Abstract
Emerging data indicate that alterations in the expression of tumour necrosis factor (TNF) superfamily members play a crucial role in the pathogenesis of intestinal inflammation. Recent results demonstrated that sustained transgenic expression of lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpesvirus entry mediator on T cells (LIGHT; TNFSF14) induced severe intestinal inflammation, suggesting a specific role of LIGHT-mediated signalling to the intestinal compartment. In order to dissect the role of LIGHT in intestinal inflammation, we used LIGHT-deficient mice in the mouse model of acute dextran sodium sulphate-induced colitis. Interestingly, LIGHT-deficient mice were characterized by strongly reduced signs of intestinal inflammation compared with wild-type mice in this experimental model. Determination of mouse LIGHT mRNA expression in colon tissues of wild-type mice revealed a strong induction of mouse LIGHT mRNA expression during acute DSS-induced colitis. We therefore generated anti-mouse LIGHT monoclonal antibodies in LIGHT-deficient mice which bind specifically to LIGHT and are capable of neutralizing the activity of LIGHT in vitro and in vivo. With these antibodies, we demonstrated that neutralization of LIGHT during acute DSS-induced colitis resulted in reduced signs of intestinal inflammation. These data suggest that LIGHT is an important mediator in intestinal inflammation and may serve as a new target for therapeutic intervention.
Keywords: DSS-induced intestinal inflammation, LIGHT, neutralizing antibodies
Introduction
LIGHT, a member of the tumour necrosis factor (TNF) superfamily, is a type II transmembrane protein expressed primarily on activated T lymphocytes, but also detectable on monocytes, granulocytes and immature dendritic cells.1,2 LIGHT can signal via two receptors of the TNF receptor (TNFR) superfamily: (i) the lymphotoxin beta receptor (LTβR) and (ii) the herpesvirus entry mediator (HVEM; TNFRSF14), and can further bind to the soluble decoy receptor 3 (DcR3; TR6).3,4 LTβR is expressed on cells of the myeloid lineage and on stromal cells, while HVEM is expressed predominantly on lymphocytes which do not express LTβR. Functionally, LIGHT signalling through HVEM has been shown to be an important costimulatory signal for activation and proliferation of T cells. LIGHT stimulates T-cell proliferation and induces interferon (IFN)-γ production, which can both be inhibited by neutralizing antibodies to HVEM or HVEM:Ig fusion protein.1 Furthermore, the blockade of LIGHT leads to a decreased cell-mediated immune response and ameliorated graft versus host disease.5 Several recent observations suggest a possible contribution of LIGHT in the development and manifestation of intestinal inflammation. Transgenic mice with enhanced expression of LIGHT on T cells spontaneously develop colitis.6 Furthermore, adoptive transfer of LIGHT transgenic mesenteric lymph node cells into recombination activating gene (RAG)−/− mice induced intestinal inflammation by activation and expansion of T helper type 1 (Th1) cells in the intestine.7
Crohn’s disease (CD) and ulcerative colitis are inflammatory bowel diseases (IBDs), characterized by a deregulated mucosal inflammatory response.8,9 Therefore, specific inhibitors of a pro-inflammatory mucosal immune response (e.g. anti-TNF therapy) are used in the treatment of patients with CD.10 However, the partial success of anti-TNF treatment in only a subset of patients with CD demonstrates the complexity of the immune-regulatory mechanisms and the need for other therapeutic targets involved in the pathogenesis of IBD.
Recent results have shown that ablation of LTβR signalling using either a functional inhibitor of LTβR activation (LTβR:Ig) or LTβR-deficient and LTαβ-deficient mice resulted in a significant aggravation of dextran sodium sulphate (DSS)-induced acute colitis. Activation of LTβR by LTαβ mainly expressed on T cells seems to be crucial for the down-regulation of the inflammatory response in this experimental model.11
In order to test the role of LIGHT, another functional ligand of the LTβR, in the development of acute intestinal inflammation we used LIGHT-deficient mice in this experimental model of acute DSS-induced colitis. As mice with genetic ablation of LIGHT developed almost no signs of intestinal inflammation, we generated monoclonal antibodies (mAbs) against mouse LIGHT capable of binding to and neutralizing mouse LIGHT in vitro and in vivo. Treatment of C57BL/6 mice with neutralizing anti-mouse LIGHT mAbs during acute DSS-induced colitis ameliorated acute intestinal inflammation in our experimental model. In conclusion, our results suggest that LIGHT plays a critical role in intestinal inflammation and thus LIGHT might serve as a new target molecule for therapeutic intervention.
