Abstract
Surround inhibition, a neural mechanism relevant for skilled motor behavior, has been shown to be deficient in the affected primary motor cortex (M1) in patients with focal hand dystonia (FHD). Even in unilateral FHD, however, electrophysiological and neuro-imaging studies have provided evidence for bilateral M1 abnormalities. Clinically, the presence of mirror dystonia, dystonic posturing when the opposite hand is moved, also suggests abnormal interhemispheric interaction. To assess whether a loss of inter-hemispheric inhibition (IHI) may contribute to the reduced surround inhibition, IHI towards the affected or dominant M1 was examined in thirteen FHD patients (seven patients with and six patients without mirror dystonia, all affected on the right hand) and twelve right-handed, age-matched healthy controls. IHI was tested at rest and during three different phases of a right index finger movement in a synergistic, as well as in a neighbouring, relaxed muscle. There was a trend for a selective loss of IHI between the homologous surrounding muscles in the phase 50ms before EMG onset in FHD patients. Post hoc analysis revealed that this effect was due to a loss of IHI in the FHD patients with mirror dystonia, while patients without mirror dystonia did not show any difference in IHI modulation compared to healthy controls. We conclude that mirror dystonia may be due to impaired IHI towards neighbouring muscles before movement onset. However, IHI does not seem to play a major role in the general pathophysiology of FHD.
Keywords: focal hand dystonia, TMS, human, motor cortex, inhibition
Introduction
Coordination of finger movement plays an important role in many daily tasks involving fine motor skills. A basic issue in the neurophysiology of motor control is how the brain generates the complex spatio-temporal commands needed to vary speed, amplitude, and direction of finger movements. This process is severely impaired in patients with focal hand dystonia (FHD), such as writer’s or musician’s cramp. Dystonia is generally regarded as a motor execution abnormality caused by a dysfunction in the cortico-striato-thalamo-cortical motor loop (Berardelli et al. 1998). Typical clinical features of FHD are task- and context-specific abnormal posturing due to sustained muscle contractions interfering with the performance of motor tasks (Chen and Hallett 1998). Neurophysiological findings in patients with FHD are characterized by loss of inhibition on multiple levels of the central nervous system (Berardelli et al. 1985; Cohen and Hallett 1988; Chen and Hallett1998). On the motor cortical level, increased cortical excitability and deficiencies in intra-cortical inhibition at rest are present in FHD patients in the dystonic hemisphere (Ridding et al. 1995; Ikoma et al. 1996;Chen et al. 1997).
In patients with FHD, there is evidence for impaired surround inhibition in M1 of the dystonic hemisphere (Hallett 2004;Sohn and Hallett 2004b;Beck et al. 2008), likely due in part to deficient inhibition from local, GABAA-mediated, inhibitory interneurons in M1 as assessed by short intracortical inhibition (SICI) (Stinear et al. 2004; Beck et al. 2008). Surround inhibition is a neural mechanism described in the retina (Angelucci et al. 2002), as well as in other sensory areas (Blakemore et al. 1970) and in models for focal epilepsy (Collins 1978). It is thought that surround inhibition enhances contrast between neural signals by facilitating the central signal and actively inhibiting the surrounding signals and has been shown to be present in M1 (Hallett 2004; Sohn et al. 2004).
Recent experiments indicate that in patients with focal, unilateral dystonia, abnormalities are still observed in both hemispheres. This suggests that the contralateral, clinically unaffected hemisphere is also involved in the pathophysiology (Meunier et al. 2001;Merello et al. 2006;Tamura et al. 2008). Using transcranial magnetic stimulation (TMS) in healthy volunteers, facilitatory and inhibitory effects in one primary motor cortex (M1) can be evoked by stimulating the other M1 (Ferbert et al. 1992;Ugawa et al. 1993). Inter-hemispheric inhibition (IHI) is regarded as a cortico-cortical phenomenon, since there is no inhibition of motor output evoked by transcranial electrical stimulation (TES) and H-reflexes are not affected by the stimulation (Ferbert et al. 1992), although there may be a subcortical contribution (Gerloff et al. 1998). The excitatory transcallosal fibers project onto a subset of the local, GABAA-mediated, inhibitory interneurons in M1 (Ferbert et al. 1992;Meyer et al. 1995;Chen 2004), which most likely differs from what is reflected by SICI, but the two mechanisms interact (Daskalakis et al. 2002).
A unique clinical phenomenon in patients with FHD is mirror dystonia, which is defined as dystonic movement or posture in the affected homologous muscle induced by a specific task (e.g., writing) performed by the unaffected hand (Sitburana and Jankovic 2008). Mirror dystonia is therefore different from mirror movements, which are contralateral involuntary homologous movements. Mirror dystonia is seen in about 50% of FHD patients (Jedynak et al. 2001) and can be very useful clinically as guidance for injection of botulinum toxin to distinguish the dystonia from compensatory movements (Singer et al. 2005).
It has been proposed that IHI and facilitation of the contralateral motor cortex via the corpus callosum may assist synchronous bilateral movements as well as suppress unwanted movements during unimanual motor performance (Schnitzler et al. 1996). However, while IHI is known to be reduced in healthy professional musicians (Ridding et al. 2000), there are no data about the role of IHI during movement generation in FHD and in mirror dystonia.
