaglandins have been shown to inhibit pro-inflammatory cytokine secretion from murine dendritic cells,11–15 and to modulate B-cell and T-cell responses.16,17 Some of these effects are mediated by prostaglandin-induced IL-10,18 whereas others directly interfere with cell signalling via the activation of G-protein-coupled receptors.10,19 Under physiological conditions, prostaglandins may regulate the extent and the quality of the pro-inflammatory response, thus preventing an overshoot and autoinflammatory responses. By contrast, deregulated overexpression of prostaglandin E2 (PGE2) by tumour cells induces sustained immunosuppression.20
Our previous studies showed that PDC infiltrate tumour tissue in head and neck cancer but become deficient in expression of TLR9 and are therefore less responsive to cytosine–phosphate–guanosine (CpG) DNA-induced IFN-I production.2 In the present study we addressed the question of whether the loss of function may be caused by prostaglandins produced by the tumour cells or by immunosuppressory cytokines produced by tumour-infiltrating lymphocytes. Our results show that, indeed, PGE2 suppresses TLR-stimulated PDC-derived interferon-α (IFN-α) and TNF secretion via cyclic AMP (cAMP) induction. Furthermore, PGE2 alters the CCR7 : CXCR4 ratio, resulting in a PDC phenotype bound to reside in stromal cell-derived factor 1 (SDF-1)-expressing tissue instead of migrating to the lymph node. Moreover, TGF-β synergizes with PGE2, leading to a complete block of IFN-α production. Our data suggest that tumour therapy with CpG DNA oligodesoxynucleotides (ODN) or TLR7 agonists may benefit from combined therapy with cyclooxygenase (COX) inhibitors and TGF-β antagonists.
Materials and methods
PDC isolation
Isolation of peripheral blood mononuclear cells (PBMC) from healthy donors was approved by the local ethics committee. PBMC were prepared by Ficoll–Hypaque (Biochrome, Hamburg, Germany) density-gradient centrifugation. PDC were labelled with anti-BDCA-4 microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany) and passed through two magnetic separation columns (LS and MS columns; Miltenyi Biotec). The purity of isolated PDC [lineage-negative, major histocompatibility complex class II (MHC II)-positive, CD123-positive and CD11c-negative) was 94–99%. PDC were cultured in RPMI 1640 (Biochrom) supplemented with 2% autologous serum, 10 mm HEPES (Sigma-Aldrich, Munich, Germany), 1·5 mm l-glutamine, 100 U/ml of penicillin, 100 μg/ml of streptomycin (all from PAA Laboratories, Vienna, Austria) and 10 ng/ml of IL-3 (R&D Systems, Wiesbaden, Germany) in 96-well round-bottom plates.
PDC stimulation
PDC (1·25 × 105 cells/ml) were pre-incubated with recombinant human PGE2 (Sigma, dissolved in ethanol) at the concentrations indicated in the diagrams and/or with TGF-β1 (Strathmann, Hamburg, Germany) at 10 ng/ml for 4 hr, and then stimulated with TLR ligands (CpG ODNs; Coley Pharmaceutical Group, Langenfeld, Germany) (small letters indicate phosphorothioate linkage and capital letters indicate phosphodiester linkage 3′ of the base): CpG-B ODN 2006 5′-tcgtcgttttgtcgttttgtcgtt-3′; and CpG-A ODN 2216 5′-ggGGGACGATCGTCgggggG-3′. The TLR7 ligands R848 and loxoribine (7-allyl-8-oxoguanosine) were purchased from Invivogen (Toulouse, France) and Sigma (München, Germany) respectively, and the final concentrations used were: CpG ODN, 3 μg/ml; R848, 0·25 μg/ml; and loxoribine, 500 μm. Butaprost and 11-deoxy-PGE1 were purchased from Cayman Chemicals (Ann Arbor, MI); sulprostone, forskolin, staurosporine and SB-431542 [all solubilized in dimethylsulphoxide (DMSO)] were purchased from Sigma. All pre-incubations with TGF-β, PGE2, EP receptor agonists and forskolin were started 4 hr before stimulation with TLR ligands, and PDC were pre-incubated with SB-431542 20 min before TGF-β priming. DMSO and ethanol used at the respective dilutions did not affect the results obtained with any of the reagents. Although PDC are not responsive to lipopolysaccharide (LPS), all reagents were tested for endotoxin, as previously described.21
Cell lines and generation of supernatants
Primary head and neck tumour tissues and tumour cell lines were provided by Professor Dr B. Wollenberg (University of Lübeck, Germany).2 The cell lines BHY and HLAC-79 originate from human oral squamous cell carcinoma; FADU, PCI and Ant-1 cell lines are derived from human hypopharynx carcinomas. The origin of the primary tumours is listed in Table 1.
Table 1.
Tumour sample origin
| Tumour sample | Origin |
|---|---|
| T 123 | Hypopharynx-larynx-carcinoma |
| T 126 | Sinus piriformis lymph node metastasis level III |
| T 128 | Primary tumour of T 126 (sinus piriformis) |
| T 129 | Cervical lymph node metastasis level III–IV (Histology: PEC) |
| T 130 | Primary tumour of T 129 |
| T 131 | Hypopharynx-larynx-carcinoma |
| T 133 | Supraglottic hypopharynx-larynx-carcinoma; stage T4a |
| T 134 | Lymph node metastasis after neck dissection of tumour |
For the generation of primary tumour supernatants the weight of the primary tumour samples was adjusted to 21 mg, and 7 mg was cultured in 500 μl of X-Vivo (Cambrex, Walkersville, MD) containing 5% fetal calf serum (FCS), 1·5 mm glutamine, 1% sodium pyruvate, 100 U/ml of penicillin and 100 μg/ml of streptomycin for 12, 24 and 48 hr. For the experiments using indomethacin, tumour cell lines were incubated for 48 hr in RPMI 1640 containing 10% FCS, 5 mm glutamine, 100 U/ml of penicillin, 100 μg/ml of streptomycin, with or without the COX-inhibitor indomethacin (1 μm) in ethanol (Sigma); medium controls were incubated in the absence or presence of indomethacin for the same length of time. Supernatants were added at a 1:1 (v/v) ratio to PDC 4 hr before stimulation with CpG DNA.
Quantification of cytokine secretion
PDC supernatants were harvested 24 h after stimulation. Cytokine concentrations in the supernatants were measured using enzyme-linked immunosorbent assays (ELISAs). The IFN-α ELISA was purchased from Bender MedSystems (Vienna, Austria), the PGE2 ELISA was from Assay Designs (Ann Arbor, MI); and all other ELISA kits (TNF, IL-6, IL-8, TGF-β, IL-10) were from BD Biosciences (Heidelberg, Germany).