Materials and methods
Animals and cell lines
Female C57BL/6 mice [wild type (WT)] were obtained from Charles River (Sulzfeld, Germany). LIGHT-deficient (LIGHT−/−) mice were generated as previously described12 and have been backcrossed more than six times to the C57BL/6 background. Animals were housed in individually ventilated cages and handled in accordance with institutional guidelines. Mice used for experiments were age, sex and weight (minimum 22 g) matched. A375 human melanoma cells were obtained from the American Type Culture collection (ATCC, Rockville, MD); BFS1 fibrosarcoma cells have been described previously.13,14
Generation of specific mAbs to mouse LIGHT
The extracellular domain of mouse and human LIGHT was expressed in Drosophila S2 cells and purified as described previously.13 LIGHT-deficient mice were immunized three times [subcutaneously (s.c.) and intraperitoneally (i.p.) on day 1 and day 22 and only i.p. on day 43] with 50 μg of mouse LIGHT. In the first two immunizations, Titermax Gold Adjuvant (Sigma Aldrich, Deisenhofen, Germany) was used as adjuvant. The fusion of splenocytes from immunized mice with mouse myeloma cells (SP2/0-Ag14) using polyethylene glycol (PEG) 1500 (Roche Diagnostics, Mannheim, Germany) was carried out 3 days after the last immunization according to established protocols.14 Hybridomas were selectively grown in hypoxantine–aminopterin–thymidine (HAT-Media Supplement; Boehringer, Mannheim, Germany), in the presence of peritoneal exudate cells as feeder cells. Hybridoma supernatants were screened for binding to mouse LIGHT by enzyme-linked immunosorbent assay (ELISA), using an anti-mouse immunoglobulin G (IgG) antibody as the detection antibody (Sigma Aldrich). Positive hybridomas were subcloned by limiting dilution, and screened for stable immunoglobulin production. Monoclonal antibodies were purified from supernatants by Protein-G column affinity chromatography (Econo System; BioRad, München, Germany) and dialysed against phosphate-buffered saline (PBS).
RNA isolation and reverse transcription–polymerase chain reaction (RT-PCR)
After removal of the first distal 1 cm of the colon for histological analysis, the second distal 1 cm of colon tissue was harvested and placed in an ice-cold RNAlater solution (Ambion, Austin, TX). RNA was extracted using the RNeasy Kit (Qiagen, Hilden, Germany) in combination with the Qiagen Shredder Kit following the manufacturer’s recommendations. RNA was transcribed using the Promega (Mannheim, Germany) Reverse Transcription System following the manufacturer’s recommendations. Quantification of mouse LIGHT mRNA was performed using a Light Cycler (Roche Molecular Systems, Mannheim, Germany) following the manufacturer’s recommendations. For standardization, 18S RNA was amplified. Primers specific for mouse LIGHT were purchased from SA Bioscience (Frederick, MD) following the manufacturer’s recommendations. Data (n = 3) are expressed as mean ± standard deviation and statistical analysis was performed using Student’s t-test. Representative data from one out of three independent experiments are shown.
Fluorescence-activated cell sorter (FACS) analysis
Spleen cells from C57BL/6 mice (WT) and LIGHT-deficient mice were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml ionomycin for 4 hr. Stimulated cells were stained with anti-CD3 and were incubated with anti-mouse LIGHT mAb 9D10 or 15B2 (10 μg/ml) followed by fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG. Dead cells were excluded by propidium iodide staining. As a control the FITC-conjugated rabbit anti-mouse IgG without anti-mouse LIGHT mAb was used. Cells were analysed using the LSRII (Becton Dickinson, San Jose, CA).