The purpose of the current study was to explore the role of the contralateral M1 in FHD. The initial hypothesis was that increased excitability and the loss of surround inhibition in the affected M1 in FHD may be due to a lack of inhibition from the contralateral M1. Therefore, IHI toward the affected M1 was assessed in an active muscle (first dorsal interosseous muscle, FDI), as well as in a surrounding muscle (abductor pollicis brevis muscle, APB) in the resting state and during different phases of a skilled movement. As mirror dystonia may be associated with impaired IHI, a subgroup analysis of the FHD patients was done comparing those with mirror dystonia (FHD-D) and those without mirror dystonia (FHD-ND). The FHD groups were compared with an age-matched control group. First, we hypothesized that IHI onto the M1 representation of the surrounding muscles would be diminished in all FHD patients due to a deficient local, inhibitory network of interneurons in FHD. Second, we expected reduced IHI onto the active muscle would be deficient in FHD patients with mirror dystonia compared to those patients without mirror dystonia.
Methods
Subjects
Thirteen FHD patients (age 44–73 years, mean 56.3 ± 2.2 years; 11 males) and twelve age-matched healthy subjects (age 39–69 years, mean 56.4 ± 2.1 years; 10 males) participated in the study. In the FHD group, seven patients showed mirror dystonia (FHD-D, age 44–73 years, mean 57.6 ± 3.4 years; all males), whereas six patients did not (FHD-ND, age 46–61 years, mean 55 ± 2.2 years; four males). The movement part of the Fahn Scale (see supplements) was assessed for the affected movement (writing in nine patients and the affected finger movement in the four musicians: three pianists and one guitarist). There was no significant difference between groups with respect to the severity on the Fahn Scale (range 0–28; 6.7 ± 3 in the FHD-D group and 8.5 ± 2 in the FHD-ND group). Healthy volunteers did not have mirror movements. All subjects were right-handed according to the Edinburgh handedness inventory (Oldfield 1971). FHD patients had only unilateral symptoms in their right, dominant hand. Participants had never been exposed to neuroleptic drugs and had no history of neuropsychiatric disorders, neurosurgery, or metal or electronic implants. Most of the patients had been treated with local injections of botulinum toxin type A in the affected muscles. The last injection had been given at least three months before the experiments (see Table 1). All participants gave their informed consent prior to the experiments, which were approved by the Institutional Review Board (IRB) of the National Institute of Neurological Disorders and Stroke (NINDS).
Table 1.
Patient demographics
| Sex | Age [years] |
MD | Type | Duration [years] |
Botulinum toxin / last injection |
|---|---|---|---|---|---|
| M | 58 | + | MC | 17 | yes / 3 months |
| M | 73 | + | WC | 10 | yes / 3 months |
| M | 51 | + | WC | 9 | yes / 3 months |
| M | 44 | + | MC | 3 | no |
| M | 56 | + | WC/MC | 5 | yes / 4 months |
| M | 63 | + | WC | 39 | no |
| M | 58 | + | MC | 5 | yes / 6 months |
| F | 52 | WC | 10 | yes / 3 months | |
| F | 45 | WC | 23 | yes / 3.5 years | |
| M | 57 | WC/MC | 21 | no | |
| M | 56 | MC | 3 | yes / 4 months | |
| M | 61 | WC | 10 | yes / 2 years | |
| M | 59 | WC | 17 | yes / 3 months |
MD = Mirror Dystonia; WC = writer’s cramp; MC = musician’s cramp
Recording
Subjects were seated in a comfortable chair with their right arm resting on a side table, which was individually adjusted. In some subjects, the wrist was supported by a towel to help the subject keep the hand muscles as relaxed as possible. Disposable surface silver-silver chloride EMG electrodes were placed on the abductor pollicis brevis muscle (APB) and the first dorsal interosseus muscle (FDI) bilaterally in a bipolar montage. Impedance was reduced below 5 kΩ. The EMG signal was amplified using a conventional EMG machine (Viking IV, Nicolet Biomedical, Madison, WI) and bandpass filtered (20–2000 Hz). The signal was digitized at a frequency of 4 kHz and fed into a computer for off-line analysis. Individual MEP amplitudes were measured in four phases (rest, premotor, phasic and tonic; see motor task). Background EMG was calculated by assessing the root mean square over 50 ms prior to MEP onset in the same four phases.
Motor task
With their right hand lying flat on a table beside them, subjects pushed down on a small force transducer (Strain Measurement Devices, Inc, Meriden, CT, model S215 load cell) with the tip of their index finger in response to an acoustic signal. This led to flexion in the meta-carpo-phalangeal joint (MCP joint) of the index finger. In preliminary tests, even healthy volunteers were unable to completely suppress EMG activity in thumb muscles when the FDI was abducted (primary movement), since the thumb is then the only finger to oppose the movement under this condition to stabilize the hand. To minimize concomitant EMG activity in APB, index finger flexion was used. FDI participates as a synergist rather than as a prime mover in this motion, but it has been shown that modulation of cortical excitability is similar in synergists and agonists (Sohn and Hallett 2004a).
In the task, subjects generated 10% of their maximum force (Fmax) during isolated flexion of the index finger as a reaction time task after the onset of an acoustic signal. The force level was individually adjusted and displayed as a line on an oscilloscope in front of them. The output of the force transducer was also displayed on the oscilloscope as feedback. The acoustic signal was present for 2 seconds and subjects maintained contraction for the duration of the tone. Subjects practiced the task at the beginning of the experiment to attain a consistent motor performance. The task used has previously been described in detail (Beck et al. 2008).
