Abstract
In the setting of high salt intake, aldosterone stimulates fibrosis in the heart, great vessels, and kidney of rats. We used uninephrectomized rats treated with angiotensin II and placed on a high salt diet to exaggerate renal fibrosis. We then tested whether mineralocorticoid receptor blockade by spironolactone or aldosterone synthase inhibition by FAD286 have similar effects on end-organ damage and gene expression. Individually, both drugs prevented the hypertensive response to uninephrectomy and high salt intake but not when angiotensin II was administered. Following 4 weeks of treatment with FAD286, plasma aldosterone was reduced, whereas spironolactone increased aldosterone at 8 weeks of treatment. Angiotensin II and high salt treatment caused albuminuria, azotemia, renovascular hypertrophy, glomerular injury, increased plasminogen activator inhibitor-1 (PAI-1), and osteopontin mRNA expression, as well as tubulointerstitial fibrosis in the kidney. Both drugs prevented these renal effects and attenuated cardiac and aortic medial hypertrophy while reducing osteopontin and transforming growth factor-β mRNA expression in the aorta. The two drugs also reduced cardiac interstitial fibrosis but had no effect on that of the perivascular region. Although spironolactone enhanced angiotensin II and salt-stimulated PAI-1 mRNA expression in aorta and heart, spironolactone and FAD286 prevented renal PAI-1 mRNA protein expression. Our study shows that mineralocorticoid receptor antagonism and aldosterone synthase inhibition similarly decrease hypertrophy and interstitial fibrosis of the kidney and heart caused by angiotensin II and high salt.
Keywords: aldosterone, mineralocorticoid receptor, plasminogen activator inhibitor-1
Recent studies highlight the important contribution of aldosterone to cardiovascular and renal morbidity and mortality. In rats, in the setting of high salt intake, aldosterone activates NFκB and stimulates an inflammatory response followed by fibrosis in the heart, great vessels, and kidney.1–6 Conversely, mineralocorticoid receptor (MR) antagonism reduces angiotensin II (Ang II)-induced activation of NF-κB, oxidative stress, inflammation and cardiac fibrosis, aortic remodeling, and renal injury in many models.1,7–10 Clinical studies confirm the relevance of these findings in humans. During chronic therapy with an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, aldosterone concentrations can ‘escape’ to pretreatment levels.11 MR antagonism decreases markers of myocardial remodeling and mortality in congestive heart failure,12,13 and decreases albuminuria in diabetic and hypertensive nephropathy.14
MR activation by corticosterone or by transactivation by the AT1 receptor may contribute to end-organ damage, and aldosterone may also act through MR-independent effects.15–19 The availability of the pharmacological aldosterone synthase inhibitor, FAD286, provides a tool to examine the role of endogenous aldosterone without blocking the MR. Fiebeler and co-workers20 report that treatment with FAD286 or adrenalectomy significantly reduced mortality, cardiac hypertrophy, albuminuria, and cardiac and renal inflammation in rats doubly transgenic for the human renin and angiotensinogen genes. In this study, we tested the hypothesis that MR antagonism and aldosterone synthase inhibition have similar effects on end-organ damage and gene expression in Ang II/salt-treated rats.
RESULTS
Blood pressure, RAAS parameters, electrolytes and renal function
Table 1 shows the mean systolic blood pressure (SBP) in each treatment group measured biweekly by tail-cuff plethysmography over the 8-week study period. SBP was significantly increased by uninephrectomy/salt or by uninephrectomy and Ang II/salt administration. Spironolactone or FAD286 reduced SBP following uninephrectomy alone, but neither drug lowered blood pressure during Ang II infusion. SBP was measured at least twice by indwelling carotid artery catheter in a small number of animals in each group between 2 and 5 weeks of therapy, and showed a similar pattern to tail-cuff plethysmography: sham (N = 3) 118.9±9.3 mm Hg, uninephrectomy/salt (N = 2) 146.3±8.2 mm Hg, uninephrectomy/salt±spironolactone (N = 5) 132.5±9.0 mm Hg, uninephrectomy/salt + FAD286 (N = 4) 128.9±15.6 mm Hg, uninephrectomy±Ang II/salt (N = 4) 147.5±11.3 mm Hg, uninephrectomy + Ang II/salt + spironolactone (N = 4) 140.8±13.0 mm Hg, and uninephrectomy + Ang II/salt-FAD286 (N = 3) 146.0±10.9 mm Hg.
