Abstract
Histological evaluation is a complex, multistep process culminating in tissue staining. All of the steps leading up to the staining affect the final quality, but too often the effects of these preparations are not given enough consideration. Fixatives in particular usually are chosen not for efficacy but for convenience and availability. This study attempts to create guidelines for selecting fixatives for bone tissue histological evaluation. We compared two of the most widely used fixatives, ethanol and formalin, in their use on mouse tibias embedded in methylmethacrylate and subsequently stained with toluidine blue, safranin O, or Von Kossa. Our results show that ethanol fixation (70%) and subsequent processing in methylmethacrylate gives better staining results for bone cell related elements than fixing in 10% neutral buffered formalin with the same processing and embedding techniques. Further we demonstrated than an additional acetone dehydration and clearing step allowed for even better visualization in bone specimens fixed with 70% ethanol. However, the additional acetone step did not enhance visualization in bone specimens fixed with 10% neutral buffered formalin. Finally, marrow elements were more easily visualized when fixed with formalin as opposed to ethanol.
Keywords: Bone cells, dehydration, fixation, fixative, histology, MMA, mouse, osteoblast, osteoclast
Introduction
A critical component for consideration when designing any experimental protocol is the endpoint. In histological evaluation the endpoint is staining, and this data-yielding step is affected by all preceding steps in the experimental procedure. Histological samples are fixed, processed, embedded, and sectioned before being stained, and all of these steps can enhance, or detract, from the final outcome of the stain quality.
Oftentimes, the fixative chosen for a particular tissue specimen is based not on the endpoint but rather on what the investigator routinely uses in his or her laboratory. Studies that determine what fixative yields the best stain for different tissues types help in making choices based on outcome, not on familiarity. Here, we compare two of the most commonly used fixatives, ethanol and formalin, to see which is more effective at fixing undecalcified bone specimens. Also, we look at how an additional processing step of acetone dehydration and clearing affects the final stain and how fixative choice influences dehydration and clearing.
This study eliminates the ambiguity in choosing a fixative for specific tissue specimens by directly comparing two primary laboratory fixatives. We examined the effects of ethanol versus formalin fixation on the staining of sections from undecalcified mouse tibiae embedded in methylmethacrylate and subsequently stained. Additionally, we determined whether an acetone dehydration and clearing step had any effect on the stain. Here we examined the following stains: toluidine blue for visualization of bone cell elements like osteoblasts, osteoclasts, osteoid, and trabecular bone; Von Kossa for examining mineralized matrix; and safranin O for examining proteoglycans. Our data show that ethanol fixation yields better staining results for visualization and analysis of bone cells such as osteoblasts and osteoclasts. Further, we show that the additional acetone dehydration and clearing step is advantageous when bone specimens are fixed with 70% ethanol, but sections remain unchanged when fixed with neutral buffered formalin. Importantly, the bone marrow cells were more readily visualized (in particular with regard to nuclear detail) when fixed with formalin.
Materials and Methods
Specimens
Intact tibiae from 10- to 12-week-old C57BL/6 mice purchased from Jackson Laboratories (Bar Harbor, ME) were stripped of soft tissue, and placed in approximately 20 mL of 10% neutral buffered formalin (NBF) at 4°C or approximately 20 mL of 70% ethyl alcohol (EtOH) for 16 h (approximately a 1:20 specimen/fixative ratio). Ten tibiae were fixed with NBF and 10 were fixed with EtOH (20 total specimens).
Tissue Processing/Handling
Table 1 summarizes our processing schedule. In brief, following fixation, tibiae were washed with phosphate-buffered saline (PBS) and either incubated in 70% EtOH (n = 5 for each NBF and EtOH fixation) for 24 h or in 70% acetone (n = 5 for each NBF and EtOH fixation) for 24 h. Then all of the tibiae were dehydrated in graded acetones (90% 1×, 100% 2×, for 1 h each). All of the aforementioned steps were performed at 4°C.
Table 1.
