Abstract
Object
Pituitary stalk hemangioblastomas are rare, and information on them is limited to a small number of case reports. To gain insight into the incidence, clinical effects, and management of pituitary stalk hemangioblastomas, the authors analyzed a series of patients with von Hippel–Lindau (VHL) disease.
Methods
Patients with VHL disease who were enrolled in a prospective National Institutes of Health natural history study were included. Clinical, imaging, and laboratory findings were analyzed.
Results
Two hundred fifty patients were included in the study (120 male and 130 female patients). In 8 patients (3%), 8 pituitary stalk hemangioblastomas were identified on MR imaging. This anatomical location was the most common supratentorial site for these lesions; 29% of all supratentorial hemangioblastomas were found there. The mean (± standard deviation) pituitary stalk hemangioblastoma volume was 0.5 ± 0.9 cm3 (range 0.08–2.8 cm3). Results of endocrine laboratory profiles were normal in all patients. All patients remained asymptomatic and none required treatment during the follow-up period (mean duration 41.4 ± 14.4 months).
Conclusions
The pituitary stalk is the most common site for the development of supratentorial hemangioblastomas in patients with VHL disease. Pituitary stalk hemangioblastomas often remain asymptomatic and do not require treatment. These findings indicate that pituitary stalk hemangioblastomas in patients with VHL disease may be managed with observation and that surgery for them can be reserved until associated signs or symptoms occur.
Keywords: hemangioblastoma, pituitary stalk, treatment options, tumor incidence, von hippel, Lindau Disease
Hemangioblastomas are benign vascular tumors of the CNS that are composed of vessels and neoplastic stromal cells.22 Hemangioblastomas can occur sporadically (66–80% of tumors) or in the context of the familial neoplasia syndrome VHL disease (20–33% of tumors).1 Whether hemangioblastomas occur sporadically or in association with VHL disease, they are histologically identical and often result from loss of function of both alleles of the VHL gene.10,23 Hemangioblastomas most frequently originate in defined site-specific regions of the CNS (that is, retina, cerebellum, brainstem, and spinal cord) and are seldom seen in the supratentorial compartment of the CNS.11
Despite the uncommon occurrence of hemangioblastomas in the supratentorial compartment of the CNS, recent reports suggest that when these tumors occur in this anatomical region, they may be found in the pituitary stalk. Current understanding of these lesions is derived from isolated case reports. Because of the limited information on the natural history of pituitary stalk hemangioblastomas, their true incidence, their clinical effects, and optimal management strategies for them have not been established. To better determine the incidence of hemangioblastomas arising in the pituitary stalk, to understand their clinical and endocrinological effects, and to help define their optimal management, we analyzed the clinical, imaging, and laboratory findings in a large series of patients with VHL disease who were followed prospectively at the NIH.
Methods
Patient Population
All patients were enrolled in a prospective natural history study of CNS lesions in VHL (NIH protocol 00-N-0140) after informed consent was obtained. In all patients VHL disease was diagnosed based on clinical and genetic criteria.11
Clinical and Imaging Evaluation
Clinical Evaluation
Patients underwent serial clinical evaluations at 6-month intervals. Findings from physical, neurological, and imaging examinations were recorded at each visit. In addition, for patients with pituitary stalk hemangioblastomas, laboratory studies including complete blood count, basic chemistry, thyroid panel, and serum cortisol, luteinizing hormone, follicle-stimulating hormone, testosterone, and prolactin levels were obtained.
Imaging Evaluation
Patients underwent serial enhanced and unenhanced MR imaging studies (including T1- and T2-weighted and FLAIR sequences) at 6-month clinical visits. Tumor location and size (volume = largest anteroposterior dimension × largest mediolateral dimension × largest dorsoventral dimension/2) were determined and recorded based on enhanced T1-weighted MR images.