Table 1.
Effect of treatment on systolic blood pressure, electrolytes, and renal function
| Sham (n=5) | Uninephrectomy (n=7) | Uninephrectomy+ spironolactone (n=6) | Uninephrectomy+ FAD286 (n=6) | Uninephrectomy+ Ang II (n=7) | Uninephrectomy+ Ang II+spironolactone (n=6) | Uninephrectomy+ Ang II+FAD286 (n=7) | |
|---|---|---|---|---|---|---|---|
| SBP (mm Hg) | 157.7±2.7 | 168.0±2.4* | 159.1±2.2 | 162.9±2.5 | 172.2±2.5* | 171.7±2.5* | 167.4±2.4* |
| Serum Na+(mmol/l) | 137.3±0.5 (N=4) | 138.4±1.1 | 137.6±2.2 (N=5) | 138.2±2.6 | 138.7±2.2 | 140.8±3.6*,‡ (N=4) | 138.3±1.3 (N=4) |
| Serum K+(mmol/l) | 5.6±0.6 | 5.1±0.2 | 5.9±0.3† | 5.5±0.3 | 5.3±0.3 | 5.40±.2 | 5.5±0.1 |
| pH | 7.47±0.03 (N=4) | 7.43±0.04 (N=4) | 7.46±0.05 (N=5) | 7.42±0.07 (N=5) | 7.44±0.07 (N=6) | 7.44±0.03 (N=3) | 7.38±0.06*‡(N=6) |
| HCO3 | 26.6±2.2 (N=4) | 28.8±2.9 (N=4) | 28.0±1.6 (N=4) | 26.9±2.5 (N=4) | 27.1±1.6 (N=4) | 27.7±0.3 (N=4) | 27.0±2.7 (N=4) |
| BUN (mg/100 ml) | 22.5±2.1 | 24.9±2.0 | 26.6±1.1 | 26.2±1.9 | 46.7±8.0*,†,‡,§,||,¶ | 23.8±0.9 | 30.8±0.6 |
| Urine K+ (mmol/l) | 132±29 | 92±25 | 72±14 | 80±18 | 58±14 | 67±18 | 95±23 |
| Urine Na+ (mmol/l) | 245±44 | 221±31 | 269±23 | 243±49 | 205±66 | 214±83 | 275±115 |
| Urine volume (ml) | 22.2±4.8 | 45.6±17.2* | 48.0±4.0* | 47.0±10.6* | 56.0±11.0* | 48.8±14.3* | 53.3±38.2* |
| Urine Na+/K+ | 2.0±0.2 | 3.1±0.4 | 4.3±0.6* | 3.4±0.4 | 4.2±1.8* | 3.7±1.0* | 4.1±2.2* |
| Urine albumin/Cr (μg/mg) | 460±226 | 1027±407 | 314±114 | 1062±488 | 3583±1606* | 2621±1074 | 2537±1694 |
Ang II, angiotensin II; Bun, blood urea nitrogen; SBP, systolic blood pressure.
P < 0.05 versus sham,
P < 0.05 versus uninephrectomy,
P < 0.05 versus uninephrectomy+spironolactone,
P < 0.05 versus uninephrectomy+FAD286,
P < 0.05 versus uninephrectomy+Ang II+spironolactone,
P < 0.05 versus uninephrectomy+Ang II+FAD286.