In four different phases of the movement, IHI was assessed in four different tests for each muscle (FDI and APB): rest (100ms before the onset of the tone); premotor (50ms before the onset of the EMG in FDI, movement initiation); phasic (the first peak of EMG in FDI, movement initiation); and tonic (1600 ms after onset of the acoustic signal, maintenance phase).
TMS
For TMS, two high-power Magstim 200 machines (Magstim Co., Whitland, Dyfed, UK) were connected to two custom-made figure-of-8 coils with an outer diameter of 8 cm and an inner loop diameter of 3.5 cm. The experiment consisted of two parts. In each part, one of the two target muscles, FDI or APB, was tested. The order of the two parts was randomized. At the beginning of each part (for FDI and APB separately), the “motor hot spot” for eliciting MEPs in FDI or APB, respectively, was determined over the right and left M1 by moving the coil over the M1 area using a slightly supra-threshold stimulation intensity. Coil orientation was tangential to the scalp with the handle pointing backwards and laterally at a 45-degree angle away from the midline. The motor hot spot was on average 3 cm lateral and 1cm posterior to Cz over the hand knob of M1. This position was marked on a tight fitting cap to ensure proper coil placement throughout the four experiments (IHI at rest, during premotor, phasic and tonic phase) performed for each target muscle. In each part, the resting motor threshold (MT) was determined (for FDI or APB), to the nearest 1% of maximal stimulator output. MT was defined as the minimal stimulus intensity required to evoke MEPs of at least 50µV in 5 out of 10 consecutive trials. MEP size was determined by averaging peak-to-peak amplitudes. Trials with a background EMG of more than 0.02 mV in APB (assessed as root mean square) over 50 ms before MEP onset were rejected.
For IHI, a 10-ms interstimulus interval was used, which was previously shown to be most effective (Ferbert et al. 1992). The four different phases (rest, premotor, phasic and tonic) were tested in four separate experiments for each muscle (FDI or APB). The test pulse was applied to the motor hotspot of FDI or APB, respectively, over the dystonic or dominant hemisphere. Both muscles were chosen as a target muscle in separate sessions and assessed in all four phases of the movement. In the beginning of each experiment, test pulse intensity was adjusted to evoke an MEP of 1.5 mV. IHI was first performed at rest. The intensity of the conditioning stimulus was adjusted to reduce the size of the test pulse to approximately 60% of the MEP induced by the test pulse (= 900µV), resulting in an IHI of 40%. This adjustment was performed to avoid a floor or ceiling effect of IHI regulation. The same intensity of conditioning pulse was used for all phases.
Statistics
Outcome measures for EMG (root mean square), MT (stimulator output), and MEP (peak-to-peak amplitudes) were analysed using a two-way repeated-measures analysis of variance (ANOVA) to compare the effect of “phase” as a within-subject factor (four levels: rest, premotor, phasic, and tonic) and “group” as a between-subject variable (two levels: FHD and controls). MEP data were not always distributed normally. Therefore, Conover’s free distribution method, a non-parametric analysis of variance (ANOVA) based on ranks {Conover et al. 1983} was used to calculate a two-way non-parametric analysis of variance (ANOVA) in order to compare the four levels of the within-subject factor PHASE (rest; premotor, phasic and tonic) and two levels of the between-subject factor GROUP (FHD and CON). If significant in the two-way analysis, mean differences were calculated between the levels using a one-way design and confidence intervals were given after Bonferroni correction for repeated comparisons.
As secondary analysis, the FHD patients were split into two groups (with and without mirror dystonia) and the similar analysis as described above was performed for MEP (peak-to-peak amplitudes) using a two-way repeated-measures analysis of variance (ANOVA). The effect of “phase” as a within-subject factor (four levels: rest, premotor, phasic, and tonic) and “group” as a between-subject variable (three levels: FHD-D, FHD-ND, and controls) was compared. Again, MEP data were not always distributed normally. Therefore, Conover’s free distribution method, a non-parametric analysis of variance (ANOVA) based on ranks {Conover et al. 1983} was used to calculate a two-way non-parametric analysis of variance (ANOVA) in order to compare the four levels of the within-subject factor PHASE (rest; premotor, phasic and tonic) and three levels of the between-subject factor GROUP (FHD-D, FHD-ND and CON). If significant in the two-way analysis, mean differences were calculated between the levels using a one-way design and confidence intervals were given after Bonferroni correction for repeated comparisons.
Data are presented as means and standard error of means. P values less than 0.05 are considered significant. For analysis, SPSS 16.0.1 was used.
Results
IHI in the agonist muscle (FDI)
IHI was calculated, and is shown as the percentage of the conditioned MEP (MEPcond) with reference to the test MEP (MEPtest) (IHI = MEPcond / MEPtest *100 [%]). The target size of MEP size was 60% for the rest condition. IHI at rest was 73 ± 7% in the control group, 63 ± 7% in FHD-D, and 62 ± 10% in FHD-ND.
Comparing FDI IHI in the three active levels to rest, there was a trend for a significant main effect for PHASE (F3,69 = 2.3, p = 0.08), without any subsequent significant contrasts (all p > 0.1), and no effect for GROUP (F1,23 = 0.06, p = 0.8) or for the GROUP-by-PHASE interaction (F3,69 = 1.6, p = 0.2).When FDI IHI was compared among all three groups (controls, FHD-D and FHD-ND), the analysis showed again a trend for a main effect of PHASE (F3,66 = 2.2, p = 0.09) indicating that IHI in the premotor phase was reduced compared to rest (contrast between rest and premotor F1,22 = 4.1, p = 0.05), while there were no other significant main effects, interactions or contrasts (all p > 0.1; see Fig. 1). Thus, the comparison of IHI in FDI showed no main effect for GROUP (F2,22 = 0.1, p = 0.9), nor GROUP-by-PHASE interaction (F6,66 = 0.9, p = 0.5; see Fig. 1).