Flow cytometry
Flow cytometric data were acquired on FACSCanto and analyzed using facsdiva software (BD Biosciences). Staining was performed following standard protocols. The monoclonal antibodies (mAbs) used, namely anti-lineage [fluorescein isothiocyanate (FITC) conjugate], anti-human leucocyte antigen (HLA)-DR [peridinin chlorophyll protein (PerCP)-Cy5.5 conjugate], anti-CD123 [phycoerythrin (PE) conjugate], anti-CD11c [allophycocyanin (APC) conjugate], anti-CD40 (FITC conjugate), anti-CD80 (PE conjugate), anti-CD86 (PE conjugate), anti-CXCR4 (PE conjugate) and anti-CCR7 (PE conjugate), were purchased from BD PharMingen (Heidelberg, Germany). Apoptosis and cell viability were assessed using annexin V (Bender MedSystems), propidium iodide (Sigma) or TOPRO-3 (iodide DNA stain) (Molecular Probes, Eugene, OR).
Migration assay
The migration assay was performed using a transwell assay. PDC migrated along the SDF-1 concentration gradient (R&D Systems) through a 0·5 μm pore membrane (Corning, Corning, NY) after incubation with or without PGE2 for 2 hr.
Real-time polymerase chain reaction
Isolation of total RNA (High Pure RNA isolation kit; Roche, Mannheim, Germany) and complementary DNA (cDNA) synthesis from messenger RNA (mRNA) (First Strand cDNA kit; Fermentas, St Leon-Rot, Germany) were performed according to the manufacturer’s protocol. Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed with a quantitative PCR mix using SYBR-Green following the standard protocol (Eurogentec; ABI PRISM 7700; Applied Biosystems, Darmstadt, Germany). RT-PCR was controlled by no-template and no-reverse transcriptase controls. Relative expression to β-actin was calculated as [1/2(cycle threshold of target gene – cycle threshold of actin)]. Primers and fragment size: EP1, forward 5′-CCAATGCTGGTGTTGGTG, reverse 5′-AATGCTGGTGTTGGTGGC (166 bp); EP2, forward 5′-CTTTCACGATTTTTGCATATATGAA, reverse 5′-CGACAACAGAGGACTGAACG (163 bp); EP3, forward 5′-CGCTCCTGATAATGATGTTGAA, reverse 5′-ACAGCAGGTAAACCCAAGGA (156 bp); EP4, forward 5′-TACAGACCCAGCCTTGCACT, reverse 5′-ACTTGCACAGCACCACGAT (181 bp); and β-actin, forward 5′-AGAGCTACGAGCTGCCTGAC, reverse 5′-AGCACTGTGTTGGCGTACAG. For COX2 mRNA expression analysis, mRNA isolation was performed as previously described.22,23 The primers were purchased from SEARCH-LC GmbH (Heidelberg, Germany).
Statistics
Data are depicted as mean ± standard error of the mean (SEM). Statistical significance of differences was determined by the paired two-tailed Student’s t-test using Microsoft Excel software. Statistically significant differences are indicated with a single asterisk (*) for a P-value of≤ 0·05 and with a double asterisk (**) for a P-value of ≤ 0·005.
Results
PGE2-containing supernatants from head and neck tumour cell lines suppress IFN-α production in human PDC
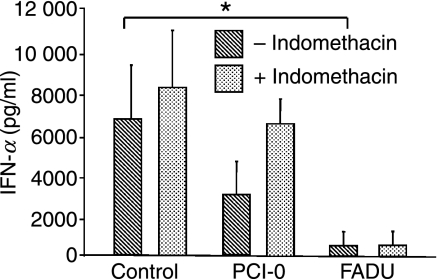
Supernatants of head and neck tumour cell lines suppressed PDC-derived IFN-α production (Fig. 1). We hypothesized that this could be caused by tumour cell-derived PGE2. We therefore examined the supernatants of head and neck cancer cell lines and from freshly isolated primary head and neck tumour samples for the presence of PGE2 and the immunosuppressory cytokines IL-10 and TGF-β. We found that nearly all tumour cell lines tested, and all primary tumours, secreted varying amounts of PGE2 (Table 2). By contrast, IL-10 and TGF-β were only found in supernatants of primary tumours, suggesting that these cytokines may be secreted by tumour-infiltrating leucocytes. Notably, the concentrations of IL-10 were highest after 12 hr (data not shown) and 24 hr of incubation (Table 2), while TGF-β was only detectable after 48 hr of incubation (Table 2).
Figure 1.
Immunomodulatory effect of cyclooxygenase (COX) inhibition on tumour cell line-mediated suppression of cytosine–phosphate–guanosine (CpG) oligodesoxynucleotide (ODN)-activated plasmacytoid dendritic cell (PDC)-derived interferon-α (IFN-α) secretion. PDC were pre-incubated with medium or with supernatants (1 : 1) from tumour cell lines PCI-0 and FADU treated with or without indomethacin before stimulation with CpG ODN 2216 for 24 hr. IFN-α production was measured using enzyme-linked immunosorbent assays (ELISAs). The mean values ± standard error of the mean (SEM) of three individual experiments are shown. P*[Medium/FADU] = 0·04.
Table 2.
Prostaglandin E2 (PGE2), transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) concentrations in the supernatants of tumour cell lines and of primary head and neck tumours
| Cell lines | PGE2 (pg/ml) | TGF-β1 (pg/ml) | IL-10 (pg/ml) | |||
|---|---|---|---|---|---|---|
| BHY | 2970 | 0 | 0 | |||
| HLAC-79 | 23 | 0 | 0 | |||
| FADU | 13 | 0 | 0 | |||
| PCI-13 | 156 | 0 | 0 | |||
| Ant-1 | 2 | 0 | 0 | |||
| PCI-1 | 181 | 0 | 0 | |||
| Tumour samples | 12 hr | 48 hr | 12 hr | 48 hr | 12 hr | 48 hr |
| T 123 | 7580 | 18 655 | 0 | 2818 | 19 | 0 |
| T 126 | 5868 | 4875 | 0 | 0 | 112 | 52 |
| T 128 | 5111 | 8687 | 0 | 597 | 266 | 66 |
| T 129 | 2671 | 3359 | 0 | 1752 | 68 | 5 |
| T 130 | 5887 | 667 | 0 | 297 | 54 | 0 |
| T 131 | 7473 | 11 963 | 0 | 0 | 388 | 0 |
| T 133 | 16 460 | 17 605 | 0 | 0 | 631 | 680 |
| T 134 | 5191 | 3774 | 0 | 0 | 0 | 52 |
Indomethacin treatment reverses inhibition of PDC-derived IFN-α production by the head and neck cancer cell line PCI-0
Next we tested whether inhibition of COX-1/2 by indomethacin restores IFN-α induction by CpG DNA in PDC exposed to tumour supernatant. The two head and neck cancer cell lines PCI-0 and FADU were treated with indomethacin and the effect of the tumour supernatants on PDC-mediated IFN-α secretion was examined. We found that pretreatment of PCI-0 tumour cells (which produce high levels of PGE2) with indomethacin reduced the capacity of the tumour supernatant to inhibit IFN-α production (Fig. 1). Consistent with the lack of PGE2 production by FADU cells, the inhibitory effect of FADU supernatants was not affected by the pretreatment with indomethacin (Fig. 1 and Table 3), suggesting that in FADU cells a different mechanism of PDC suppression is in place.