Processing schedule
| Solution | Time in solution | Temp |
|---|---|---|
| Fixative | ||
| 10% NBF or 70% EtOH | 16 h | RT |
| Wash | ||
| PBS | 15 min | RT |
| Dehydration procedure | ||
| 70% Acetone or 70% EtOH | 24 h | 4°C |
| 90% acetone | 1 h | 4°C |
| 100% acetone | 1 h | 4°C |
| 100% acetone | 1 h | 4°C |
| Infiltration procedure | ||
| MMA infiltration solution (vacuum dessicator) | 48 h | 4°C |
| MMA embedding solution (waterbath in radiant oven) | 48 h | 37°C |
As has been detailed elsewhere (1–3), after dehydration, tibiae were placed in a 20-mL glass vial containing 15 mL of infiltration medium containing 85% destabilized MMA (Sigma, St. Louis, MO), 15% dibutyl phthalate (Sigma), and 0.15% benzoyl peroxide (Polysciences, Inc., Warrington, PA).
After 48 h of infiltration in a vacuum dessicator at 4°C, tibiae were removed from infiltration MMA and were placed on prepolymerized bases/layers, covered with 8 mL of fresh catalyzed MMA (85% destabilized MMA, 15% dibutyl phthalate, and 5% benzoyl peroxide catalyst), and incubated for 2 d in a waterbath, which was placed in a 37°C radiant heat oven (Labline, Melrose Park, IL). Glass vials were removed from the oven, cooled at −20°C for 30 min, and the specimen blocks were removed by breaking the glass vial. It should be noted that this infiltration and embedding protocol has consistently produced (during the last 10 years) well-polymerized blocks of appropriate hardness to provide good sections. Clearly the cutting properties of the embedded material and the quality of the sections greatly depend on the infiltration and polymerization steps. Our laboratory has worked to optimize each of these steps which we have previously documented (4). Like was described by Lebeau et al (5) in these studies a low percentage of initiator (benzoyl peroxide) and a platicizer (dibutyl phthalate) were used. In our hands, we have found more uniform and rapid polymerization with using destabilized MMA. Because hazards exist with destabilized MMA, multiple precautions are taken including that only small quantities of MMA are destabilized at a time.
Specimen blocks were then trimmed and sanded, and 4-μm sections were obtained with the use of a Leica™ 2165 Microtome (Leica, Heidelberg, Germany), and a tungsten-carbide knife, D-profile (Dorn & Hart Microedge, Villa Park, IL). Sections were then placed on chrome-alum-gel coated slides and incubated overnight at 37°C in a radiant heat oven to adhere sections to the slide as previously described (2,6). Sections mounted on individual slides were covered with a plastic film, and slides were stacked and clamped prior to incubation overnight.
Histochemical Staining
All histochemical stains were performed on the sections mounted on chromalum-gel–coated slides. Histochemical staining procedures for toluidine blue, Von Kossa, and safranin O, have been previously described (2,6–11). For each of the histochemical stains evaluated, slides were batch processed so that all slides were stained concurrently.
Toluidine Blue Staining
After overnight incubation, 4-μm tibia sections on chromalum-gel-coated slides were deplasticized at room temperature with Cellosolve acetate (Fisher Scientific, Pittsburgh, PA; 2×, 25 min each). Next, sections were rehydrated in graded ethanols: 70% for 5 min, 40% for 5 min, and then brought to distilled water for 5 min.
Toluidine blue stain was prepared as described by Baron et al (6). The pH of the stain was carefully adjusted to 3.7 for ideal differentiation of osteoid and mineralized bone. Once rehydrated, bone sections were immersed in stain for 5 min at room temperature, then rinsed with citric acid buffer (pH 3.7), and blotted dry. Slides were dipped 2× in butanol (tert-butyl alcohol, Fisher), 1× in butanol/toluene (50:50), and 2× in toluene, before mounting with Permount™ (Fisher).
Von Kossa Staining
Von Kossa staining was performed as previously described (6). In brief, sections mounted on chromalum-gel–coated slides were deplasticized and brought to distilled water as described previously. A 5% solution of silver nitrate (Sigma) was prepared and filtered. Slides were immersed in the silver nitrate solution for 30 min at room temperature in the dark. After this incubation, slides were rinsed three times in distilled water and immersed in a 5% sodium carbonate-formaldehyde solution (mix 75 mL of distilled water, 25 mL of 37% formaldehyde, then dissolve 5 g of sodium carbonate) for 2 minutes. Slides are then washed for 10 min in running tap water and then counterstained for 20 min in methyl green pyronin (Sigma). After counterstaining, slides were washed 2× with freshly boiled, cooled distilled water (1 min/wash), dehydrated in graded ethanols, cleared in xylene, and mounted with Permount™ (Fisher). Mineralized tissues are stained black, and osteoid appears red.