Figure 1. Paired Pulse TMS APB.
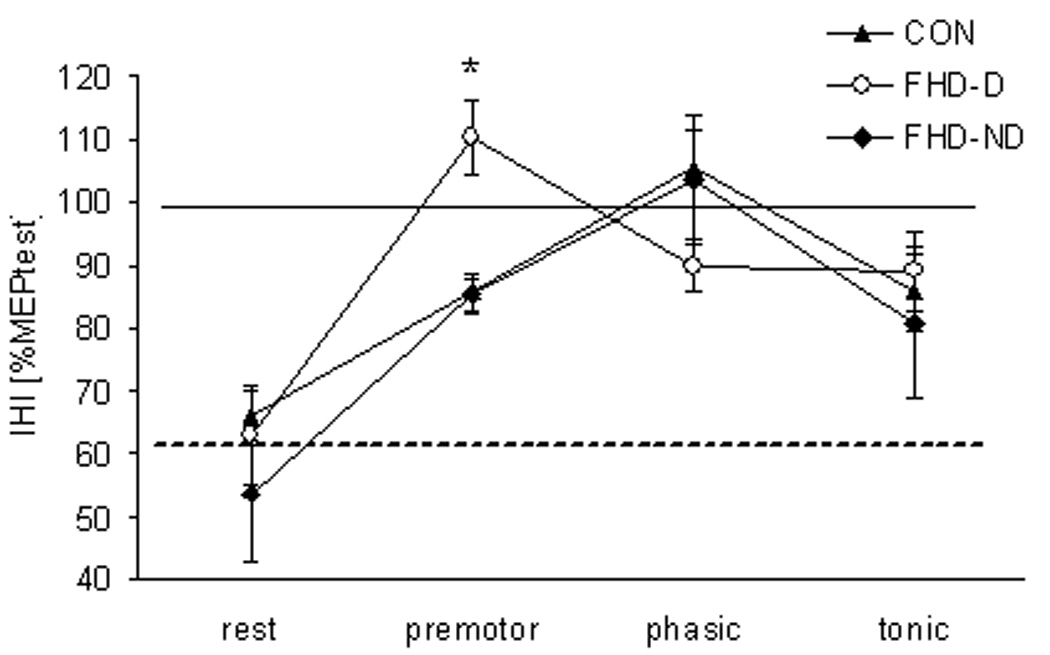
Mean and standard errors for the conditioned MEP in APB for all three groups (CON, FHD-D, and FHD-ND). While there was no difference among groups at rest, the FHD-D group shows loss of inhibition in the premotor phase, while the other two groups maintained some inhibition in this phase.
The analysis of the FDI IHI test MEP size did not reveal any significant main effects or interactions (all p > 0.1; rest: CON 1.8 ± 0.2mV, FHD-D 1.7 ± 0.1mV, FHD-ND 1.5 ± 0.2mV; premotor: CON 1.8 ± 0.2mV, FHD-D 1.6 ± 0.3mV, FHD-ND 1.5 ± 0.2mV; phasic: CON 1.7 ± 0.1mV, FHD-D 1.5 ± 0.4mV, FHD-ND 1.4 ± 0.1mV; tonic: CON 1.5 ± 0.1mV, FHD-D 1.2 ± 0.1mV, FHD-ND 1.4 ± 0.2mV).
IHI in the surrounding muscle (APB)
The target size of MEP size was 60% for the rest condition. In fact, the mean of the induced IHI in APB was 65 ± 4% for CON, 63 ± 8% for FHD-D and 54± 11% for FHD-ND. Comparing APB IHI in all FHD patients to controls, there was a significant main effect for PHASE (F3,69 = 24.6, p < 0.001), but not for GROUP (F1,23 = 0.025, p = 0.9). The GROUP-by-PHASE interaction showed a trend for significance indicating that IHI may be reduced in the premotor phase in the FHD patients (F3,69 = 2, p = 0.09).
When APB IHI was then compared among controls, FHD-ND and FHD-D during the four different phases, there was no significant main effect for GROUP (F1,22 = 1.4, p = 0.27). However, there was a highly significant main effect for PHASE (F3,66 = 23.8, p < 0.001), and for the GROUP-by-PHASE interaction (F6,66 = 2.5, p = 0.03; see Fig. 2). Contrasts between phases for the GROUP-by-PHASE interaction showed that IHI was modulated differently in the three groups during the premotor phase (F1,22 = 4.2, p = 0.03, see Fig. 3), in that APB IHI was abolished in the FHD-D group, as revealed by pairwise comparisons of means from each phase (rest vs. premotor: −62%; 95% CI ± −91%, −34%; p = 0.001; phasic vs. premotor: −27%; 95% CI ± −55%, 1%; p = 0.06; all other comparisons not significant; see Fig. 1, Fig. 2). During the phasic phase, IHI was abolished without a difference between groups (contrast between rest and phasic F1,22 = 72.7, p < 0.001; means for CON 105 ± 6%; FHD-D 90 ± 8%; FHD-ND 103 ± 8%, see Fig. 1). During the tonic phase, IHI was reduced as compared to rest without a difference between groups (contrast between rest and tonic F1,22 = 40, p < 0.001; means for CON 86 ± 6%; FHD-D 89 ± 8%; FHD-ND 81 ± 9%, see Fig. 1).