Immunoprecipitation
Recombinant human and mouse LIGHT (5 μg each) both expressed in DrosophilaS2 cells as described earlier13 and fused to a C-terminal myc-tag for detection were incubated in protein extraction buffer (RIPA buffer) and pre-cleared by the addition of Protein-G sepharose (GE Healthcare, Munich, Germany) for 15 min at room temperature. The supernatant was supplemented with 5 μg of the monoclonal antibodies 15B2 and 9D10, respectively, or with no antibody as a negative control. After incubation for 1 hr at 4° proteins were precipitated by adding 5 μg of Protein-G sepharose and additionally incubated for 1 hr at 4°. Precipitated proteins were washed twice, dissolved in sodium dodecyl sulphate (SDS) sample buffer and subjected to SDS gel electrophoresis. Western blot analysis was performed using anti-myc antibodies (Invitrogen, Groningen, the Netherlands) followed by peroxidase-conjugated anti-mouse IgG as a secondary antibody.
CXCL chemokine (C-X-C motif) ligand 2 (CXCL2) and CXCL8 expression induced by mouse and human recombinant LIGHT
Human melanoma cells (A375) and mouse fibrosarcoma cells (BFS-1) were incubated in quadruplicates for 4 hr with medium alone or medium supplemented with mouse LIGHT or human LIGHT (10 μg/ml), respectively, after incubation with increasing concentrations of the monoclonal antibody 15B2 or 9D10 for 15 min. The induced expression of CXCL8 and CXCL2 was measured in the supernatants by ELISA (all from R&D Systems, Minneapolis, MN). Mean values of quadruplicates ± standard deviation are given. Representative data from one out of three independent experiments are shown.
Induction and treatment of colitis
For induction of acute colitis, mice received 1·5% DSS (ICN Biomedicals, Aurora, OH) dissolved in drinking water for 7 days, as described previously.15,16 Body weight was recorded daily. For therapeutic studies, mice with DSS-induced acute colitis received 100 μg of affinity-purified anti-mouse LIGHT mAbs (i.p. from day 1 to day 6). Mouse IgG (100 μg; Sigma Aldrich) was used as a control.
Assessment of histological score
Mice were killed by cervical dislocation and their colons were removed and washed with PBS. The distal third of the colon was cut longitudinally and fixed in 10% formalin in PBS overnight. Longitudinal sections of the paraffin-embedded material were made. One 2-μm section was cut; the next section was cut at a distance of 100 μm. After another 100 μm, a third section was cut. The sections were stained with haematoxylin-eosin. Histological analysis was performed in a blinded fashion. All sections obtained were evaluated. Mice were scored individually, with each score representing the mean of three sections. Histology was scored as described previously:17 epithelium: 0, normal morphology; 1, loss of goblet cells; 2, loss of goblet cells in large areas; 3, loss of crypts; 4, loss of crypts in large areas; infiltration: 0, no infiltrate; 1, infiltrate around crypt basis; 2, infiltrate reaching to lamina muscularis mucosae; 3, extensive infiltration reaching the lamina muscularis mucosae and thickening of the mucosa with abundant oedema; 4, infiltration of the lamina submucosa. The total histological score represents the sum of the epithelium and infiltration scores and ranges from 0 to 8.
Statistics
Statistical analysis was performed using Student’s t-test [for the quantitative RT-PCR (qRT-PCR) and ELISA] or the Mann–Whitney rank sum test (for the histological score and change of body weight). Differences were considered significant at a P-value of P < 0·05.