Safranin O Staining
For safranin O staining (7,8,10,11), sections were deplasticized in two changes of acetone, 5 min each, then rehydrated through one change of 70% ethanol (5 min), 40% ethanol (5 min), and washed in distilled water for 5 min. Slides were then stained with Weigert’s iron hematoxylin working solution for 7 min and then washed in running distilled water for 10 min. After the water wash, slides were stained with 0.001% fast green solution for 3 min, and then rinsed quickly with 1% acetic acid solution. Slides were then stained with 0.1% safranin O solution for 5 min. After staining with safranin O solution, slides were dehydrated in two changes each of 95% ethanol, absolute ethanol and xylene for two minutes in each solution, then mounted in Permount™ (Fisher).
Results
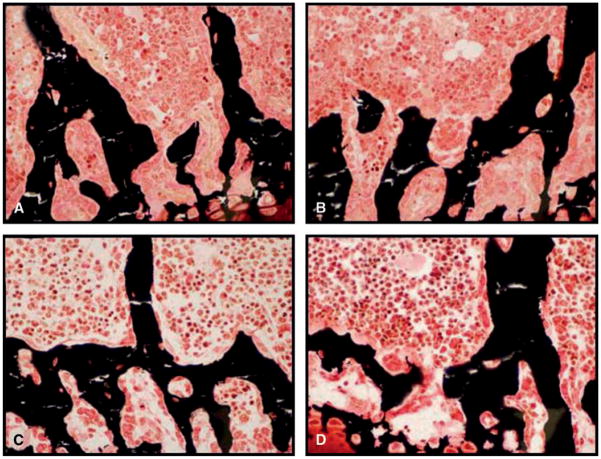
Figure 1 illustrates the importance of selecting an appropriate fixative to obtain high-quality histology sections of undecalcified bone specimens stained with Von Kossa (essentially identical findings were observed with toluidine blue and safranin O staining, data not shown). In this study we examined the effects of EtOH or formalin fixation. Figures 1A and 1B show representative micrographs of specimens fixed with 10% NBF. Figures 1C and 1D are micrographs of specimens fixed with 70% EtOH. Specimens shown in Figures 1A and 1C were subjected to an additional dehydration/clearing step in 70% acetone, whereas specimens in Figures 1B and 1D were bathed in 70% EtOH during that step.
Figure 1.
Tibiae from 10- to 12-week-old C57BL/6 mice were either fixed in 10% NBF (A and B) or 70% EtOH (C and D) and stained with Von Kossa. Some specimens were subjected to an additional dehydration/clearing step in 70% acetone (A and C). Bone marrow elements and bone cells/trabecular bone were evaluated histologically. Original magnification for all panels, 750×.
Bone marrow elements were best preserved in specimens fixed with 10% NBF (Figures 1A and 1B). Nuclear detail was easily visualized and nuclear chromatin condensation was reduced compared with the other specimens. However, the marrow was not as well cleared of fat as was seen in Figure 1C. In addition, the tissue was dry and there were numerous cracks and microfractures. Of note, the bone cell elements, such as osteoblasts, osteoclasts, and the newly secreted bone matrix or osteoid were more difficult to visualize in the formalin-fixed specimens. Interestingly, little difference was observed when specimens fixed with 10% NBF were followed by an additional dehydration/clearing step in 70% acetone.
The marrow was best cleared in specimens fixed in 70% EtOH followed by an additional dehydration/clearing step (Figure 1C); however, condensation of nuclear chromatin was evident and nuclear detail was lacking. There were fewer cracks or microfractures in the trabecular elements as compared with Figure 1A and it was much easier to distinguish osteoblasts and osteoclasts from bone (as compared with Figures 1A and 1B). Specimens fixed with 70% EtOH without an additional dehydration step (Figure 1D) also exhibited nuclear condensation and a lack of nuclear detail. However, the trabecular bone in these sections showed good integrity without significant microfractures or cracks. In these sections the osteoblasts and osteoclasts were most easily visualized adjacent to trabecular bone.
In general, if one is interested in the bone cells and trabecular bone quality, EtOH fixation would be recommended and fewer microfractures are seen in specimens not subjected to the additional 70% acetone incubation. However, if one is interested in examining bone marrow cells, then fixation in 10% NBF is recommended.