Figure 2. Paired Pulse TMS FDI.
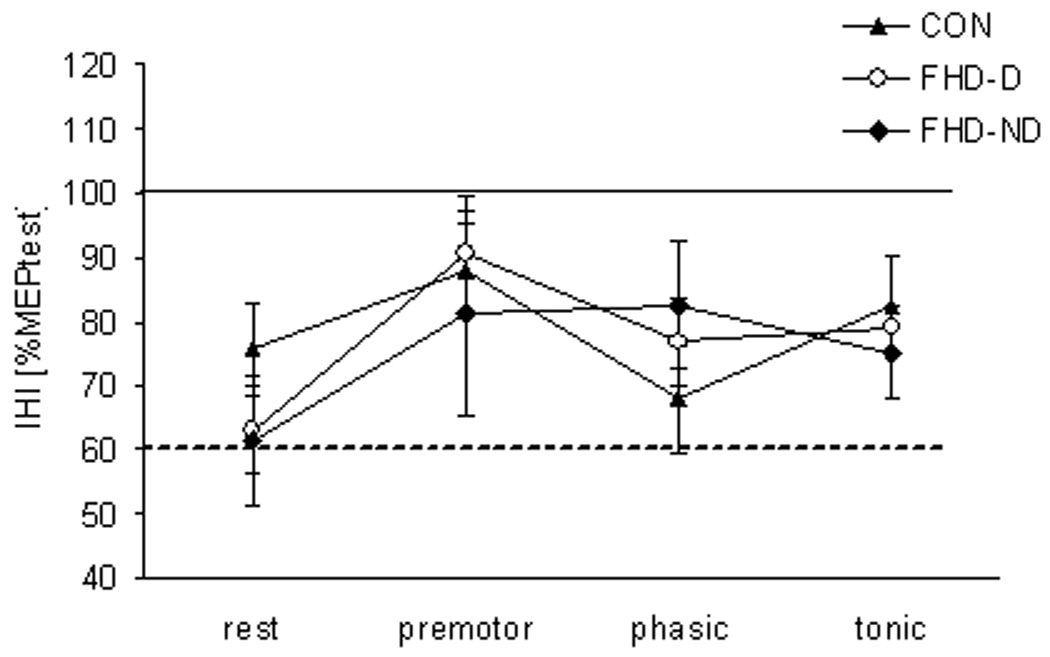
Mean and standard errors for the conditioned MEP in FDI for all three groups (CON, FHD-D, and FHD-ND). There was no difference in IHI in FDI among groups or phases.
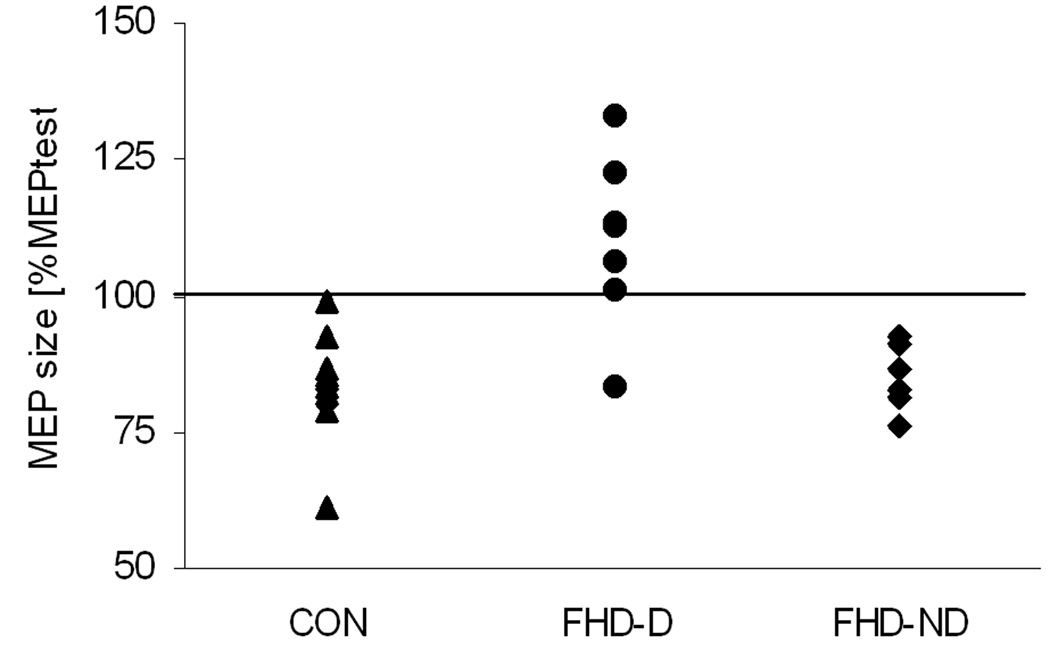
Figure 3. IHI in APB during the premotor phase.
The individual values for all participating subjects divided into the three groups (▲CON, ● FHD-D and ♦ FHD-ND) show that six of seven FHD-MM patients had an inter-hemispheric facilitation instead of an inhibition in the premotor phase. None of the subjects in the control group or the FHD-NM patients showed facilitation.