Results
Amelioration of acute intestinal inflammation by LIGHT deficiency
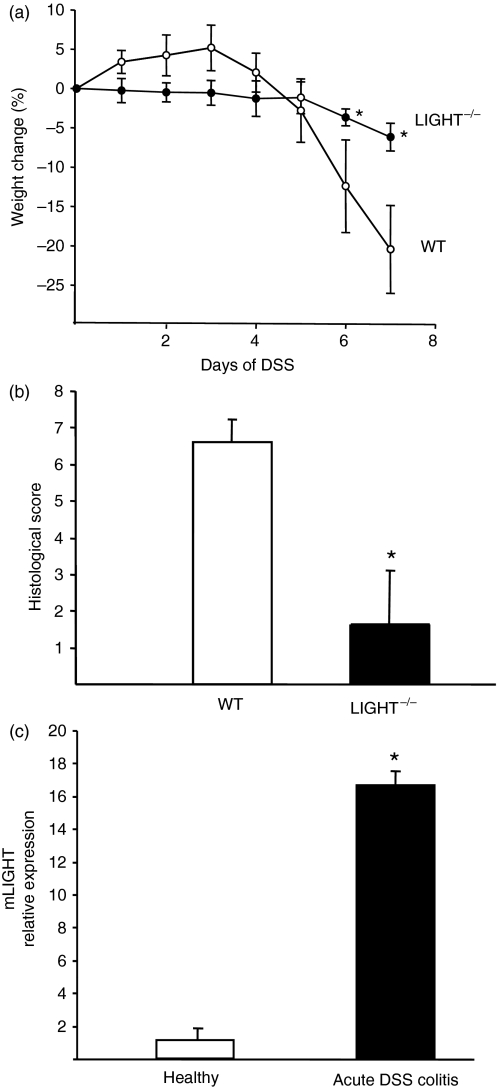
To determine the role of LIGHT in the development of colitis, we induced acute DSS-induced colitis in LIGHT-deficient mice and wild-type mice. After 7 days of 1·5% DSS treatment, LIGHT-deficient mice demonstrated reduced signs of intestinal inflammation characterized by significantly lower weight loss after day 6 in the LIGHT-deficient mice compared with the wild-type mice (Fig. 1a). Reduced ulceration, nearly no loss of crypts and goblet cells and an ameliorated inflammatory infiltrate were the histological findings in LIGHT-deficient mice after DSS treatment, resulting in a significantly decreased histological score compared with wild-type mice (Fig. 1b). These data clearly demonstrate that mice congenitally devoid of LIGHT expression show reduced signs of acute DSS-induced intestinal inflammation. In order to assess the expression of LIGHT during acute DSS-induced colitis, we determined the relative mRNA expression level in colon tissue after 7 days of DSS-induced colitis compared with the expression level in healthy control animals. As shown in Fig. 1c, treatment of C57BL/6 mice with 1·5% DSS for 7 days resulted in a strong induction of mouse LIGHT mRNA expression in colon tissue compared with healthy control animals.
Figure 1.
Effect of LIGHT deficiency in acute dextran sodium sulphate (DSS)-induced colitis. (a) Weight loss of C57BL/6 mice (n = 5) and LIGHT-deficient mice (n = 5) during DSS-induced acute colitis. Data are expressed as mean ± standard deviation (SD) and statistical significance was determined using the Mann–Whitney rank sum test. Differences were considered significant at P < 0·05. Representative data from one out of three independent experiments are shown. (b) Histological score of colon sections from C57BL/6 (n = 5) mice and LIGHT-deficient mice (n = 5) at day 7 after the induction of acute DSS-induced colitis. Statistical significance was determined using the Mann–Whitney rank sum test. Differences were considered significant at P < 0·05. Representative data from one out of three independent experiments are shown. (c) Quantitative reverse transcription–polymerase chain reaction (RT-PCR) of mouse LIGHT expression in colon tissue derived from C57BL/6 mice (n = 3) either untreated or treated with 1·5% DSS for 7 days. Data are expressed as mean ± SD. Representative data from one out of three independent experiments are shown.
Anti-mouse LIGHT mAbs bind and neutralize soluble mouse LIGHT but do not bind to the transmembrane form of mouse LIGHT
To determine whether neutralization of LIGHT can reduce the signs of intestinal inflammation in vivo, we generated monoclonal antibodies (mAbs) against mouse LIGHT. Recombinant mouse LIGHT was expressed and purified as reported previously13 for recombinant human LIGHT, and used for immunization of LIGHT-deficient mice.