Discussion
Histological, histochemical, and immunohistochemical analysis of bone are useful techniques for examining growth and development as well as diseases associated with bone. Unfortunately, the fixation, processing, embedding, and all specimen-handling procedures, may be more suitable for one or two of these types of analyses, to the exclusion of the others (1–20). For example, it is well understood that fixative selection and fixation time affects the histological appearance of morphological details in bone specimens embedded in MMA (5,21,22). Along the same lines, with regard to histochemical and immunohistochemical detection in bone, the fixation step has been identified as the most problematic step as it must be appropriately adapted for the size and hardness of the tissue specimen. Recently Hannouche (23) reported that the optimization of fixation steps is important for the detection of enzyme activity or antigens on undecalcified bone specimens. Clearly, the multiple studies focused on optimizing histological, histochemical, enzymatic and immunohistochemical analyses speak to the technical difficulties associated with such procedures and the continuing need for improvement.
Although we recognize that every step from the choice of fixative through the mounting of slides is critical to obtaining high-quality data, in this study we focused on the effects of two fixatives and two dehydrants on the quality of sub sequent histological stains. The purpose of fixation is to harden tissue specimens and preserve their microarchitecture, as much as possible, for investigation. Fixatives must minimize the enzymatic breakdown of cellular molecules, and protect the tissue from microorganisms (24). Physical means of fixation include heat fixation, microwave fixation, freeze drying and chemical fixations. The latter falls into the two broad categories: coagulant fixatives and noncoagulant, or cross-linking, fixatives. Coagulant fixatives make proteins insoluble by coagulating them, and because a major component of cell membranes and cytoskeletons are lipoproteins and fibrous proteins, this protects the tissue architecture from degradation (25). Cross-linking fixatives join proteins with other proteins and with nucleic acids, and also cross-link nucleic acids with each other, again stabilizing the tissue architecture for histological evaluation (26). Every fixative used in the laboratory has a different capability for staining certain organelles and maintaining tissue integrity, and the choice of fixative should be based on the tissue being stained, and the specific organelle or molecule being examined.
Ethanol is a widely used coagulant fixative that acts as a dehydrant. It removes free water from the tissue specimen so proteins can coagulate and precipitate (27). Formalin is the aqueous solution of formaldehyde and is the most widely used diagnostic pathological fixative. It acts to cross-link and, thus, stabilize, a wide range of cellular molecules including nuclear proteins and nucleic acids (28–31).
Although ethanol and formalin are from two different fixative classes, each with their own strengths and weaknesses, investigators often use ethanol and formalin interchangeably. Perhaps this is because they are both readily available, and studies like this one proving one better than the other for specific tissue specimens are lacking. Here we show that fixative choice does in fact make a significant difference. Our data demonstrate that ethanol fixation and subsequent processing in MMA yields better staining results for bone cell related elements than fixing in 10% neutral buffered formalin with the same processing and embedding techniques. However, the marrow elements stained better when fixed with formalin and showed nice nuclear detail. These results are not surprising since ethanol is designed to highlight fibrous cytoskeletal proteins common in bone, while formalin cross-links nucleic acids, and would thus be expected to produce good nuclear detail.
In addition to comparing two fixatives, this study also looked at adding an additional step of clearing and dehydrating with acetone. The acetone made no difference in the final quality of the bone tissue element stains. All three stains looked better, with respect to bone cells, when fixed with ethanol. However, the bone marrow elements were best visualized when fixed with formalin. Importantly, when ethanol-fixed specimens underwent an additional acetone step, the marrow cells were more easily visualized, likely owing to the fact that acetone is a more mild dehydrant than ethanol, and it also acts as a clearant. This observation was not as apparent in formalin-fixed specimens.
Although not formally tested here, we would also like to briefly discuss the likely effects of increasing time in fixation or dehydration. With extended formalin fixation, cross-linking of proteins continues to occur long after the fixative has penetrated the tissue. This continued cross-linking may cause the tissue to harden, resulting in overhardened tissue (32). Tissue that is overfixed is less penetrable to stain, resulting in poorer staining quality. It is known that formalin reduces the quantity of cationic amino groups available for binding with stain. For example, the reaction of formaldehyde with amino group in tissue can interfere with the binding of eosin to the tissue since eosin binds to these amino groups in tissues (32). With extended ethanol fixation, the tissue can also be overhardened. Further, as ethanol also acts as a dehydrant, excessive shrinkage of the tissue may also occur. Importantly, for bone specimens, differential shrinkage of calcified structures vs. cells may occur, resulting in bone cells such as osteoblasts and osteoclasts pulling away from the edges of the osteoid or mineralized bone matrix, respectively. Cell shrinkage due to extended ethanol fixation or extended dehydration processing can result in cell morphology alterations (32). Finally, fixation influences staining rate; therefore, the length of time needed to stain the tissue may also be affected by the fixation duration (32).