The analysis of the APB IHI test MEP size did not reveal any significant main effects or interactions (all p > 0.1; rest CON 1.5 ± 0.1mV, FHD-D 1.7 ± 0.2mV, FHD-ND 1.6 ± 0.1mV; premotor CON 1.3 ± 0.1mV, FHD-D 1.2 ± 0.2mV, FHD-ND 1.6 ± 0.1mV; phasic CON 1.4 ± 0.1mV, FHD-D 1.5 ± 0.1mV, FHD-ND 1.6 ± 0.2mV; tonic CON 1.2 ± 0.1mV, FHD-D 1.4 ± 0.1mV, FHD-ND 1.5 ± 0.2mV).
Background EMG
Background EMG was not different between groups as indicated by a non-significant main effect for GROUP, as well as the GROUP-by-PHASE interaction and the GROUP-by-PHASE-by-MUSCLE interaction (all p > 0.1, see Table 2). The main effect for PHASE was not significant. A significant main effect for MUSCLE (F = 7.36, p = 0.013) and the PHASE-by-MUSCLE interaction (F = 5.4, p = 0.012) reflected differential modulation between FDI and APB. While simple contrasts did not reveal differences between the three different phases and rest in APB, background EMG increased in FDI for the phasic phase (F = 7.1, p = 0.01) and showed a trend to be enhanced during the tonic phase (F = 3.4, p = 0.08).
Table 2.
Background EMG
| rest | premotor | phasic | tonic | ||
|---|---|---|---|---|---|
| FDI | CON | 11 ± 1 | 12 ± 1 | 14 ± 4* | 12 ± 2 |
| FHD-D | 13 ± 2 | 13 ± 2 | 17 ± 2* | 14 ± 2 | |
| FHD-ND | 12 ± 1 | 12 ± 1 | 14 ± 1* | 12 ± 1 | |
| APB | CON | 12 ± 1 | 12 ± 1 | 12 ± 1 | 12 ± 1 |
| FHD-D | 11 ± 1 | 11 ± 1 | 13 ± 1 | 11 ± 1 | |
| FHD-ND | 11 ± 1 | 11 ± 1 | 12 ± 1 | 11 ± 1 | |
Shown are mean values and standard errors for the background EMG in FDI and APB; the four phases are shownin µV. Background EMG was not different between phases in APB, while a significant increase during the phasic phase in FDI indicates this muscle’s role as synergist in the task. There were no differences between the three groups.
indicates a significant difference for the factor phase.
Discussion
The results of the current study showed a selective, time-dependent reduction of IHI between homologous surrounding muscles in patients with mirror dystonia. IHI was exclusively reduced in FHD patients with mirror dystonia in the phase before EMG onset. During all other phases and between the synergistic muscles, IHI was not different between groups. If FHD patients were compared to controls as one group, there was only a trend for a loss of IHI in APB in the premotor phase, but the secondary analysis revealed that the reduction of IHI was due to a complete loss of IHI in six of the seven FHD-D patients.
As second part of our hypothesis, mirror dystonia was assumed to be due to additional loss of IHI between synergistic homologous muscles. This was hypothesized with the knowledge that IHI is mediated via the local inhibitory interneurons in M1 (Ferbert et al. 1992), which have recently been shown to be deficient in FHD (Beck et al. 2008). The current results show no loss of IHI in FDI, even when the groups are split into FHD-D and FHD-ND.
Despite evidence for abnormalities in the contralateral, non-dystonic hemisphere in focal, unilateral types of dystonia (Meunier et al. 2001; Merello et al. 2006; Tamura et al. 2008), considering the current results it seems unlikely that IHI plays a key role in the pathophysiology of FHD. Instead, IHI appeared to be deficient depending on the subset of local inhibitory interneurons in the dystonic M1 that are affected by the disorder. The main finding of this study is that the clinical phenomenon of mirror dystonia seems to be associated with deficient IHI onto the surrounding muscle of the affected side before EMG onset.
Timing of inter-hemispheric interactions
For single pulse TMS over the contralateral M1, inhibition of MEP can be observed starting from 80 –100 ms before movement in the homologous muscle, which is more pronounced when directed toward the dominant M1 (Leocani et al. 2000;Liepert et al. 2001). This time interval before EMG onset seems to be relevant for the interaction between the two primary motor cortices (Duque et al. 2005). In that study, MEP size in the contralateral, relaxed homologous muscle was inhibited by mirroring its primary movement and was facilitated when the index finger was moved in the opposite direction.
In the current study, two different target muscles were assessed: a synergist muscle (FDI) and a surrounding muscle (APB). IHI was slightly reduced when testing FDI in the premotor phase compared to rest in all groups. Concerning the time interval, this finding is consistent with the previous reports in healthy volunteers (Duque et al. 2005).
For APB, IHI decreased from rest to the premotor phase and then completely disappeared during the phasic phase in the control group and patients without mirror dystonia. This finding for the timing is again consistent with previous reports in healthy volunteers using single pulse TMS to assess the interaction between the two M1 areas (Duque, et al. 2005). However, in patients with mirror dystonia, IHI changed from inhibition to facilitation in the premotor phase instead. During the tonic phase, IHI was restored without difference between groups.