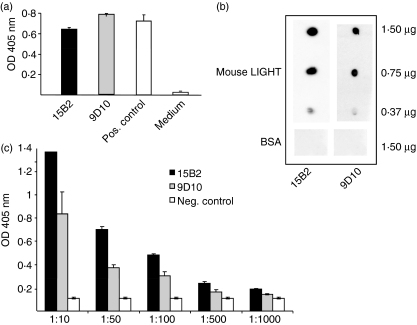
Fusion of splenocytes from the immunized LIGHT-deficient mice with mouse myeloma cells was performed according to established protocols.14,18 The two independent clones 9D10 and 15B2 were screened positive for specific binding to mouse LIGHT in ELISA coated with mouse LIGHT (5 μg/ml) as compared with medium alone as a negative control (Fig. 2a). Specific binding of the purified mAbs to mouse LIGHT was further demonstrated by diluting out the binding to mouse LIGHT as compared with mouse IgG as a negative control (Fig. 2c).
Figure 2.
Characterization of anti-mouse LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpesvirus entry mediator on T cells) monoclonal antibodies (mAbs). (a) Enzyme-linked immunosorbent assay (ELISA) using coated mouse LIGHT (2 μg/ml) and supernatants of the hybridomas 9D10 and 15B2 (dilution 1 : 100). Sera of immunized mice at a dilution of 1 : 10 000 served as a positive control and conditioned RPMI medium as a negative control. For detection, an alkaline phosphatase-conjugated anti-mouse immunoglobulin G (IgG) was used. (b) Dot blot analysis using supernatants of the hybridomas 9D10 and 15B2 and mouse LIGHT at the indicated concentrations including bovine serum albumin (BSA) as a negative control. Mouse LIGHT protein was detected using peroxidase-conjugated anti-mouse IgG as a secondary antibody. (c) ELISA using coated mouse LIGHT (2 μg/ml) and a dilution series of purified 9D10 and 15B2. Mouse IgG served as a negative control. For detection, an alkaline phosphatase-conjugated anti-mouse IgG was used.
Furthermore, both monoclonal antibodies recognized mouse LIGHT in dot blot assays in a concentration-dependent manner, indicating specific binding to the extracellular domain of mouse LIGHT, while no binding could be detected using bovine serum albumin (BSA) as antigen (Fig. 2b). In contrast, no specific binding to mouse LIGHT could be detected by western blot analysis using 9D10 and 15B2 (data not shown), indicating that both antibodies recognize only the native form of mouse LIGHT.
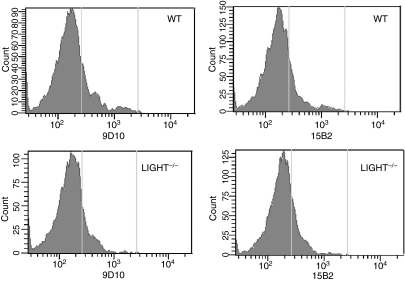
To further test whether both mAbs are capable of specifically binding to membrane-bound LIGHT, flow cytometry analysis was performed using spleen cells derived from wild-type and LIGHT-deficient mice. It was shown previously by others that activated spleen cells express LIGHT mRNA. Although we could detect LIGHT mRNA expression in activated spleen cells by RT-PCR (data not shown) we were not able to detect specific binding to stimulated spleen cells derived from wild-type mice cells with either of the two monoclonal antibodies 9D10 and 15B2 when compared with activated spleen cells derived from LIGHT-deficient mice (Fig. 3). Although the expression of membrane-bound LIGHT on these activated spleen cells can only be determined by RT-PCR, our results seem to indicate that both hybridomas (9D10 and 15B2) produce anti-mouse LIGHT mAbs that may not recognize the membrane form of mouse LIGHT expressed on the cell surface.
Figure 3.