Table 3.
Prostaglandin E2 (PGE2) concentrations in tumour cell line supernatants treated without indomethacin
| Cell line | PGE2 (pg/ml) |
|---|---|
| PCI-0 | 1470 |
| PCI-0 + indomethacin | 76 |
| FADU | 39 |
| FADU + indomethacin | 34 |
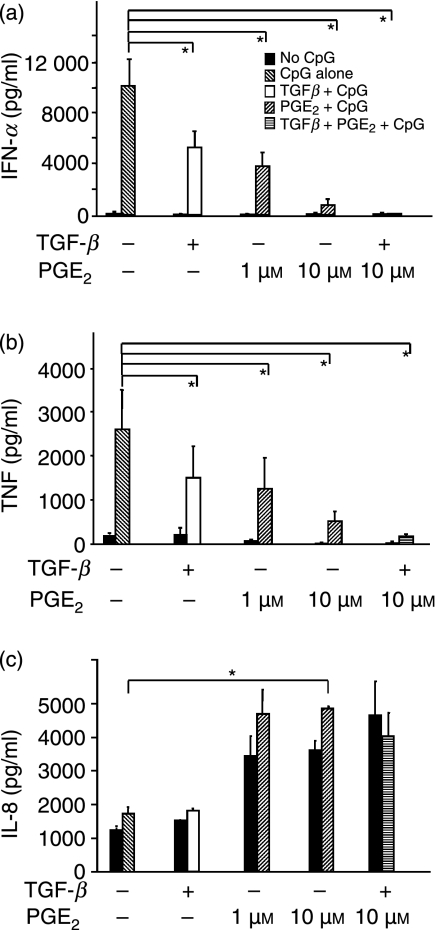
Recombinant PGE2 inhibits IFN-α and TNF production but stimulates IL-8 in TLR9-stimulated human PDC
As these results indicated that PGE2 synthesis may represent a central factor for tumour-mediated suppression of PDC function we next studied whether recombinant human PGE2 interferes with TLR9-triggered PDC-derived IFN-α production. Indeed, we found that PGE2 inhibited CpG ODN 2006-induced IFN-α and TNF secretion in a concentration-dependent manner (Figs 2a,b). Interestingly, this effect was only seen when PDC were pre-incubated with PGE2 for at least 2 hr, and 4 hr of pre-incubation was most effective (data not shown).
Figure 2.
Effect of human recombinant prostaglandin E2 (PGE2) and transforming growth factor-β (TGF-β) on cytosine–phosphate–guanosine oligodesoxynucleotide (CpG-ODN)-stimulated interferon-α (IFN-α), TNF and interleukin-8 (IL-8) production in plasmacytoid dendritic cells (PDC). PDC were pre-incubated with PGE2 and TGF-β before stimulation with toll-like receptor (TLR). After 24 hr, the production of IFN-α, TNF and IL-8 production was measured using enzyme-linked immunosorbent assays (ELISAs). Black bars show unstimulated conditions; grey (CpG alone), white (TGF + CpG) and striped (PGE2 + CpG or TGF + PGE2 + CpG) bars show values after stimulation with CpG ODN 2006, respectively. The mean values ± standard error of the mean (SEM) of four individual donors are shown. (a) IFN-α. P*[CpG/TGF + CpG] = 0·007; P*[CpG/PGE2 1 μm+ CpG] = 0·009; P*[CpG/PGE2 10 μm+ CpG] = 0·007; P*[CpG/PGE2 10 μm+ TGF + CpG] = 0·009 (b) TNF.P*[CpG/TGF + CpG] = 0·009; P*[CpG/PGE2 1 μm+ CpG] = 0·007; p*[CpG/PGE2 10 μm + CpG] = 0·03; P*[Medium/PGE2 10 μm+ TGF + CpG] = 0·03; (c) IL-8.P*[CpG/PGE2 10 μm+ CpG] = 0·03.
Moreover, PGE2 increased IL-8 secretion (Fig. 2c) in TLR9-stimulated cells and in unstimulated PDC. It should also be noted that IL-6 induction was observed in the majority of donors; however, individual donors displayed PGE2-dependent downregulation of IL-6.
TGF-β synergizes with PGE2 in blocking IFN-α and TNF secretion
Immunosuppressive cytokines secreted by tumour-infiltrating leucocytes may add to the PGE2-induced IFN-α suppression. To examine this, PDC were pre-incubated with PGE2, TGF-β, or both, before stimulation with CpG ODN 2006. We found that TGF-β alone inhibits both IFN-α and TNF secretion in TLR9-stimulated PDC, but does not influence IL-8 secretion (Fig. 2c). Furthermore, TGF-β and PGE2 synergistically suppressed IFN-α and TNF production, resulting in a near to complete block of IFN-α and TNF production (Figs 2a,b). As with TGF-β alone, the addition of TGF-β to PGE2 did not affect IL-8 and IL-6 production (Fig. 2c and data not shown).
