Abstract
Hemangioblastomas are frequently associated with peritumoral edema caused by extravasation of plasma ultra-filtrate through permeable neoplastic vessels. The authors report the clinical and imaging findings in a 62-year-old man with von Hippel–Lindau disease who presented with rapid (within 24 hours) loss of color vision and near-complete loss of left eye vision (acuity too poor to test). Serial MR imaging demonstrated a stable vascular tumor in the medioinferior aspect of the left optic nerve, associated with progressive edema extending from the nerve through to the bilateral optic radiations. Complete resection of the lesion was performed via an extended transsphenoidal approach, and histological examination confirmed the lesion was a hemangioblastoma. Postoperatively, the patient recovered color vision and had improvement in visual acuity (20/320). Serial imaging in this unique case captured the progressive extravasation of peritumoral edema that tracked and defined the parallel white matter tracts of first- and second-order neurons of the optic system, causing vision loss. Tumor resection led to resolution of the edema and improvement in visual function.
Keywords: edema, hemangioblastoma, optic nerve, tumor, von Hippel‒Lindau disease
Recent clinical, imaging, and molecular biological evidence has indicated that CNS peritumoral edema is formed by the extravasation of plasma ultra-filtrate through leaky tumor vessels.1,9,12 Once fluid leaks out of the permeable tumor vasculature into the interstitial spaces of the CNS parenchyma (vasogenic edema), its anatomical distribution is determined by the properties of surrounding tissue. Within the CNS, interstitial infusion studies3,11 and diffusion tensor imaging2,16,23 have shown that parallel fibers of white matter tracts provide low-resistance pathways for interstitial fluid distribution, and the propagation of edema occurs preferentially along these pathways. We describe the unique clinical and imaging findings in a patient with acute visual loss due to an optic nerve hemangioblastoma associated with peritumoral edema that preferentially followed the optic fiber tracks, and we discuss the implications these findings have for the treatment of hemangioblastomas in this location.
Case Report
History and Examination
This 62-year-old man with VHL disease (diagnosed based on clinical criteria and genetic analysis) was evaluated at the National Institutes of Health after he had experienced rapid (within a 24-hour period) loss of vision in the left eye 1 week earlier. At an outside institution he had been placed on high-dose steroids after vision loss, and he reported intermittent subjective improvement. On admission to our facility, a detailed ophthalmological examination of the right eye revealed 20/16 visual acuity, loss of color vision, an afferent pupillary defect, and the ability to count fingers in the left eye only (acuity was too poor to test). Retinal examination of the left eye revealed no sign of cupping, retinal detachment, or vascular occlusion. Serial high-resolution T1-weighted MR imaging of the brain revealed a stable enhancing lesion associated with the left optic nerve, just proximal to the optic canal (Fig. 1). Fluid attenuated inversion recovery MR imaging demonstrated progressive edema of the left optic nerve that coursed preferentially along the interconnected white matter tracts of the optic system including the chiasm, bilateral optic nerves, bilateral lateral geniculate bodies, and optic radiations (Fig. 2).
Fig. 1.
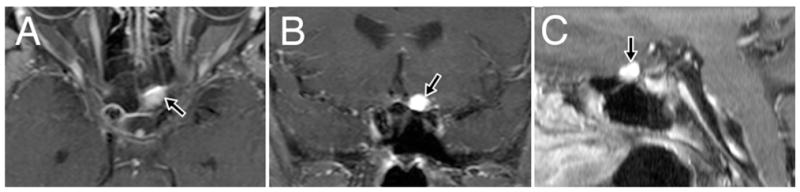
Preoperative axial (A), coronal (B), and sagittal (C) post–contrast enhanced T1-weighted MR images demonstrating the enhancing hemangioblastoma (arrows) on the medioinferior aspect of the left optic nerve.