Fluorescence-activated cell sorter (FACS) analysis of anti-mouse LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpesvirus entry mediator on T cells) monoclonal antibodies (mAbs) using spleen cells derived from C57BL/6 mice and LIGHT-deficient mice. A flow cytometry analysis was performed of mouse LIGHT expression on stimulated CD3+ spleen cells. Stimulated spleen cells [50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml ionomycin for 4 hr] were stained with an anti-CD3 antibody and the purified mAbs 9D10 and 15B2. A fluorescein isothiocyanate (FITC)-labelled anti-mouse immunoglobulin G (IgG) (Fab-specific) was used as a secondary antibody.
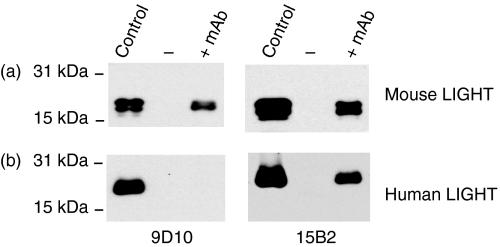
In order to test the specific binding of 9D10 and 15B2 to soluble mouse and human LIGHT, immunoprecipitation assays were performed. As shown in Fig. 4a, both monoclonal antibodies 9D10 and 15B2 are capable of specific binding to soluble mouse LIGHT. Interestingly, 15B2 also seems to bind to soluble human LIGHT, as revealed by immunoprecipitation, while the mAb 9D10 only recognizes soluble mouse LIGHT (Fig. 4b). Taken together, our data indicate that both mAbs recognize soluble mouse LIGHT as revealed by ELISA, dot blot and immunoprecipitation assays, while 15B2 additionally seems to cross-react with human LIGHT.
Figure 4.
Immunoprecipitation of mouse and human LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpesvirus entry mediator on T cells) by anti-mouse LIGHT monoclonal antibodies (mAbs). Mouse LIGHT (a) or human LIGHT (b), both expressed with a C-terminal myc-tag, was incubated with or without 5 μg of 9D10 and 15B2, respectively. Precipitated LIGHT protein was detected using an anti-myc antibody (9E10) followed by a peroxidase-conjugated anti-mouse immunoglobulin G (IgG) secondary antibody.
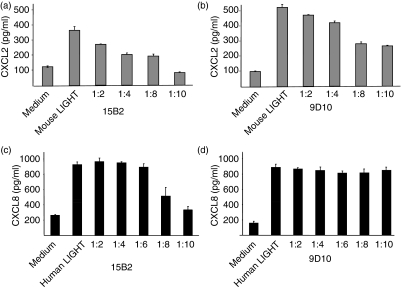
Neutralization of LIGHT inhibits the induction of CXCL8/CXCL2 expression by tumour cells
Earlier reports demonstrated that human LIGHT induced CXCL8 secretion in A375 melanoma cells and, furthermore, that mouse LIGHT induced CXCL2 expression in mouse fibrosarcoma cells (BFS-1) via activation of the lymphotoxin beta receptor.13,14 Accordingly, the neutralization capacity of 9D10 and 15B2 in vitro was functionally tested in cellular systems. BFS-1 cells were stimulated with mouse LIGHT with increasing concentrations of either 9D10 or 15B2. Both mAbs inhibited the release of CXCL2, indicating that both mAbs are capable of neutralizing mouse LIGHT in these in vitro assays (Fig. 5a,b). Stimulation of human melanoma cells (A375) with human LIGHT in the presence of increasing concentrations of these mAbs revealed that 9D10 does not neutralize human LIGHT, while in contrast 15B2 was also capable of neutralizing human LIGHT, as revealed by a decreased CXCL8 induction (Fig. 5c,d). These results support the observation of 15B2 cross-reacting with human LIGHT in the immunoprecipitation assays.
Figure 5.