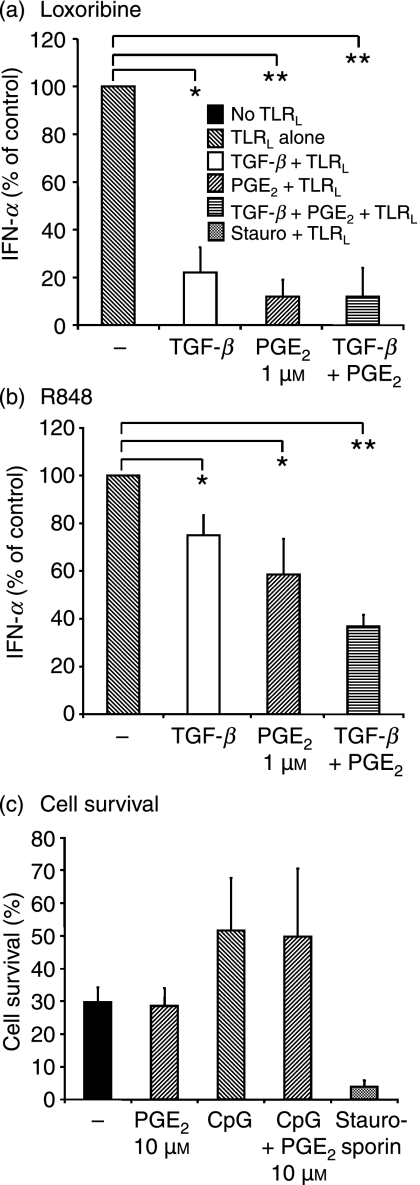
PGE2- and TGF-β-induced suppression of TLR9-induced IFN-α production also applies to TLR7 ligation but does not affect cell survival
PDC-derived IFN-α production can be stimulated via both TLR7 and TLR9 expressed on PDC. We found that PGE2 and TGF-β also inhibited IFN-α production induced by another TLR9 ligand, namely type A CpG ODN 2216 (data not shown), the TLR7 ligand loxoribine (Fig. 3a) and the TLR7/8 ligand R848 (Fig. 3b). Again, the combination of PGE2 and TGF-β was most effective. It should also be noted that loxoribine-induced IFN-α secretion was much more easily blocked than R848-mediated IFN-α, which was only partially reduced to 37 ± 5%. One possible explanation for this is that R848 has been proposed to activate the inflammasome, possibly by engaging cytosolic RNA recognition receptors in addition to TLR7 and TLR8.24
Figure 3.
Effect of prostaglandin E2 (PGE2) and transforming growth factor-β (TGF-β) on toll-like receptor 7 (TLR7)-mediated cytokine secretion and on cell viability. Plasmacytoid dendritic cells (PDC) were pre-incubated with PGE2 and TGF-β before stimulation with TLR7. Interferon-α (IFN-α) secretion was quantified in supernatants, 24 hr after stimulation with TLR7, using enzyme-linked immunosorbent assays (ELISAs). Grey (TLR ligand (TLRL) alone, white (TGF + TLRL) and striped (PGE2 + TLRL or TGF + PGE2 + TLRL) bars show the results for PDC stimulated with loxoribine (a) or R848 (b). All values were normalized to sole TLR7 ligation (= 100%; grey bar). The mean values ± standard error of the mean (SEM) of four individual donors are shown. (a) Loxoribine.P* [loxoribine/TGF + loxoribine] = 0·01; P** [loxoribine /PGE2 + loxoribine] = 0·0003; P** [loxoribine /TGF + PGE2 + loxoribine] = 0·002. (b) R848. P*[R848/TGF + R848] = 0·02; [R848/PGE2 + R848] = 0·02; P** [R848/TGF + PGE2 + R848] = 0·004. (c) Cell survival. Cell viability was determined in unstimulated and in cytosine–phosphate–guanosine oligodesoxynucleotide (CpG ODN) 2006-stimulated PDC, with and without PGE2 (10 μm) pre-incubation, after 18 hr. Viable cells were defined as annexin V negative and propidium iodide negative. The graph depicts the percentage of viable cells in the different conditions summarized from n = 4 experiments.
As with CpG ODN 2006, TLR7-induced IL-6 and IL-8 secretion were preserved (data not shown), indicating that PGE2- and TGF-β-treated PDC are viable albeit the fact that other researchers have associated the use of higher concentrations of PGE2 (10 μm) with apoptosis.9 To exclude that the reduction in IFN-α and TNF was caused by decreased cell survival, we additionally controlled cell viability. We did not find differences in the percentages of annexin V- and propidium iodide-negative cell populations when PDC were treated overnight with or without PGE2 (10 μm) (Fig. 3c). Notably, PDC survival was enhanced when PDC were stimulated with the TLR9 agonist CpG ODN 2006, regardless of the presence or absence of PGE2. Furthermore, TGF-β also had no effect on PDC survival (data not shown).
EP receptor expression in human PDC
The biological effects of PGE2 are mediated via the protein G-coupled receptors EP1–4. To understand in greater detail the underlying molecular mechanisms responsible for the effects of PGE2 on human PDC, we next performed a real-time RT-PCR analysis of EP receptor mRNA expression. We found that PDC express EP2 at high levels and express EP3 and EP4 at comparably low levels (Fig. 4a). These results suggest a dominant role of EP2 in human PDC.
Figure 4.
Role of EP receptors and cyclic AMP (cAMP) in prostaglandin E2 (PGE2)-mediated suppression of toll-like receptor (TLR)-induced interferon-α (IFN-α) secretion. (a) EP receptor messenger RNA (mRNA) expression. Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed from mRNA obtained from freshly isolated whole peripheral blood mononuclear cells (PBMC) and plasmacytoid dendritic cells (PDC). The diagram shows the mean from six independent donors ± standard error of the mean (SEM). Mean values are shown as numbers. (b and c) PDC were pre-incubated with PGE2, forskolin or EP receptor agonists for 4 hr before stimulation with cytosine–phosphate–guanosine oligodesoxynucleotide (CpG ODN) 2006. After 24 hr, IFN-α production was determined in the supernatants. The diagram summarizes the mean values ± SEM of n = 4 individual donors. Only values for CpG ODN-stimulated samples are shown. Individual values were normalized to the untreated CpG ODN control. (b) Forskolin. **P (CpG/CpG+Forskolin) = 0·004. (c) EP-receptor agonists: sulprostone (EP1 and EP3 agonist) and 11-deoxy-PGE1 (EP2 and EP4 agonist). *P (CpG/CpG+PGE2) = 0·01; *P (CpG/CpG+100nm 11-deoxy-PGE1) = 0.04; *P (CpG/CpG+100 nm Sulprostone) = 0·01. (d) Inhibition of smad signaling. PDC were pre-incubated with SB-431542 (SB-4315.) for 20 min before the addition of transforming growth factor-β (TGF-β) for a 4-hr pre-incubation period and the addition of CpG ODN 2006 for another 24 hr. IFN-α production in the supernatants was measured using enzyme-linked immunosorbent assays (ELISAs). Only CpG ODN-stimulated conditions are shown. The mean values ± SEM of four individual donors are shown. P** [medium/TGF-β] = 0·0001.