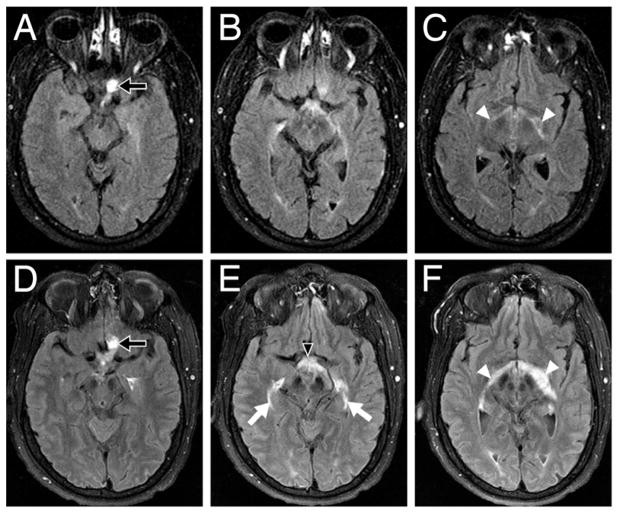
Fig. 2.
Axial T1-weighted FLAIR MR images demonstrating the progression of hemangioblastoma-induced peritumoral edema involving the optic pathways from August 2005 to January 2007. In August 2005 (A, B, and C), edema surrounding the left optic nerve hemangioblastoma (A, arrow) with mild edema (C, arrowheads) was noted along the optic fibers bilaterally. In January 2007 (D, E, and F), 5 days before surgery, the size of the hemangioblastoma had remained stable (D, arrow), but a significant increase in edema in the left optic nerve extended through the optic chiasm (E, arrowhead) and lateral geniculate bodies (F, arrowheads) and into the bilateral optic radiations (E, arrows).
Operation
The tumor was removed via a sublabial extended transsphenoidal approach,7 which provided excellent exposure to the vascular tumor situated in the medioinferior portion of the left optic nerve. Circumferential microsurgical dissection of the lesion revealed that it arose from within, and separated the fibers of, the left op tic nerve (Fig. 3). The tumor was excised en bloc, and histopathological analysis confirmed that it was a hemangioblastoma.
Fig. 3.
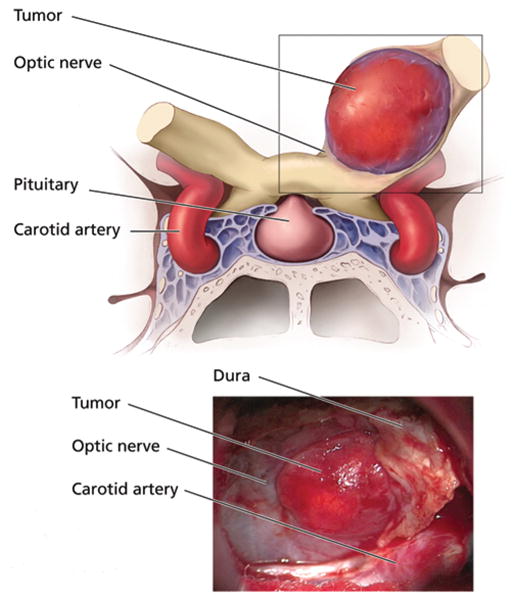
Upper: Coronal illustration showing the relationship of the hemangioblastoma to the optic apparatus and surrounding anatomical structures. Lower: Intraoperative photograph showing the vascular hemangioblastoma arising from within the left optic nerve and separating the fibers of the medioinferior aspect of the nerve.
Postoperative Course
After surgery, the patient regained color vision, had resolution of the afferent pupillary defect, and experienced improvement in left eye visual acuity (20/320). Steroids were subsequently tapered. Magnetic resonance imaging performed 7 days after surgery confirmed complete re section of the hemangioblastoma and resolution of the edema (Fig. 4).