Clearly, how we treat and preserve tissue specimens can have a marked impact on our results. Although this study is particularly relevant to those interested in bone biology because of the specimens chosen, its lessons are widely applicable. Investigators must consider their object of evaluation before choosing a fixative and processing their tissue.
Acknowledgments
This work was supported by the Department of Orthopaedic Surgery at Indiana University School of Medicine, the Department of Orthopaedics and Rehabilitation at Yale University School of Medicine, and by NIH AR46032, the Physiology Core of the Yale Core Center for Musculoskeletal Disorders.
References
- 1.Kacena MA, Troiano NW, Coady CE, Horowitz MC. Decalcification of mounted bone sections enhances immunohistochemical staining. J Histotechnol. 2003;26:105–109. [Google Scholar]
- 2.Kacena MA, Troiano NW, Coady CE, Horowitz MC. Effects of ethanol post-fixation on the histological, histochemical, and immunohistochemical analysis of murine bone specimens. J Histotechnol. 2004;27:15–20. [Google Scholar]
- 3.Kacena MA, Troiano NW, Wilson KM, Coady CE, Horowitz MC. Evaluation of two different methylmethacrylate processing, infiltration, and embedding techniques on the histological, histochemical, and immunohistochemical analysis of murine bone specimens. J Histotechnol. 2004;27:119–130. [Google Scholar]
- 4.Kacena MA, Halfon JK, Coady CE, Nelson T, Troiano NW. Optimization of methylmethacrylate infiltration duration in undecalcified murine bone specimens. J Histotechnol. 2006;29:21–27. [Google Scholar]
- 5.Lebeau A, Muthmann H, Sendelhofert A, Diebold J, Lohrs U. Histochemistry and immunohistochemistry on bone marrow biopsies. A rapid procedure for methyl methacrylate embedding. Pathol Res Pract. 1995;191:121–129. doi: 10.1016/S0344-0338(11)80561-3. [DOI] [PubMed] [Google Scholar]
- 6.Baron R, Vignery A, Neff L, Silverglate A, Santa Maria A. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker R, editor. Bone Histomorphometry: Techniques and Interpretation. Boca Raton, FL: CRC Press Inc; 1983. pp. 13–35. [Google Scholar]
- 7.Lillie RD, Fulmer HM. Histopathologic Technic and Practical Histochemistry. 4. New York: McGraw-Hill; 1976. p. 657. [Google Scholar]
- 8.Conn HJ, Lillie RD. Biological Stains. 9. Baltimore: Waverly Press; 1977. p. 387. [Google Scholar]
- 9.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. 2. St. Louis: The C.V. Mosby Company; 1980. Bone; pp. 89–96. [Google Scholar]
- 10.Arrington JB, Prophet EB. Solution Preparation. In: Prophet EB, Mills B, Arrington JB, Sobin LH, editors. AFIP Laboratory Methods in Histotechnology. Washington, DC: American Registry of Pathology; 1992. p. 18. [Google Scholar]
- 11.Gaffney E. Carbohydrates. In: Prophet EB, Mills B, Arrington JB, Sobin LH, editors. AFIP Laboratory Methods in Histotechnology. Washington, DC: American Registry of Pathology; 1992. p. 167. [Google Scholar]
- 12.Beckstead JH. Optimal antigen localization in human tissues using aldehyde-fixed plastic-embedded sections. J Histochem Cytochem. 1985;9:954–958. doi: 10.1177/33.9.4020104. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell D, Ibrahim S, Gusterson BA. Improved immunohistochemical localization of tissue antigens using modified meth-acarn fixation. J Histochem Cytochem. 1985;33:491–495. doi: 10.1177/33.5.3921605. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MB, Miller TR, Beckstead JH. Enzyme histochemistry and thyroid neoplasia. Am J Clin Pathol. 1986;85:668–673. doi: 10.1093/ajcp/85.6.668. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Mukai K, Watanabe S, Goto M, Shimosato Y. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 1986;125:431–435. [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt GM, Schmidt SP. The effects of different fixatives for immunofluorescent and immunoperoxidase localization of factor VIII-related antigen in canine carotid artery and vascular prostheses. Histochem J. 1986;18:351–356. doi: 10.1007/BF01675215. [DOI] [PubMed] [Google Scholar]