PGE2-mediated IFN-α suppression can be mimicked by activation of adenylate cyclase and by EP2 agonists
EP2, EP3 and EP4 have been demonstrated to activate adenylate cyclase and thereby increase cAMP levels in the cell.10,25 Assuming that this signalling pathway may be involved in the inhibition of TLR-induced IFN-α production in human PDC, we tested whether the synthetic cAMP inductor forskolin26 would mimic the PGE2 effects on PDC. Indeed, pre-incubation with forskolin can substitute for PGE2 in inhibiting IFN-α secretion from human PDC (Fig. 4b).
Next, the effect of different PGE2 receptor agonists was tested. Human PDC were pre-incubated with sulprostone (EP1 and EP3) and with 11-deoxy PGE1 (EP2 and EP4). A significant suppression of PDC-derived IFN-α secretion was seen with both substances, starting with a concentration of 100 nm (Fig. 4c). Interestingly, the EP2 agonist, butaprost, was only effective at high concentrations (10 μm) (data not shown). As with PGE2, TLR-induced IL-6 secretion was preserved by all PGE2 analogues (data not shown).
Taken together, these data indicate that PGE2-induced inhibition of IFN-α secretion is probably caused by a cAMP-dependent mechanism because forskolin can substitute for PGE2. Thus, EP1 is unlikely to be involved, a conclusion supported by the lack of EP1 mRNA expression. Nevertheless, sulprostone and 11-deoxy-PGE1 both exerted an inhibitory effect on IFN-α production. Thus, the functional assays support a redundant role of EP2, EP3 and EP4. Moreover, synergistic action of these receptors may be necessary because stimulation with the selective EP2 agonist, butaprost, showed little effect when it was used at comparable concentrations (data not shown).
TGF-β executes its effect on PDC-derived IFN-α via recruitment of smad proteins
To assess whether the effects of TGF-β are mediated via receptor-mediated activation of the smad signaling cascade we decided to block smad-mediated signal transduction. We found that, indeed, the ALK-5 inhibitor SB-431542 (an inhibitor of TGF-β-induced smad signaling) reverses the IFN-α blocking effect of recombinant TGF-β (Fig. 4d). It should, however, also be noted that high concentrations of SB-431542 increased the levels of CpG-induced IFN-α in the absence of exogenous TGF-β (Fig. 4d), thus indicating that endogenous TGF-β may be involved in controlling IFN production in PDC.
Evidence for an autoinhibitory control of IFN-α secretion by endogenous PGE2
Moreover, exposure of PDC to indomethacin also resulted in slightly enhanced IFN-α levels (Fig. 1). This finding was confirmed by detection of COX-2 mRNA expression in human PDC (data not shown) and PGE2 production in PDC supernatants (56 pg/ml in unstimulated PDC; 86 pg/ml in CpG-stimulated PDC). Downregulation of endogenous PGE2 via COX-2 inhibition may therefore result in dissolution of autoinhibition of IFN-α production. Taken together, these data illustrate that blockade of PGE2 synthesis and/or of TGF-β signalling enhance TLR-mediated stimulation of IFN-α production via direct effects on PDC and indirect effects on the tumour and the infiltrating leucocytes.
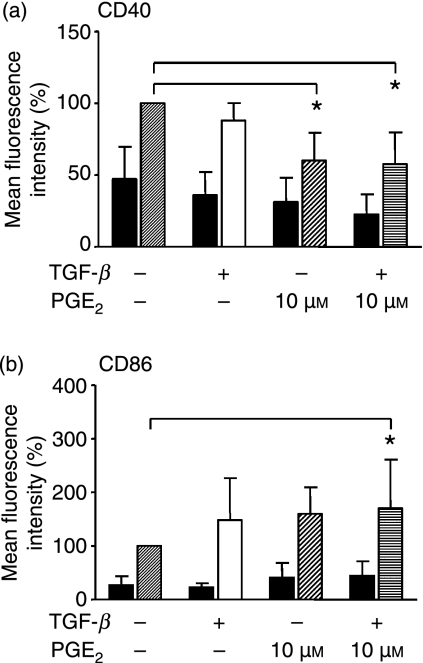
PGE2 and TGF-β modulate the expression of costimulatory molecules
PDC can costimulate T-effector cells and T-regulatory cells via costimulatory molecules such as CD80, CD86 and CD40; additionally, an upregulation of MHC II molecules precedes its antigen-presenting capacity. We were therefore interested whether, in addition to cytokine secretion, PGE2 and TGF-β would affect the costimulatory properties of human PDC. To test this, we performed a flow cytometric characterization of PDC surface markers after pre-incubation with PGE2 and TGF-β, with and without TLR9 stimulation. We found that PGE2 and TGF-β increased the expression of CD86 but suppressed the expression of CD40 in unstimulated and TLR9-stimulated PDC (Fig. 5). The reduction in CD40 surface expression was most prominent with PGE2 (Fig. 5a), while an increase in CD86 surface expression was statistically significant only when both substances were combined (Fig. 5b).
Figure 5.
Influence of prostaglandin E2 (PGE2) and transforming growth factor-β (TGF-β) on the expression of costimulatory molecules. Plasmacytoid dendritic cells (PDC) were pre-incubated with or without PGE2 and/or TGF-β. Expression of cell-surface molecules was measured by flow cytometric analysis of live gated cells after 18 hr. Unstimulated cells (black bars) were compared with cytosine–phosphate–guanosine oligodesoxynucleotide (CpG ODN)-stimulated PDC (grey, white, pattered and striped bars). Mean fluorescence intensities (MFI) were normalized to CpG ODN-stimulated PDC (= 100%). Mean values are shown as the percentage of CpG ODN-stimulated PDC ± standard deviation (SD) from n = 4 (a) and n = 5 (b) independent experiments. (a) CD40 expression: P* [CpG:CpG + PGE2] = 0·026; P* [CpG : CpG + PGE2 + TGF-β] = 0·03.(b) CD86 expression: P* [CpG : CpG + PGE2 + TGF-β] = 0·05).