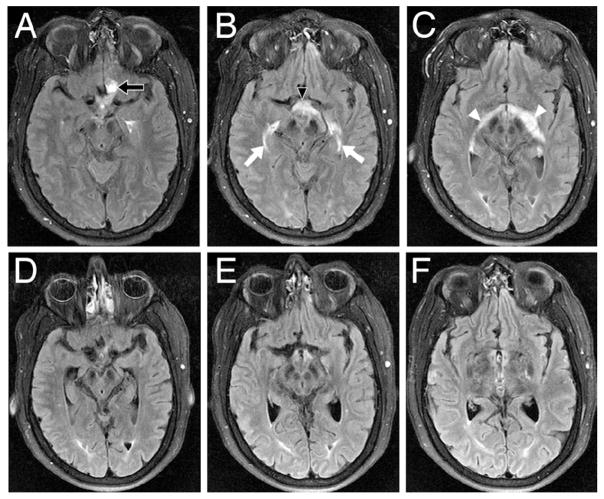
Fig. 4.
Axial FLAIR MR images (A, B, and C) obtained preoperatively, revealing significant edema surrounding the left optic nerve hemangioblastoma (A, arrow) that tracked into the optic chiasm (B, arrowhead) and along the bilateral optic system (C, arrows and arrowheads). Axial FLAIR MR images (D, E, and F) obtained 7 days after surgery, demonstrating resolution of edema.
Discussion
Optic Nerve Hemangioblastomas in VHL Disease
Von Hippel–Lindau disease is an autosomal dominant neoplastic disorder that results from a germline mutation or deletion of the VHL tumor suppressor gene on the short arm of chromosome 3.8,21 It has a prevalence of ~ 1 in 39,000 live births15 and is associated with a variety of visceral lesions and CNS tumors.10 Common visceral lesions include renal cell carcinomas or cysts (25–60% of cases), pheochromocytomas (10–20% of cases), pancreatic tumors or cysts (35–70% of cases), and cystadenomas of the adnexal reproductive organs (25–60% of cases). Central nervous system tumors include hemangioblastomas of the retina (25–60% of cases), cerebellum (44–72% of cases), brainstem (10–25% cases), spinal cord (13–50% of cases), and lumbosacral nerve roots (2–5% of cases) as well as endolymphatic sac tumors (10% of cases).
Despite the frequent association of CNS hemangioblastomas with VHL disease, optic nerve hemangioblastomas have been reported in only 9 cases of VHL disease (mean age at discovery 33.4 ± 15.0 years) in the English literature, including the present case.4–7,14,17–19 Although 1 optic nerve hemangioblastoma was discovered at autopsy, 9 tumors had been resected from 8 patients (1 patient with bilateral tumors; Table 1). Preoperatively, vision was significantly impaired in 7 patients (8 eyes) and remained unchanged after resection in all cases for which outcome was reported, except the present case in which vision improved. This improvement in vision in our patient may be related to edema as the cause of visual loss (as opposed to progressive and compressive growth) and the relatively short interval from visual loss to resection. As in our case, tumors in the other cases were found to originate from within the substance of the nerve, except in patients with normal vision preoperatively.7,17
TABLE 1.
Literature summary of optic nerve hemangioblastomas in patients with VHL disease
| Authors & Year | Age (yrs), Sex | Symptoms | Lesion Side | Resection Performed | Tumor Origin | Visual Outcome |
|---|---|---|---|---|---|---|
| Stefani & Rothemund, 1974 | 20, M | total atrophy of rt optic nerve before death | rt | no | intraneural | not applicable |
| In et al., 1982 | 23, F | blindness, pulsating exophthalmos & absent direct-light reflex | lt | yes | intraneural | unchanged |
| Nerad et al., 1988 | 24, F | blindness, proptosis, papilledema, decreased sensation in V1 | lt | yes | intraneural | unchanged |
| Ginzburg et al., 1992 | 44, M | bilat visual loss & optic disc pallor | bilat | yes | intraneural | unchanged |
| Rubio et al., 1994 | 43, F | absence of rt pupillary light reflex & absence of rt visual evoked response | rt | yes | intraneural | not reported |
| Kerr et al., 1995 | 40, F | visual acuity 14/224 in rt eye w/afferent defect | rt | yes | intraneural | unchanged |
| Raila et al., 1997 | 30, F | asymptomatic | lt | yes | peripheral | unchanged |
| Kouri et al., 2000 | 15, F | asymptomatic | lt | yes | peripheral | unchanged |
| present case | 62, M | loss of color vision, vision for finger countting only in lt eye, & afferent defect | lt | yes | intraneural | improved |
Edema Formation and Distribution
The biological phenomenon associated with peritumoral edema formation and propagation along low-resistance white matter pathways was demonstrated by serial imaging in the present case. Edema formation from a single source (the tumor) was established on early imaging studies based on the emanation of interstitial fluid from the edges of the hemangioblastoma. Confirmation of the lesion as the sole source of edema was provided by the fact that resection led to the resolution of edema throughout the optic system. The preferential distribution of progressive peritumoral edema along the parallel white matter tracts of the visual system was seen on serial FLAIR MR imaging and extended from the left optic nerve through the optic chiasm into the optic tracts to the lateral geniculate bodies, across the neuronal synapse, and into the bilateral optic radiations.