Results
General Patient Characteristics
A total of 250 patients with VHL disease were included in the study protocol (120 male and 130 female patients). Twenty-seven (11%) of these patients had 28 supratentorial hemangioblastomas identified on MR imaging. The most common supratentorial anatomical site for hemangioblastoma development was the pituitary stalk. Eight of the supratentorial hemangioblastomas (29%) were located in the pituitary stalk (Table 1) in 8 patients (4 female and 4 male). The mean (± standard deviation) age of patients with pituitary stalk hemangioblastomas at entry into the study was 38 ± 13 years (range 11–74 years). The mean follow-up duration was 41.4 ± 14.4 months.
TABLE 1.
Distribution of 28 supratentorial hemangioblastomas in 250 patients with VHL disease
| Anatomical Site | No. of Tumors (%) |
|---|---|
| pituitary stalk | 8 (29) |
| hippocampus | 6 (21) |
| optic nerve/chiasm | 4 (14) |
| sylvian fissure | 2 (7) |
| corpus callosum | 1 (4) |
| other | 7 (25) |
Imaging Characteristics
The pituitary stalk hemangioblastomas enhanced vividly on T1-weighted MR imaging sequences obtained after addition of contrast material (Fig. 1). The tumors could be clearly seen arising from the pituitary stalk posterior to and separate from the optic chiasm. The mean tumor volume was 0.5 ± 0.9 cm3 (range 0.08–2.8 cm3).
Fig. 1.
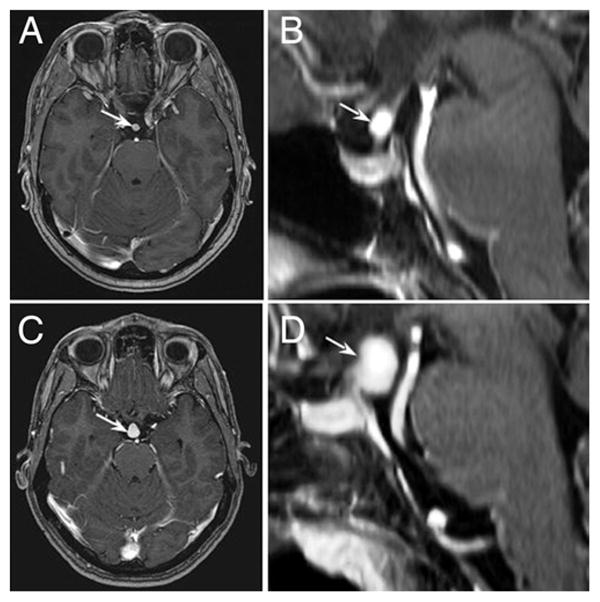
Illustrative cases. Axial (A) and sagittal (B) T1-weighted, contrast-enhanced MR images of a 5-mm pituitary stalk hemangioblastoma (arrows) obtained in a 49-year-old woman with VHL disease. Axial (C) and sagittal (D) T1-weighted, contrast-enhanced MR images of a large pituitary stalk hemangioblastoma (arrows) obtained in a 35-year-old woman with VHL disease.
Clinical and Laboratory Findings
No patient presented with or developed signs or symptoms attributable to a pituitary stalk tumor. Detailed neurological and ophthalmological examination did not reveal deficits attributable to the pituitary stalk hemangioblastomas during the follow-up period. Laboratory endocrine profiles were normal in all patients. The 4 women in the study had menses during childbearing years.
Discussion
Von Hippel–Lindau Disease
Von Hippel–Lindau disease is an inherited neoplasia syndrome that is transmitted in an autosomal dominant manner and is highly penetrant (> 90% penetrance by 60 years of age).13 This disease is caused by a germline mutation or deletion in the VHL tumor suppressor gene located on the short arm of chromosome 3.10,23 Patients with VHL disease are predisposed to develop a variety of visceral and CNS lesions. Visceral lesions include renal cell carcinomas and cysts, pancreatic tumors and cysts, and pheochromocytomas and cystadenomas of the reproductive adnexal organs (broad ligament and epididymis).11 The CNS lesions include hemangioblastomas and endolymphatic sac tumors.2,12,14
Hemangioblastomas in the CNS are the most common manifestation of VHL disease (found in ~ 80% of all patients with the disease) and are often multiple in nature.11 Whether they occur sporadically or in patients with VHL disease, hemangioblastomas are distributed in a highly conserved, region-specific manner within the CNS that includes the retina, brainstem, spinal cord, and cerebellum. Although past reports indicate that the incidence of supratentorial hemangioblastomas is low, sporadic isolated case reports seem to indicate that hemangioblastomas in this anatomical region are not uncommonly found in the pituitary stalk.4,24 We describe the incidence, clinical effects, and management strategies for hemangioblastomas of the pituitary stalk.