PGE2 and TGF-β induce the expression of CXCR4
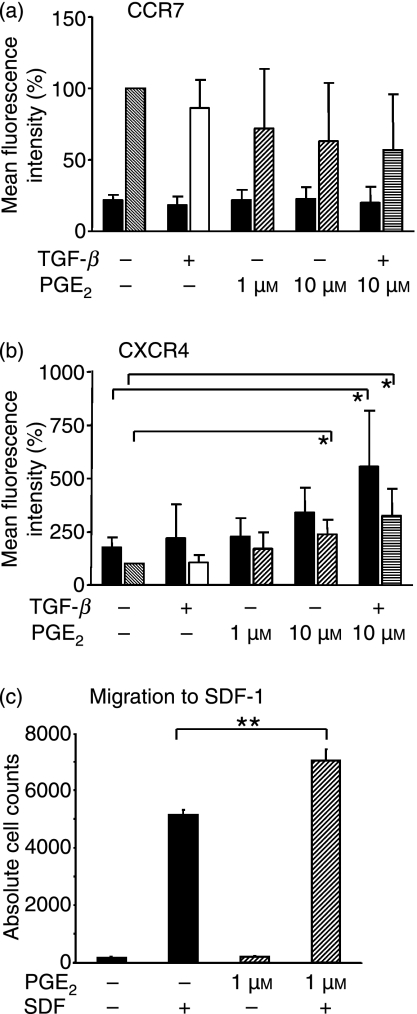
TLR-induced activation of PDC has been demonstrated to induce the lymph node homing receptor CCR7.27,28 As PDC have been shown to costimulate T cells in the regional lymph nodes,29–36 we were interested in whether tumour-infiltrating PDC exposed to PGE2 and TGF-β in tumour tissue are capable of migrating to the lymph nodes. We therefore analyzed the effect of PGE2 and TGF-β on the expression of the lymph node homing receptor CCR7 and on the SDF-1 receptor CXCR4. Our results showed a clear trend but no statistical significance for the PGE2-mediated inhibition of TLR9-mediated upregulation of CCR7 (Fig. 6a). In marked contrast, PGE2 enhanced CXCR4 expression in unstimulated and TLR9-stimulated PDC (Fig. 6b). Both effects were more pronounced when PGE2 was combined with TGF-β (Figs 6a,b). Consistent with CXCR4 upregulation, we observed increased migration of PDC in response to the CXCR4 ligand, SDF-1, when pretreated with PGE2 (Fig. 5c). We conclude that the decrease in the CCR7 : CXCR4 ratio may result in retention of PDC in the tumour tissue.
Figure 6.
Migratory potential of prostaglandin E2 (PGE2) and transforming growth factor-β (TGF-β)-treated plasmacytoid dendritic cells (PDC). (a and b) CCR7 and CXCR4 expression. PDCs were pre-incubated with PGE2 and TGF-β and stimulated with cytosine–phosphate–guanosine oligodesoxynucleotide (CpG ODN) 2006 (grey, white, patterned and striped bars), or were left unstimulated (black bars), for 18 hr. The expression of CCR7 and CXCR4 was measured using flow cytometry. The diagrams depict the average mean fluorescence intensities (MFI) ± standard deviation (SD) of n = 4 experiments in per cent because values were normalized to CpG ODN-treated PDC (= 100%; grey bars). (a) CCR7. (b) CXCR4. P* [CpG : CpG + PGE2] = 0·026; P* [CpG : CpG + PGE2 + TGF-β] = 0·037; P* [untreated : PGE2 + TGF-β] = 0·048. (c) Migration assay. Stromal-derived factor-1 (SDF-1)-induced migration was analyzed in untreated PDC and in PDC pretreated with PGE2 for 2 hr. The diagram shows the values of one representative experiment of n = 2 performed in triplicate ± standard error of the mean (SEM). P** [untreated/PGE2] = 0·002.
Discussion
Tumour escape from immune recognition represents one of the key issues to be faced in tumour therapy. Among the numerous mechanisms involved in immune escape, the secretion of prostaglandins (such as PGE2) and the induction of immunosuppressive cytokines from tumour-infiltrating leucocytes play central roles in many tumour types.20,37 One possible approach for immune stimulation in tumour patients is treatment with CpG DNA ODNs.2,38,39 This therapeutic strategy is based on selective activation of PDC that produce IFN-α, resulting in enhanced innate and adaptive immune responses. This approach depends on the presence and the intact function of the PDC in tumour tissue. As several publications indicated that PDC are functionally deficient in the tumour setting,2,31,40,41 therapeutic targeting of the PDC may require prior restoration of its full function. In a mouse model for colon carcinoma, Vicari et al.42 provided evidence that the administration of an antibody to IL-10R potentiates the efficacy of CpG ODNs by reversing IL-10-induced immunosuppression. Our study aimed to achieve a better understanding of the impact of PGE2 and TGF-β, two other key immunosuppressive factors found in tumour tissue, on the function of human PDC.
Here we demonstrate that PGE2 and TGF-β act synergistically to form a complete block of IFN-α and TNF secretion in PDC. In marked contrast to IFN-α and TNF, IL-6 and IL-8 production are enhanced in PGE2- and TGF-β-treated PDC, confirming that the viability of PDC is not reduced, as further shown by staining with annexin V and propidium iodide (PI) (Fig. 3c). Therefore, our results do not support the view that the reduction in IFN-α is caused by PGE2-induced apoptosis.9
Induction of IL-6 by PGE2 is a well-known phenomenon described in the literature.43,44 Both IL-6 and IL-8 are pleiotropic cytokines that promote immune-cell survival and chemotaxis, but also enhance tumour cell proliferation and angiogenesis.45–48 Thus, it is in favour of the tumour to support IL-6 and IL-8 secretion while inhibiting IFN-α and TNF, which are considered as immunologically more potent cytokines as a result of their ability to prime innate and adaptive immune cells and to shape the adaptive immune response. Interestingly, very recently, a similar alteration of PDC function has been described in murine Peyer’s patch PDC. It is therefore very likely that the effects we describe for the tumour setting will also be valid in the intestinal and the pulmonary mucosa where high levels of PGE2, IL-10 and TGF-β govern the immune response.49
Tumour-derived PGE2 secretion is accompanied by the secretion of other immunomodulatory factors, including SDF-1, the ligand for CXCR4. In this context it is interesting to note that PGE2 upregulates the expression of CXCR4 on human PDC (Fig. 6b). The functionality of the CXCR4 receptor could be demonstrated using a migration assay showing that, indeed, PDC migration along the SDF-1 gradient is augmented by PGE2 treatment (Fig. 6c). Thus, PDC can be retained in the tumour tissue via PGE2-induced sensitization for SDF-1.50 In further support of PDC retention in the tumour tissue there was a clear trend for the suppression of the upregulation of the lymph node-homing receptor, CCR7, in the presence of PGE2 (and TGF-β) (Fig. 6a). As a consequence, PGE2-exposed PDC are unlikely to present antigen and to prime T cells in the regional lymph nodes.