Neutralization of mouse and human LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpesvirus entry mediator on T cells) by anti-mouse LIGHT monoclonal antibodies (mAbs) in vitro. Mouse fibrosarcoma cells (BFS-1) were incubated in quadruplicate for 4 hr with medium alone or in medium supplemented with mouse LIGHT (10 μg/ml) and incubated for 15 min with increasing concentrations of 15B2 (a) or 9D10 (b). CXCL chemokine (C-X-C motif) ligand 2 (CXCL2) expression in the supernatants was measured by enzyme-linked immunosorbent assay (ELISA). Human melanoma cells (A375) were incubated in quadruplicate for 4 hr with medium alone or in medium supplemented with human LIGHT (10 μg/ml) and incubated for 15 min with increasing concentrations of 15B2 (c) or 9D10 (d). CXCL8 expression in the supernatants was measured by ELISA. Mean values of quadruplicates ± standard deviation are given. Representative data from one out of three independent experiments are shown.
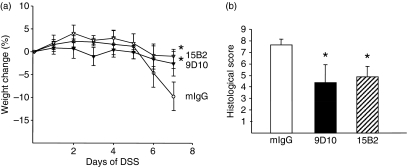
Neutralization of LIGHT reduces DSS-induced acute intestinal inflammation
In order to test the hypothesis that neutralization of LIGHT can result in down-regulation of the inflammatory response, we tested both neutralizing mAbs in our experimental in vivo model. Treatment of mice with either 9D10 or 15B2 during the course of DSS-induced acute colitis reduced the intestinal inflammation compared with the control group (Fig. 6). This was illustrated by a significantly reduced weight loss on day 7 during DSS treatment (Fig. 6a). Furthermore, anti-LIGHT-treated mice were characterized by a significantly lower histological score reflecting a reduced inflammatory infiltrate and reduced epithelial damage (Fig. 6b). Taken together, these results clearly demonstrate that both mAbs 9D10 and 15B2 can modulate the pathology associated with LIGHT in vivo.
Figure 6.
Neutralization of mouse LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpesvirus entry mediator on T cells) using specific monoclonal antibodies (mAbs) in dextran sodium sulphate (DSS)-induced acute colitis. Acute colitis was induced with 1·5% DSS in C57BL/6 mice. Mice (n = 5) received 100 μg of purified mAb 9D10 and 15B2 intraperitoneally (i.p.) for 6 days. The control group (n = 5) received 100 μg of mouse immunoglobulin G (IgG). Daily loss of body weight (a) and the histological score at day 7 (b) were determined. Statistical significance was determined using the Mann–Whitney rank sum test. Differences were considered significant at P < 0·05. Representative data from one out of three independent experiments are shown.
Discussion
The current hypothesis is that intestinal inflammation results from an inappropriate mucosal immune response. However, the exact molecular mechanisms and the cell subsets involved have not been fully identified. Our data clearly demonstrate that neutralizing LIGHT is capable of ameliorating the signs of DSS-induced intestinal inflammation.
It was previously established that LIGHT-mediated intestinal inflammation is dependent on both signalling receptors, LTβR as well as HVEM, as LIGHT-transgenic T cells do not induce intestinal inflammation when transferred into mice congenitally devoid of either LTβR or HVEM.7 Furthermore, it has been suggested that LIGHT can synergize with CD40 activation to stimulate the maturation of dendritic cells and thus bridge the innate and adaptive immune responses.19
LIGHT expression has also been reported to provide costimulatory signals resulting in proliferation and cytokine production and the induction of IFN-γ production in peripheral blood as well as lamina propria T cells.20 Several studies have suggested a role of monocytes and dendritic cells in the onset and severity of IBD. For example, the depletion of monocytes and dendritic cells in DSS-induced experimental colitis resulted in the amelioration of disease symptoms.21 However, whether induced expression of LIGHT in these cell populations is involved in the onset or severity of intestinal inflammation remains to be determined.
Interestingly, an alternatively spliced transcript of LIGHT which is devoid of the transmembrane region has also been detected in activated T cells, resulting in an intracellular form of LIGHT.22 This alternative intracellular form of LIGHT might represent a molecular mechanism for reducing the expression of membrane-bound LIGHT and consequently limiting LIGHT-mediated signalling cascades. Furthermore, these results suggest that soluble LIGHT rather than the membrane-bound form plays a critical role in inducing an acute inflammation in our experimental model.