These results reflect a difference in the modulation of the premotor phase between two types of muscles (synergist versus surrounding muscle) as well as between the two patient groups. In the current results, lack of IHI in patients with mirror dystonia occurred in the same (premotor) phase before EMG onset in which interactions between the two M1 areas have been described previously (Duque, et al. 2005) suggesting that similar pathways were involved. While mirror dystonia is clinically observable throughout all active phases, it has already been shown that surround inhibition on the M1 level is only present during movement initiation and not during the maintenance of a contraction (Beck et al. 2008). Taken together with the reported interhemispheric interaction starting from 80 – 100 ms before EMG onset (Leocani et al. 2000; Liepert et al. 2001;Duque et al. 2005), the current results further support the notion that the crucial, deficient phase on the motor cortical level is movement selection during movement initiation. Dystonic overflow occurring in this phase may then secondarily activate subcortical circuits projecting to non-synergistic muscles.
Cortical (inter)neurons involved, inhibitory pathways
IHI is thought to be mediated via excitatory transcallosal projections, which then activate the local inhibitory interneurons in M1 (Ferbert et al. 1992). Therefore, three structures, the corpus callosum as well as the dystonic and the contralateral M1 are possible candidates to cause deficient IHI. Since lack of IHI was limited to one specific phase during the movement, it seems unlikely that the corpus callosum, as the connecting fibre tract, is deficient. Previous studies have already demonstrated a functional impairment of the intra-cortical inhibitory networks in the affected M1 in FHD (Molloy et al. 2002; Stinear and Byblow 2004;Beck et al. 2008). Other studies indicated bilateral abnormalities (Meunier et al. 2001;Merello et al. 2006;Tamura et al. 2008). The inhibitory interneurons in M1, which are activated by TMS, play an important role in shaping the output from M1. In healthy people, SICI is reduced in active muscles and enhanced in surrounding muscles (Sohn and Hallett 2004a), may be increased volitionally (Liepert et al. 2001), and contributes to surround inhibition before and during movement onset (Stinear and Byblow 2004; Beck et al. 2008). Thus, SICI as well as IHI are context-dependent and modulated in the phase preceding the movement.
While it is unclear if there is a difference in SICI between patients with and without mirror dystonia, the current study showed a time-specific lack of IHI in the FHD-D group only. This may indicate that the interaction between dystonic and contralateral M1 may not be generally abnormal in FHD patients. Since there was no significant difference in symptom severity, the results suggest incomplete malfunction of local inhibitory interneurons in the dystonic M1 on which the transcallosal fibers project. Depending on the subtypes of affected interneurons, SICI or IHI would be impaired. In view of the previous studies, it is more likely that the pathology lies in the dystonic M1, although pathology in the contralateral M1 can not be completely excluded. However, IHI was not up-regulated in the surrounding muscle, as one would expect, if it contributed to the generation of surround inhibition, putting into question its contribution to surround inhibition and thus also the possibility that IHI plays a causal role in the pathophysiology of FHD.
Cortical excitability in mirror dystonia
Lateralization of voluntary movements requires a mature motor system (Cincotta and Ziemann 2008). In unilateral FHD, there is only limited electrophysiological data about mirror dystonia, while mirror movements in PD are well-described. Mirror movements in PD are more common on the less affected side (Vidal et al. 2003; Espay et al. 2005) and are thought to be due to impaired basal ganglia output to energize the cortical mechanisms that are relevant in movement preparation and execution (Berardelli et al. 2001;Cincotta and Ziemann 2008). In contrast, mirror dystonia specifically describes the induction of a dystonic movement or posture by the task-specific activation of the homologous contralateral (non-affected) muscle and mostly occurs on the pathologic side thereby revealing the affected limb (Sitburana and Jankovic2008). Similar to the pathophysiology in PD, this phenomenon may correspond with increased cortical excitability or deficient cortical inhibition in the dystonic M1 that is well-known in FHD (Ikoma et al. 1996; Stinear and Byblow 2004;Hallett 2004; Sohn and Hallett 2004b; Butefisch et al. 2005). As shown in our study, IHI toward the dystonic M1 was specifically diminished in surrounding muscles in the patients with mirror dystonia, whereas it was preserved in FHD patients without mirror dystonia and in the control group.
In conclusion, our findings indicate that mirror dystonia in FHD may emerge from a deficient restriction of excitatory input from the contralateral hemisphere. This impairment is most likely due to deficient intramotor-cortical inhibition in the dystonic hemisphere, but further studies are needed to better characterize which specific interneurons are involved. Another investigation that should be done is to evaluate IHI in the dystonic hand in the time period prior to movement of the non-dystonic hand. Since FHD patients without mirror dystonia did not show the same time-specific loss of IHI, this pathway does not seem to play a major role in the general pathophysiology of FHD.
Table 3.
MT for FDI and APB
| MTa | FDI | APB | ||
|---|---|---|---|---|
| right | left | right | left | |
| CON | 46 ± 3 | 47 ± 2 | 47 ± 4 | 47 ± 2 |
| FHD-D | 48 ± 4 | 44 ± 2 | 49 ± 5 | 48 ± 2 |
| FHD-ND | 39 ± 5 | 43 ± 2 | 42 ± 1 | 44 ± 2 |
Means and standard errors for MT, given in percent of maximum stimulator output, were not different between FDI and APB nor between sides. “FDI right”refers to the motor hot spot for FDI over the right hemisphere projecting to FDI of the left hand.
Acknowledgments
We thank D. Schoenberg for skilful editing. This work was supported by the Deutsche Forschungsgemeinschaft (DFG; BE-3792/1) and by the Intramural Research Program of the NINDS, NIH.