- 17.Azumi N, Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol. 1987;88:286–296. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- 18.Pollard K, Lunny D, Holgate CS, Jackson P, Bird CC. Fixation, processing, and immunochemical reagent effects on preservation of T-lymphocyte surface membrane antigens in paraffin-embedded tissue. J Histochem Cytochem. 1987;35:1329–1338. doi: 10.1177/35.11.3309048. [DOI] [PubMed] [Google Scholar]
- 19.Randall HW, Bogdanffy MS, Morgan KT. Enzyme histochemistry of the rat nasal mucosa embedded in cold glycol methacrylate. Am J Anat. 1987;179:10–17. doi: 10.1002/aja.1001790103. [DOI] [PubMed] [Google Scholar]
- 20.Sakr WA, Zarbo RJ, Jacobs JR, Crissman JD. Distribution of basement membrane in squamous cell carcinoma of the head and neck. Hum Pathol. 1987;18:1043–1050. doi: 10.1016/s0046-8177(87)80221-6. [DOI] [PubMed] [Google Scholar]
- 21.Bernhards J, Weitzel B, Werner M, Rimpler M, Georgii A. A new histological embedding method by low-temperature polymerisation of methyl methacrylate allowing immuno- and enzyme histochemical studies on semi-thin sections of undecalcified bone marrow biopsies. Histochem. 1992;98:145–154. doi: 10.1007/BF00315873. [DOI] [PubMed] [Google Scholar]
- 22.Erben RG. Embedding of bone samples in methylmethacrylate: An improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem Cytochem. 1997;45:307–313. doi: 10.1177/002215549704500215. [DOI] [PubMed] [Google Scholar]
- 23.Hannouche D, Raould A, Nizard RS, Sedel L, Petite H. Embedding of bone samples in methylmethacrylate: A suitable method for tracking LacZ mesenchymal stem cells in skeletal tissues. J Histochem Cytochem. 2007;55:255–262. doi: 10.1369/jhc.6A7063.2006. [DOI] [PubMed] [Google Scholar]
- 24.Titford M. Safety considerations in the use of fixatives. J Histotechnol. 2001;24:165–171. [Google Scholar]
- 25.Horobin RW. Histochemistry. Stuttgart: Gustav Fischer; 1982. An explanatory outline of histochemistry and biophysical staining. [Google Scholar]
- 26.Grizzle WE. Theory and practice of silver staining in histopathology. J Histotechnol. 1996;19:183–195. [Google Scholar]
- 27.Eltoum I, Fredenburgh J, Myers RB, Grizzle WE. Introduction to the theory and practice of fixation of tissues. J Histotechnol. 2001;24:173–189. [Google Scholar]
- 28.McGhee JD, von Hippel PH. Formaldehyde as a probe of DNA structure. I. Reaction with exocyclic amino groups of DNA bases. Biochem. 1975;14:1281–1296. doi: 10.1021/bi00677a029. [DOI] [PubMed] [Google Scholar]
- 29.McGhee JD, von Hippel PH. Formaldehyde as a probe of DNA structure. II. Reaction with endocyclic imino groups of DNA bases. Biochem. 1975;14:1297–1303. doi: 10.1021/bi00677a030. [DOI] [PubMed] [Google Scholar]
- 30.McGhee JD, von Hippel PH. Formaldehyde as a probe of DNA structure.3. Equilibrium denaturation of DNA and synthetic polynucleotides. Biochem. 1977;16:3267–3276. doi: 10.1021/bi00634a001. [DOI] [PubMed] [Google Scholar]
- 31.Chang YT, Loew GH. Reaction mechanisms of formaldehyde with endocyclic amino groups of nucleic acid bases. J Am Chem Soc. 1994;116:3548–3555. [Google Scholar]
- 32.Carson FL. Histotechnology, A Self-Instructional Text. 2. Singapore: American Society for Clinical Pathology Press; 2007. Fixation; pp. 1–24. [Google Scholar]