Serum potassium concentrations and urinary sodium and potassium excretion were similar among Ang II/salt-treated groups (Table 1). Ang II/salt significantly increased serum blood urea nitrogen and urine albumin excretion. Treatment with either spironolactone or FAD286 attenuated this effect.
As illustrated in Table 2, Ang II/salt treatment significantly increased circulating aldosterone concentrations at 4 weeks. FAD286, but not spironolactone, prevented this effect. In contrast, at 8 weeks, plasma aldosterone was increased only in the uninephrectomy + Ang II/salt + spironolactone group. Corticosterone concentrations were similar among treatment groups at 4 weeks. Although corticosterone concentrations were higher in the uninephrectomy + FAD286 group at 8 weeks, corticosterone concentrations were not different among the Ang II/salt-treated groups. Circulating active plasminogen activator inhibitor-1 (PAI-1) antigen concentrations were increased in the uninephrectomy + Ang II/salt + spironolactone group at 4 weeks, whereas by 8 weeks, PAI-1 concentrations were increased only in the uninephrectomy + Ang II/salt group.
Table 2.
Effect of treatment on aldosterone, corticosterone, and plasminogen activator inhibitor-1
| Sham (n=4) | Uninephrectomy (n=7) | Uninephrectomy+ spironolactone (n=6) | Uninephrectomy+ FAD286 (n=6) | Uninephrectomy+ Ang II (n=6) | Uninephrectomy+Ang II +spironolactone (n=6) | Uninephrectomy+Ang II +FAD286 (n=7) | |
|---|---|---|---|---|---|---|---|
| Aldosterone (pg/ml) | |||||||
| 4 weeks | 208±75 | 168±40 | 142±18 | 131±36 | 472±159*,†,‡,§,|| | 374±160‡,§ | 139±10 |
| 8 weeks | 185±50 | 151±41 | 89±23 | 101±19 | 229±68 | 305±135‡,§,|| | 121±41 |
| Corticosterone (ng/ml) | |||||||
| 4 weeks | 444±49 | 506±86 | 468±35 | 436±79 | 504±83 | 453±45 | 447±52 |
| 8 weeks | 289±96 | 313±51 | 231±45 | 389±30‡,|| | 287±60 | 299±59 | 228±40 |
| PAI-1 (ng/ml) | |||||||
| 4 weeks | 0.8±0.2 | 0.6±0.3 | 0.4±0.1 | 0.6±0.1 | 1.2±0.4 | 2.7±1.0*,†,‡,§,||,¶ | 0.7±0.1 |
| 8 weeks | 0.9±0.2 | 0.8±0.3 | 0.2±0.0 | 0.4±0.1 | 4.0±2.9‡,§,# | 0.5±0.1 | 0.7±0.3 |
P < 0.05 versus sham,
P < 0.05 versus uninephrectomy,
P < 0.05 versus uninephrectomy+spironolactone,
P < 0.05 versus uninephrectomy+FAD286,
P < 0.05 versus uninephrectomy+Ang II+FAD286,
P < 0.05 versus uninephrectomy+Ang II,
P < 0.05 versus uninephrectomy+Ang II+spironolactone.
Tissue morphology and histology
Uninephrectomy caused significant hypertrophy of the remaining kidney during high salt intake, and there was no effect of spironolactone or FAD286 on this response (Figure 1). Ang II infusion caused a further increase in kidney weight. Spironolactone or FAD286 significantly and equivalently prevented Ang II/salt-induced renal hypertrophy. Ang II/salt infusion induced glomerulosclerosis, tubulointerstitial fibrosis, and renal artery hypertrophy. Either spironolactone or FAD286 prevented these effects, and there was no difference in the degree of glomerulosclerosis, tubulointerstitial fibrosis, or renal artery hypertrophy in spironolactone-versus FAD286-treated rats.
Figure 1. Effect of treatment on kidney injury.