Clinical Implications
Based on the clinical and imaging findings in the present case, there are several implications for the biological features and treatment of optic nerve hemangioblastomas in VHL disease. First, optic nerve hemangioblastomas appear to frequently arise from within the nerve (Table 1). This observation is consistent with the previously reported intraneural origin of hemangioblastomas in the lumbosacral and spinal nerve roots.13,20 Second, although mass effect or ischemia could have contributed to vision loss in our patient, it is likely that peritumoral edema, in the absence of tumor growth, was the primary cause of visual decline. This finding is compatible with previous reports of hemangioblastoma-associated edema and symptom formation in other regions of the CNS.12,22 Third, resection of the hemangioblastoma was performed to prevent progressive edema from affecting interrelated optic fibers of the right eye and to alleviate the edema within the left eye fiber tracts in an effort to restore vision. Because the neurological deficits were the result of edema propagation, they were reversible by simply removing the source of edema (tumor). Fourth, similar to hemangioblastomas in other regions of the CNS, several years elapsed from the time of initial edema formation to actual sign and symptom development.12,22 Although this occurrence suggests that asymptomatic edema formation alone does not require immediate surgery, it can serve as an early indicator of potential visual loss if progression on imaging and/or symptom formation occurs.
Conclusions
The optimal surgical timing and management of optic nerve hemangioblastomas in VHL disease have not been determined and will depend on the ability to cure these tumors with preservation of visual function. Although the management of these lesions (particularly small and asymptomatic tumors) in VHL disease has traditionally been conservative—because the tumors are not considered malignant, grow slowly, and have an uncertain natural history—some features, including progressive tumor growth and/or edema, may indicate situations in which surgery could be useful in preventing visual loss.
Acknowledgments
Sources of support: This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Abbreviations used in this paper
- CNS
central nervous system
- VHL
von Hippel–Lindau
References
- 1.Baggenstos M, Butman JA, Oldfield EH, Lonser RR. Role of edema in peritumoral cyst formation. Neurosurg Focus. 2007;22(5):E9. doi: 10.3171/foc.2007.22.5.10. [DOI] [PubMed] [Google Scholar]
- 2.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 3.Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg. 1999;90:315–320. doi: 10.3171/jns.1999.90.2.0315. [DOI] [PubMed] [Google Scholar]
- 4.Ginzburg BM, Montanera WJ, Tyndel FJ, Griesman JA, McLennan MK, TerBrugge KG, et al. Diagnosis of von Hippel-Lindau disease in a patient with blindness resulting from bilateral optic nerve hemangioblastomas. AJR Am J Roentgenol. 1992;159:403–405. doi: 10.2214/ajr.159.2.1632366. [DOI] [PubMed] [Google Scholar]
- 5.In S, Miyagi J, Kojho N, Kuramoto S, Uehara M. Intraorbital optic nerve hemangioblastoma with von Hippel-Lindau disease. Case report. J Neurosurg. 1982;56:426–429. doi: 10.3171/jns.1982.56.3.0426. [DOI] [PubMed] [Google Scholar]