Thus, in acute DSS-induced colitis, soluble LIGHT might contribute to increasing inflammation via activation of macrophages in the intestine in response to mucosal injury by DSS. Blocking LIGHT and its downstream signalling pathways may be an effective approach for the treatment of intestinal inflammation.
Acknowledgments
This work was supported by the DFG in part by He3116/4-1 and FOR 876 to TH.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Harrop JA, McDonnell PC, Brigham-Burke M, et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273:27548–56. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 2.Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 3.Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274:13733–6. doi: 10.1074/jbc.274.20.13733. [DOI] [PubMed] [Google Scholar]
- 4.Zhai Y, Guo R, Hsu TL, et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102:1142–51. doi: 10.1172/JCI3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamada K, Shimozaki K, Chapoval AI, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6:283–9. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, Cheroutre H, Ware CF. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167:6330–7. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Anders RA, Wang Y, Turner JR, Abraham C, Pfeffer K, Fu YX. The critical role of LIGHT in promoting intestinal inflammation and Crohn’s disease. J Immunol. 2005;174:8173–82. doi: 10.4049/jimmunol.174.12.8173. [DOI] [PubMed] [Google Scholar]
- 8.Sartor RB. Innate immunity in the pathogenesis therapy of IBD. J Gastroenterol. 2003;38(Suppl. 15):43–7. [PubMed] [Google Scholar]
- 9.Sartor RB. Clinical applications of advances in the genetics of IBD. Rev Gastroenterol Disord. 2003;3(Suppl. 1):S9–17. [PubMed] [Google Scholar]
- 10.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 11.Jungbeck M, Stopfer P, Bataille F, Nedospasov SA, Mannel DN, Hehlgans T. Blocking lymphotoxin beta receptor signalling exacerbates acute DSS-induced intestinal inflammation – opposite functions for surface lymphotoxin expressed by T and B lymphocytes. Mol Immunol. 2008;45:34–41. doi: 10.1016/j.molimm.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–24. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hehlgans T, Mannel DN. Recombinant, soluble LIGHT (HVEM ligand) induces increased IL-8 secretion and growth arrest in A375 melanoma cells. J Interferon Cytokine Res. 2001;21:333–8. doi: 10.1089/107999001300177529. [DOI] [PubMed] [Google Scholar]
- 14.Hehlgans T, Muller P, Stopfer P, Mannel DN. Activation of the lymphotoxin-beta receptor induces NFkappaB-dependent interleukin-6 and MIP-2 secretion in mouse fibrosarcoma cells. Eur Cytokine Netw. 2003;14:103–7. [PubMed] [Google Scholar]
- 15.Obermeier F, Dunger N, Deml L, Herfarth H, Scholmerich J, Falk W. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur J Immunol. 2002;32:2084–92. doi: 10.1002/1521-4141(200207)32:7<2084::AID-IMMU2084>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 17.Stopfer P, Obermeier F, Dunger N, et al. Blocking lymphotoxin-beta receptor activation diminishes inflammation via reduced mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression and leucocyte margination in chronic DSS-induced colitis. Clin Exp Immunol. 2004;136:21–9. doi: 10.1111/j.1365-2249.2004.02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulman M, Wilde CD, Kohler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978;276:269–70. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- 19.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479–86. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 20.Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR. LIGHT expression by mucosal T cells may regulate IFN-gamma expression in the intestine. J Immunol. 2004;173:251–8. doi: 10.4049/jimmunol.173.1.251. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe N, Ikuta K, Okazaki K, et al. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis Sci. 2003;48:408–14. doi: 10.1023/a:1021960401290. [DOI] [PubMed] [Google Scholar]
- 22.Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF. Genomic characterization of LIGHT reveals linkage to an immune response locus on chromosome 19p13.3 and distinct isoforms generated by alternate splicing or proteolysis. J Immunol. 2001;167:5122–8. doi: 10.4049/jimmunol.167.9.5122. [DOI] [PubMed] [Google Scholar]