References
- Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Prog.Brain Res. 2002;136:373–388. doi: 10.1016/s0079-6123(02)36031-x. [DOI] [PubMed] [Google Scholar]
- Beck S, Pirio Richardson S, Shamim E, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J. Neurosci. 2008;28:10363–10369. doi: 10.1523/JNEUROSCI.3564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Day BL, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985;108(Pt 3):593–608. doi: 10.1093/brain/108.3.593. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7):1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Carpenter RH, Georgeson MA. Lateral inhibition between orientation detectors in the human visual system. Nature. 1970;228:37–39. doi: 10.1038/228037a0. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Boroojerdi B, Chen R, Battaglia F, Hallett M. Task-dependent intracortical inhibition is impaired in focal hand dystonia. Mov Disord. 2005;20:545–551. doi: 10.1002/mds.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp.Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M. Focal dystonia and repetitive motion disorders. Clin.Orthop.Relat Res. 1998 Jun;351:102–106. [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Canos M, Hallett M. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin.Neurophysiol. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Hallett M. Hand cramps: clinical features and electromyographic patterns in a focal dystonia. Neurology. 1988;38:1005–1012. doi: 10.1212/wnl.38.7.1005. [DOI] [PubMed] [Google Scholar]
- Collins RC. Use of cortical circuits during focal penicillin seizures: an autoradiographic study with [14C]deoxyglucose. Brain Res. 1978;150:487–501. doi: 10.1016/0006-8993(78)90815-6. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformation as a bridge between parametric and non-parametric statistics (1982) J Am Stat Assoc. 1982;35:124–129. [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J.Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb.Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Li JY, Johnston L, Chen R, Lang AE. Mirror movements in parkinsonism: evaluation of a new clinical sign. J.Neurol.Neurosurg.Psychiatry. 2005;76:1355–1358. doi: 10.1136/jnnp.2005.062950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J.Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CM. Alien hand phenomena: a review with the addition of six personal cases. Can.J.Neurol.Sci. 2000;27:192–203. doi: 10.1017/s0317167100000834. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J.Physiol. 1998;510(Pt 1):249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Dystonia: abnormal movements result from loss of inhibition. Adv.Neurol. 2004;94:1–9. [PubMed] [Google Scholar]
- Ikoma K, Samii A, Mercuri B, Wassermann EM, Hallett M. Abnormal cortical motor excitability in dystonia. Neurology. 1996;46:1371–1376. doi: 10.1212/wnl.46.5.1371. [DOI] [PubMed] [Google Scholar]
- Jedynak PC, Tranchant C, de Beyl DZ. Prospective clinical study of writer's cramp. Mov Disord. 2001;16:494–499. doi: 10.1002/mds.1094. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin.Neurophysiol. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Merello M, Carpintiero S, Cammarota A, Meli F, Leiguarda R. Bilateral mirror writing movements (mirror dystonia) in a patient with writer's cramp: functional correlates. Mov Disord. 2006;21:683–689. doi: 10.1002/mds.20736. [DOI] [PubMed] [Google Scholar]
- Meunier S, Garnero L, Ducorps A, Mazieres L, Lehericy S, du Montcel ST, Renault B, Vidailhet M. Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann.Neurol. 2001;50:521–527. doi: 10.1002/ana.1234. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Grafin v E, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118(Pt 2):429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Molloy FM, Sohn YH, Hallett M. Surround inhibition is impaired in patients with focal hand dystonia during movement preparation. Mov Disord. 2002;17 Suppl 5:304–305. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Nordstrom MA. Reduced interhemispheric inhibition in musicians. Exp.Brain Res. 2000;133:249–253. doi: 10.1007/s002210000428. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J.Neurol.Neurosurg.Psychiatry. 1995;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Kessler KR, Benecke R. Transcallosally mediated inhibition of interneurons within human primary motor cortex. Exp.Brain Res. 1996;112:381–391. doi: 10.1007/BF00227944. [DOI] [PubMed] [Google Scholar]
- Singer C, Papapetropoulos S, Vela L. Use of mirror dystonia as guidance for injection of botulinum toxin in writing dysfunction. J.Neurol.Neurosurg.Psychiatry. 2005;76:1608–1609. doi: 10.1136/jnnp.2004.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitburana O, Jankovic J. Focal hand dystonia, mirror dystonia and motor overflow. J.Neurol.Sci. 2008;266:31–33. doi: 10.1016/j.jns.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann.Neurol. 2004a;56:595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp.Brain Res. 2004b;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired modulation of intracortical inhibition in focal hand dystonia. Cereb.Cortex. 2004;14:555–561. doi: 10.1093/cercor/bhh017. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Matsuhashi M, Lin P, Ou B, Vorbach S, Kakigi R, Hallett M. Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov Disord. 2008;23:558–565. doi: 10.1002/mds.21870. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Hanajima R, Kanazawa I. Interhemispheric facilitation of the hand area of the human motor cortex. Neurosci.Lett. 1993;160:153–155. doi: 10.1016/0304-3940(93)90401-6. [DOI] [PubMed] [Google Scholar]
- Vidal JS, Derkinderen P, Vidailhet M, Thobois S, Broussolle E. Mirror movements of the non-affected hand in hemiparkinsonian patients: a reflection of ipsilateral motor overactivity? J.Neurol.Neurosurg.Psychiatry. 2003;74:1352–1353. doi: 10.1136/jnnp.74.9.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]