Previous Studies
Wanebo et al.24 described 3 cases of pituitary stalk hemangioblastomas in a series of 160 patients with VHL disease who had CNS hemangioblastomas. Because the primary purpose of that study was to examine the natural history of brainstem, spinal cord, and cerebellar hemangioblastomas, no additional information was provided on the patients with pituitary stalk hemangioblastomas. Aside from that study, there have been several reports of hemangioblastomas arising in the region of the sella turcica,3,7,8,17–21,25 as well as 5 prior reports that specifically described cases of hemangioblastomas that arose from the pituitary stalk (Table 2).4–6,9,16 Similar to reports describing age at presentation in patients with hemangioblastomas in other regions of the CNS, the average patient age at discovery of the hemangioblastomas arising in pituitary stalk was 33.4 years (range 28–51 years). These patients were all female; 3 (60%) had VHL disease. The most common signs and symptoms associated with these cases included abnormalities in menstruation (60%) and headache (40%). Resection of the hemangioblastoma was reported in 4 patients (80%); resection was complete in 3 patients (75%) and incomplete in 1 (25%). Two of the 3 patients who underwent complete resection lost all pituitary function after surgery. The third patient had panhypopituitarism before surgery, which persisted after surgery. The patient who underwent partial hemangioblastoma resection maintained normal pituitary function after surgery.
TABLE 2.
Literature review of 5 reported cases of pituitary stalk hemangioblastoma*
| Authors & Year | Age (yrs), Sex | Signs & Symptoms | Preop Endocrine Function | VHL | Extent of Resection | FU |
|---|---|---|---|---|---|---|
| Grisoli et al., 1984 | 28, F | HA, galactorrhea, obesity, alopecia | elevated prolactin w/TRH stimulation (14–27 ng/ml) | no | total | postop panhypopituitarism |
| Neumann et al., 1989 | 35, F | HA, amenorrhea, diabetes insipidus | NA | no | NA | NA |
| Kouri et al., 2000 | 20, F | amenorrhea, diabetes insipidus | panhypopituitarism | yes | total | stable panhypopituitarism |
| Goto et al., 2001 | 33, F | irregular menstruation | “slight decrease in the response of LH & FSH to the GnRH stimulation test” | yes | partial | normal pituitary function |
| Fomekong et al., 2007 | 51, F | rt inferior temporal quadrantanopia | elevated prolactin (80.7 ng/ml) | yes | total | postop panhypopituitarism; recovery of visual loss |
FSH = follicle-stimulating hormone; FU = follow-up; GnRH = gonadotropin-releasing hormone; HA = headache; LH = luteinizing hormone; NA = not available; TRH = thyrotropin-releasing hormone.
Current Study
Incidence
Eleven percent of our patients with VHL disease harbored a supratentorial hemangioblastoma. The most common site in which hemangioblastomas occur in the supratentorial compartment is the pituitary stalk (29% of all supratentorial hemangioblastomas and 3% of all patients with VHL). The frequency of supratentorial and pituitary stalk tumors described in the current study is higher than predicted and/or described in prior reports of hemangioblastomas in patients with VHL and in those with sporadic disease. Previous studies have generally found the incidence of supratentorial hemangioblastomas to be in the range of 1–6%, with the predicted incidence of pituitary stalk hemangioblastomas far less than 1%.6,15 The increased occurrence of hemangioblastomas found in our series may be related to the prospective design of the study, the larger number of patients in the series, the long-term follow-up of the patients in the study (mean 41.4 ± 14.4 months), the lower imaging resolution in some of the prior studies (often based on CT scans or lower-resolution MR images), or the focus of previous studies on the more common sites for hemangioblastomas (that is, the brainstem, cerebellum, and spinal cord).