- 6.Kerr DJ, Scheithauer BW, Miller GM, Ebersold MJ, McPhee TJ. Hemangioblastoma of the optic nerve: case report. Neurosurgery. 1995;36:573–581. doi: 10.1227/00006123-199503000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Kouri JG, Chen MY, Watson JC, Oldfield EH. Resection of suprasellar tumors by using a modified transsphenoidal approach. Report of four cases. J Neurosurg. 2000;92:1028–1035. doi: 10.3171/jns.2000.92.6.1028. [DOI] [PubMed] [Google Scholar]
- 8.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 9.Lohle PN, Wurzer HA, Seelen PJ, Kingma LM, Go KG. The pathogenesis of cysts accompanying intra-axial primary and metastatic tumors of the central nervous system. J Neurooncol. 1998;40:277–285. doi: 10.1023/a:1006170129761. [DOI] [PubMed] [Google Scholar]
- 10.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 11.Lonser RR, Gogate N, Morrison PF, Wood JD, Oldfield EH. Direct convective delivery of macromolecules to the spinal cord. J Neurosurg. 1998;89:616–622. doi: 10.3171/jns.1998.89.4.0616. [DOI] [PubMed] [Google Scholar]
- 12.Lonser RR, Vortmeyer AO, Butman JA, Glasker S, Finn MA, Ammerman JM, et al. Edema is a precursor to central nervous system peritumoral cyst formation. Ann Neurol. 2005;58:392–399. doi: 10.1002/ana.20584. [DOI] [PubMed] [Google Scholar]
- 13.Lonser RR, Wait SD, Butman JA, Vortmeyer AO, Walther MM, Governale LS, et al. Surgical management of lumbosacral nerve root hemangioblastomas in von Hippel-Lindau syndrome. J Neurosurg. 2003;99:64–69. doi: 10.3171/spi.2003.99.1.0064. [DOI] [PubMed] [Google Scholar]
- 14.Nerad JA, Kersten RC, Anderson RL. Hemangioblastoma of the optic nerve. Report of a case and review of literature. Ophthalmology. 1988;95:398–402. doi: 10.1016/s0161-6420(88)33184-2. [DOI] [PubMed] [Google Scholar]
- 15.Neumann HP, Wiestler OD. Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991;337:1052–1054. doi: 10.1016/0140-6736(91)91705-y. [DOI] [PubMed] [Google Scholar]
- 16.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 17.Raila FA, Zimmerman J, Azordegan P, Fratkin J, Parent AD. Successful surgical removal of an asymptomatic optic nerve hemangioblastoma in von Hippel-Lindau disease. J Neuroimaging. 1997;7:48–50. doi: 10.1111/jon19977148. [DOI] [PubMed] [Google Scholar]
- 18.Rubio A, Meyers SP, Powers JM, Nelson CN, de Papp EW. Hemangioblastoma of the optic nerve. Hum Pathol. 1994;25:1249– 1251. doi: 10.1016/0046-8177(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 19.Stefani FH, Rothemund E. Intracranial optic nerve angioblastoma. Br J Ophthalmol. 1974;58:823–827. doi: 10.1136/bjo.58.9.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tender GC, Butman JA, Oldfield EH, Lonser RR. Thoracic spinal nerve hemangioblastoma. Case illustration. J Neurosurg Spine. 2004;1:142. doi: 10.3171/spi.2004.1.1.0142. [DOI] [PubMed] [Google Scholar]
- 21.Wait SD, Vortmeyer AO, Lonser RR, Chang DT, Finn MA, Bhowmick DA, et al. Somatic mutations in VHL germline deletion kindred correlate with mild phenotype. Ann Neurol. 2004;55:236–240. doi: 10.1002/ana.10807. [DOI] [PubMed] [Google Scholar]
- 22.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 23.Witwer BP, Moftakhar R, Hasan KM, Deshmukh P, Haughton V, Field A, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg. 2002;97:568–575. doi: 10.3171/jns.2002.97.3.0568. [DOI] [PubMed] [Google Scholar]