What is more, our data indicate that co-stimulation of T-effector cells will be impaired as a result of the suppression of CD40 expression on the PDC surface (Fig. 5a). In marked contrast, the expression of CD86, which can serve as a ligand for the negative regulatory receptor cytotoxic T-lymphocyte antigen-4 (CTLA-4), a molecule expressed on T cells, is enhanced (Fig. 5b), and may even promote the co-stimulation of T-regulatory cells.51 This, again, would support a functional shift to a tolerogenic role of the PDC at the tumour site.
Two other immunosuppressive factors present in tumour tissue are IL-10 and TGF-β, the major source for which are tumour-infiltrating leukocytes (TIL). The induction of IL-10 by PGE2 is well known.11,18 It has been reported that tumour-derived IL-10 levels correlate with decreased numbers and an immature PDC phenotype in tumour patients.52 The strong variations observed in the secretion of soluble immunomodulatory substances in individual tumour samples and different tumour cell lines are probably caused by cellular origin, patient immune status and tumour stage, and may additionally be influenced by the protocol used to generate the tumour cell line. In spite of this, our analysis of tumour cell line supernatants and of primary tumour supernatants allows the conclusion that TGF-β and IL-10 production may be derived from tumour-infiltrating leukocytes because both of these cytokines were found in primary tumour sample supernatants but not in tumour cell line supernatants (Table 2). Nevertheless, PDC in the tumour environment will be exposed to PGE2 as well as to IL-10 and TGF-β, and our data provide evidence that the mutual presence synergistically enhances their effects as we found that TGF-β augments the effects of PGE2.
The PGE2 signalling pathway is initiated by the activation of one or more EP receptors. All of these receptors have been described in the immune system. EP1 expression has so far been shown to be restricted to kidney, lung and stomach as well as to tumour tissue. Recently, EP1 expression has also been found on mast cells. In the current study we detected EP2 mRNA expression in human PDC while expression of EP3 and EP4 mRNA was minimal and expression of EP1 mRNA was absent. Our data indicate that EP2, EP3 and EP4 may exert redundant effects because the PGE2 effects could be mimicked by the EP3 agonist (11-deoxy-PGE1) as well as by EP2 and EP4 agonists (sulprostone and butaprost, respectively). Furthermore, forskolin, an adenylate cyclase activator that induces cAMP, was able to mimic the effects of PGE2. cAMP regulation has been shown to be involved in all EP receptor pathways except for EP1. This finding is also supported by the fact that PGE2 did not induce calcium flux in human PDC (M. Schäfer, unpublished observation), an observation also described for other cell types.53,54
According to our in vitro data and several recently published clinical studies, tumour patients may benefit from treatment with COX inhibitors blocking PGE2 release from the tumour.55,56 As not all tumour cell lines examined expressed PGE2 and only those respond to COX inhibitors, COX inhibitors will only be useful in patients with PGE2-secreting tumours. However, our study shows that COX inhibition with indomethacin not only blocks tumour cell-derived PGE2 synthesis, but also enhances PDC-derived TLR-induced IFN-α production in the absence of tumour supernatant. We found that PDC express COX-2 (data not shown) and secrete endogenous PGE2 (see the Results). COX inhibitors may therefore possess an additional beneficial effect, separate from that of COX inhibition, in tumour cells.
Of note, the TGF-β inhibitor, SB-431542,57,58 blocks Alk5 and smad phosphorylation. Via blockade of smad phosphorylation the TGF-β receptors, as well as the sphingosine-1-phosphate (S1P) receptors, are inhibited.59 SB-431542 not only reversed the suppression of IFN-α by exogenous recombinant TGF-β, but also enhanced CpG-induced IFN-α in PDC in the absence of exogenous TGF-β. Thus, our data provide evidence for an autocrine feedback loop regulating PDC-derived IFN-α secretion via the smad-pathway. Together these data suggest that a combination of COX-1/2 inhibitors and inhibition of TGF-β may improve the therapeutic activity of CpG oligonucleotides. However, because of a different TLR9 expression pattern in mice compared with humans (activation of myeloid cells and the direct induction of IL-12 by CpG ODN in mice but not in humans), the predictive value of testing this strategy in mouse tumour models is limited.
Acknowledgments
This work was supported by grants BMBF Biofuture 0311896, SFB 704, SFB 670 and KFO115 to G.H., the Dr Mildred Scheel Stiftung 10-2074/WO2 to B.W. and G.H., the German Research Foundation (DFG) Graduiertenkolleg 1202 to M.S., S.E. and G.H., and grants from DFG (DI898/1-1), the Krebs- and Scharlachforschung Mannheim and the Medical Faculty of the University of Heidelberg (Olympia-Morata program) to I.B.-D.
Disclosures
The authors declare no competing financial interests.