Clinical and Management Implications
Based on their anatomical location, there are several critical clinical implications for pituitary stalk hemangioblastomas. Magnetic resonance imaging of the pituitary region in patients with VHL disease is mandatory for successful diagnosis and follow-up of pituitary stalk hemangioblastomas. Serial detailed visual field testing is important to monitor these tumors to detect potential enlargement and visual loss. Similarly, detailed clinical and laboratory endocrinological follow-up is important in patients with VHL who harbor pituitary stalk tumors. Finally, the optimal surgical timing and management of pituitary stalk hemangioblastomas in VHL disease has not been determined, but depends on the capacity to cure these tumors while preserving pituitary function. Previous reports indicate that complete resection of pituitary stalk hemangioblastomas often produces panhypopituitarism. Because of this significant potential surgery-related morbidity, and the slow growth, uncertain natural history, and lack of clinical effects of these lesions, we have successfully managed hemangioblastomas in patients with VHL disease in a conservative manner, with observation on serial imaging, and with endocrine and visual assessment.
Conclusions
The pituitary stalk is the most common site for the development of supratentorial hemangioblastomas in VHL disease. Patients with pituitary stalk hemangioblastomas often remain asymptomatic and do not require treatment. Patients with VHL disease who harbor pituitary stalk hemangioblastomas may be observed without surgery, which can be reserved until symptoms or signs caused by compression of anatomically adjacent structures occurs.
Abbreviations in this paper
- CNS
central nervous system
- NIH
National Institutes of Health
- VHL
von Hippel–Lindau
Footnotes
Disclosure
The Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the NIH supported this research.
References
- 1.Browne TR, Adams RD, Roberson GH. Hemangioblastoma of the spinal cord: review and report of five cases. Arch Neurol. 1976;33:435–441. doi: 10.1001/archneur.1976.00500060041009. [DOI] [PubMed] [Google Scholar]
- 2.Butman JA, Kim HJ, Baggenstos M, Ammerman JM, Dambrosia J, Patsalides A, et al. Mechanisms of morbid hearing loss associated with tumors of the endolymphatic sac in von Hippel-Lindau disease. JAMA. 2007;298:41–48. doi: 10.1001/jama.298.1.41. [DOI] [PubMed] [Google Scholar]
- 3.Dan NG, Smith DE. Pituitary hemangioblastoma in a patient with von Hippel-Lindau disease. Case report. J Neurosurg. 1975;42:232–235. doi: 10.3171/jns.1975.42.2.0232. [DOI] [PubMed] [Google Scholar]
- 4.Fomekong E, Hernalsteen D, Godfraind C, D’Haens J, Raftopoulos C. Pituitary stalk hemangioblastoma: the fourth case report and review of the literature. Clin Neurol Neurosurg. 2007;109:292–298. doi: 10.1016/j.clineuro.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Goto T, Nishi T, Kunitoku N, Yamamoto K, Kitamura I, Takeshima H, et al. Suprasellar hemangioblastoma in a patient with von Hippel-Lindau disease confirmed by germline mutation study: case report and review of the literature. Surg Neurol. 2001;56:22–26. doi: 10.1016/s0090-3019(01)00482-7. [DOI] [PubMed] [Google Scholar]
- 6.Grisoli F, Gambarelli D, Raybaud C, Guibout M, Leclercq T. Suprasellar hemangioblastoma. Surg Neurol. 1984;22:257–262. doi: 10.1016/0090-3019(84)90010-7. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda M, Asada M, Yamashita H, Ishikawa A, Tamaki N. A case of suprasellar hemangioblastoma with thoracic meningioma. No Shinkei Geka. 2001;29:679–683. (Jpn) [PubMed] [Google Scholar]
- 8.Kachhara R, Nair S, Radhakrishnan VV. Sellar-sphenoid sinus hemangioblastoma: case report. Surg Neurol. 1998;50:461–464. doi: 10.1016/s0090-3019(97)00197-3. [DOI] [PubMed] [Google Scholar]
- 9.Kouri JG, Chen MY, Watson JC, Oldfield EH. Resection of suprasellar tumors by using a modified transsphenoidal approach. Report of four cases. J Neurosurg. 2000;92:1028–1035. doi: 10.3171/jns.2000.92.6.1028. [DOI] [PubMed] [Google Scholar]
- 10.Latif F, Tory K, Gnarra J, Yao M, Duh F-M, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 11.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059– 2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 12.Lonser RR, Kim HJ, Butman JA, Vortmeyer AO, Choo D, Oldfield E. Tumors of the endolymphatic sac in von Hippel-Lindau disease. N Engl J Med. 2004;350:2481–2486. doi: 10.1056/NEJMoa040666. [DOI] [PubMed] [Google Scholar]
- 13.Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 14.Manski TJ, Heffner DK, Glenn GM, Patronas NJ, Pikus AT, Katz D, et al. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA. 1997;277:1461–1466. doi: 10.1001/jama.277.18.1461. [DOI] [PubMed] [Google Scholar]
- 15.Morello G, Bianchi M. Cerebral hemangioblastomas: review of literature and report of two personal cases. J Neurosurg. 1963;20:254–264. doi: 10.3171/jns.1963.20.3.0254. [DOI] [PubMed] [Google Scholar]
- 16.Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg. 1989;70:24–30. doi: 10.3171/jns.1989.70.1.0024. [DOI] [PubMed] [Google Scholar]
- 17.Niemela M, Lim YJ, Soderman M, Jaaskelainen J, Lindquist C. Gamma knife radiosurgery in 11 hemangioblastomas. J Neurosurg. 1996;85:591–596. doi: 10.3171/jns.1996.85.4.0591. [DOI] [PubMed] [Google Scholar]
- 18.Peker S, Kurtkaya-Yapicier O, Sun I, Sav A, Pamir MN. Suprasellar haemangioblastoma. Report of two cases and review of the literature. J Clin Neurosci. 2005;12:85–89. doi: 10.1016/j.jocn.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Rho YM. Von Hippel-Lindau’s disease: a report of five cases. Can Med Assoc J. 1969;101:135–142. [PMC free article] [PubMed] [Google Scholar]
- 20.Rumboldt Z, Gnjidic Z, Talan-Hranilovic J, Vrkljan M. Intrasellar hemangioblastoma: characteristic prominent vessels on MR imaging. AJR Am J Roentgenol. 2003;180:1480–1481. doi: 10.2214/ajr.180.5.1801480. [DOI] [PubMed] [Google Scholar]
- 21.Sawin PD, Follett KA, Wen BC, Laws ER., Jr Symptomatic intrasellar hemangioblastoma in a child treated with subtotal resection and adjuvant radiosurgery. Case report. J Neurosurg. 1996;84:1046–1050. doi: 10.3171/jns.1996.84.6.1046. [DOI] [PubMed] [Google Scholar]
- 22.Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH, et al. von Hippel-Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol. 1997;28:540–543. doi: 10.1016/s0046-8177(97)90075-7. [DOI] [PubMed] [Google Scholar]
- 23.Wait SD, Vortmeyer AO, Lonser RR, Chang DT, Finn MA, Bhowmick DA, et al. Somatic mutations in VHL germline deletion kindred correlate with mild phenotype. Ann Neurol. 2004;55:236–240. doi: 10.1002/ana.10807. [DOI] [PubMed] [Google Scholar]
- 24.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of central nervous system hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:82– 94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 25.Wasenko JJ, Rodziewicz GS. Suprasellar hemangioblastoma in Von Hippel-Lindau disease: a case report. Clin Imaging. 2003;27:18– 22. doi: 10.1016/s0899-7071(02)00537-5. [DOI] [PubMed] [Google Scholar]