References
- 1.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann E, Wollenberg B, Rothenfusser S, et al. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–87. [PubMed] [Google Scholar]
- 3.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–10. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 5.Toutirais O, Chartier P, Dubois D, Bouet F, Leveque J, Catros-Quemener V, Genetet N. Constitutive expression of TGF-beta1, interleukin-6 and interleukin-8 by tumor cells as a major component of immune escape in human ovarian carcinoma. Eur Cytokine Netw. 2003;14:246–55. [PubMed] [Google Scholar]
- 6.Biron CA. Interferons alpha and beta as immune regulators – a new look. Immunity. 2001;14:661–4. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 7.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–68. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 8.Kumagai K, Takeda K, Hashimoto W, et al. Interleukin-12 as an inducer of cytotoxic effectors in anti-tumor immunity. Int Rev Immunol. 1997;3:229–56. doi: 10.3109/08830189709116854. [DOI] [PubMed] [Google Scholar]
- 9.Son Y, Ito T, Ozaki Y, et al. Prostaglandin E is a negative regulator on human plasmacytoid dendritic cells. Immunology. 2006;119:36–42. doi: 10.1111/j.1365-2567.2006.02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimoto Y, Narumiya S. Prostaglandin E receptor. J Biol Chem. 2007;282:11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 11.Harizi H, Norbert G. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell Immunol. 2004;228:99–109. doi: 10.1016/j.cellimm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda E, Sugiura T, Okada K, Zeki K, Yamashita U. Prostaglandin E2 up-regulates macrophage-derived chemokine production but suppresses IFN-inducible protein-10 production by APC. J Immunol. 2001;166:1650–8. doi: 10.4049/jimmunol.166.3.1650. [DOI] [PubMed] [Google Scholar]
- 13.Meja KK, Barnes PJ, Giembycz MA. Characterization of the prostanoid receptor(s) on human blood monocytes at which prostaglandin E2 inhibits lipopolysaccharide-induced tumour necrosis factor-alpha generation. Br J Pharmacol. 1997;122:149–57. doi: 10.1038/sj.bjp.0701360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seldon PM, Giembycz MA. Suppression of granulocyte/macrophage colony-stimulating factor release from human monocytes by cyclic AMP-elevating drugs: role of interleukin-10. Br J Pharmacol. 2001;134:58–67. doi: 10.1038/sj.bjp.0704238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223:120–32. doi: 10.1016/s0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 16.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 17.Roper RL, Graf B, Phipps RP. Prostaglandin E2 and cAMP promote B lymphocyte class switching to IgG1. Immunol Lett. 2002;84:191–8. doi: 10.1016/s0165-2478(02)00185-2. [DOI] [PubMed] [Google Scholar]
- 18.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–63. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 19.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–29. [PubMed] [Google Scholar]
- 20.Rolland PH, Martin PM, Jacquemier J, Rolland AM, Toga M. Prostaglandin production and metabolism in human breast cancer. Adv Prostaglandin Thromboxane Res. 1980;6:575–80. [PubMed] [Google Scholar]
- 21.Bekeredjian-Ding I, Inamura S, Giese T, Moll H, Endres S, Sing A, Zahringer U, Hartmann G. Staphylococcus aureus protein A triggers T cell-independent B cell proliferation by sensitizing B cells for TLR2 ligands. J Immunol. 2007;178:2803–12. doi: 10.4049/jimmunol.178.5.2803. [DOI] [PubMed] [Google Scholar]
- 22.Bekeredjian-Ding I, Roth SI, Gilles S, Giese T, Ablasser A, Hornung V, Endres S, Hartmann G. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J Immunol. 2006;176:7438–46. doi: 10.4049/jimmunol.176.12.7438. [DOI] [PubMed] [Google Scholar]
- 23.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–50. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 24.Kanneganti TD, Ozoren N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 25.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 26.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA. 1981;78:3363–7. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–67. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;2:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 29.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Bendriss-Vermare N, Chaperot L, Peoc’h M, et al. In situ leukemic plasmacytoid dendritic cells pattern of chemokine receptors expression and in vitro migratory response. Leukemia. 2004;18:1491–8. doi: 10.1038/sj.leu.2403452. [DOI] [PubMed] [Google Scholar]
- 31.Cox K, North M, Burke M, Singhal H, Renton S, Aqel N, Islam S, Knight SC. Plasmacytoid dendritic cells (PDC) are the major DC subset innately producing cytokines in human lymph nodes. J Leukoc Biol. 2005;78:1142–52. doi: 10.1189/jlb.1103532. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura K, Kadowaki N, Kitawaki T, Uchiyama T. Virus-stimulated plasmacytoid dendritic cells induce CD4+ cytotoxic regulatory T cells. Blood. 2006;107:1031–8. doi: 10.1182/blood-2005-04-1737. [DOI] [PubMed] [Google Scholar]
- 33.Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Briere F, Trinchieri G, Caux C. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198:823–30. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang GX, Lian ZX, Kikuchi K, Liu YJ, Ansari AA, Ikehara S, Gershwin ME. CD4- plasmacytoid dendritic cells (pDCs) migrate in lymph nodes by CpG inoculation and represent a potent functional subset of pDCs. J Immunol. 2005;174:3197–203. doi: 10.4049/jimmunol.174.6.3197. [DOI] [PubMed] [Google Scholar]
- 35.Yoneyama H, Matsuno K, Zhang Y, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–28. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 36.Yoneyama H, Matsuno K, Toda E, et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–35. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–23. [PubMed] [Google Scholar]
- 38.Heckelsmiller K, Beck S, Rall K, et al. Combined dendritic cell- and CpG oligonucleotide-based immune therapy cures large murine tumors that resist chemotherapy. Eur J Immunol. 2002;32:3235–45. doi: 10.1002/1521-4141(200211)32:11<3235::AID-IMMU3235>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 40.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, Lebecque S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–9. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 41.Treilleux I, Blay JY, Bendriss-Vermare N, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–74. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 42.Vicari AP, Chiodoni C, Vaure C, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–9. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo MA. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990;2:205–10. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Lin JX, Vilcek J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem. 1988;263:6177–82. [PubMed] [Google Scholar]
- 45.Bellocq A, Antoine M, Flahault A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 46.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto M, Kawamata H, Kawai K, Oyasu R. Enhancement of transformation in vitro of a nontumorigenic rat urothelial cell line by interleukin 6. Cancer Res. 1995;55:4581–5. [PubMed] [Google Scholar]
- 48.Voorzanger N, Touitou R, Garcia E, Delecluse HJ, Rousset F, Joab I, Favrot MC, Blay JY. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–505. [PubMed] [Google Scholar]
- 49.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. Cutting edge: Peyer’s patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol. 2007;179:2690–4. doi: 10.4049/jimmunol.179.5.2690. [DOI] [PubMed] [Google Scholar]
- 50.Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 51.Kurtz J, Raval F, Vallot C, Der J, Sykes M. CTLA-4 on alloreactive CD4 T cells interacts with recipient CD80/86 to promote tolerance. Blood. 2009;113:3475–84. doi: 10.1182/blood-2008-01-133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckebaum S, Zhang X, Chen X, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10:7260–9. doi: 10.1158/1078-0432.CCR-04-0872. [DOI] [PubMed] [Google Scholar]
- 53.Kaminuma O, Mori A, Ogawa K, Kikkawa H, Nakata A, Ikezawa K, Okudaira H. Cyclic AMP suppresses interleukin-5 synthesis by human helper T cells via the downregulation of the calcium mobilization pathway. Br J Pharmacol. 1999;127:521–9. doi: 10.1038/sj.bjp.0702558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW264.7 cells. Proc Natl Acad Sci USA. 2004;101:1840–5. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Backlund MG, Mann JR, Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69(Suppl 1):28–32. doi: 10.1159/000086629. [DOI] [PubMed] [Google Scholar]
- 56.Sinicrope FA. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Mol Carcinog. 2006;45:447–54. doi: 10.1002/mc.20232. [DOI] [PubMed] [Google Scholar]
- 57.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 58.Laping NJ, Grygielko E, Mathur A, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 59.Xin C, Ren S, Kleuser B, et al. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem. 2004;279:35255–62. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]