Effect of treatment on (a) kidney weight, (b) glomerular sclerosis, (c) tubulointerstitial fibrosis, and (d) renal vascular modeling. For post hoc comparisons, *P < 0.01 versus sham, †P < 0.05 versus uninephrectomy (Neph), ‡P < 0.05 versus uninephrectomy + spironolactone (SPL), §P < 0.05 versus uninephrectomy + FAD286, ||P < 0.05 versus uninephrectomy + Ang II + spironolactone, ¶P < 0.05 versus uninephrectomy + Ang II + FAD286. Masson’s trichrome (original magnification ×100) of the kidney in (e) control, (f) Ang II/salt-treated, (g) Ang II/salt + spironolactone-treated, and (h) Ang II/salt + FAD286-treated. The asterisks in (f) denote areas of tubulointerstitial fibrosis.
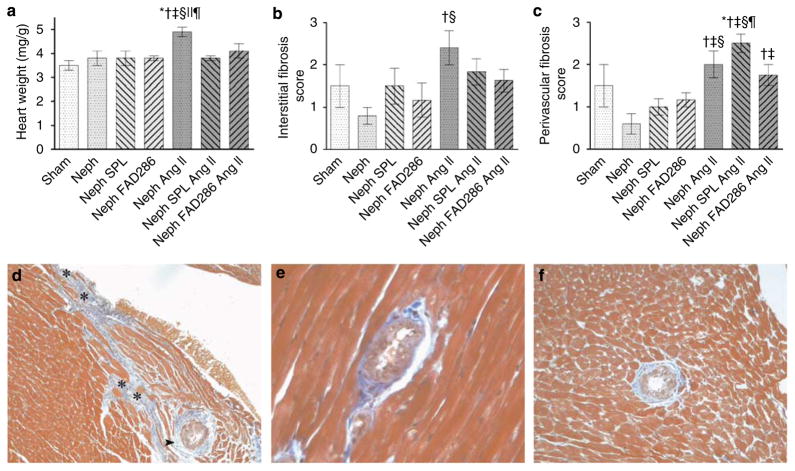
Uninephrectomy and high salt alone did not affect heart-to-body weight ratio. Ang II/salt caused cardiac hypertrophy, which was prevented by both spironolactone and FAD286 (Figure 2a). In the heart, Ang II/salt induced interstitial fibrosis, which was more pronounced in the subendocardial regions and perivascular fibrosis (Figure 2b–f). Either spironolactone or FAD286 prevented the development of interstitial fibrosis. Neither drug significantly decreased Ang II/salt-stimulated perivascular fibrosis; however, FAD286 reduced Ang II/salt-induced perivascular fibrosis compared with spironolactone (Figure 2c).
Figure 2. Effect of treatment on cardiac injury.
Effect of treatment on (a) heart weight, (b) cardiac interstitial fibrosis, and (c) perivascular adventitial fibrosis. For post hoc comparisons, *P < 0.01 versus sham, †P < 0.05 versus uninephrectomy (Neph), ‡P < 0.05 versus uninephrectomy + spironolactone (SPL), §P < 0.05 versus uninephrectomy + FAD286, ||P < 0.05 versus uninephrectomy + Ang II + spironolactone, ¶P < 0.05 versus uninephrectomy + Ang II + FAD286. (d) Focal area of subendocardial interstitial and perivascular fibrosis in heart from Ang II-treated rat (Masson’s trichrome, original magnification ×100). Persistent perivascular fibrosis in Ang II/salt-treated rats receiving (e) spironolactone (×400) and (f) FAD286. The asterisks in (d) denote cardiac interstitial fibrosis; arrowhead denotes perivascular/periadventitial fibrosis.
Ang II/salt caused aortic medial thickening, which was prevented during treatment with spironolactone or FAD286 (medial area 10,629±819, 9667±908, and 7749±981 μm2 in Ang II/salt, Ang II/salt + spironolactone, and Ang II/salt-FAD286, respectively; P < 0.05 for Ang II/salt versus all other groups). Adventitial thickness was statistically similar among the treatment groups.
Expression of profibrotic genes
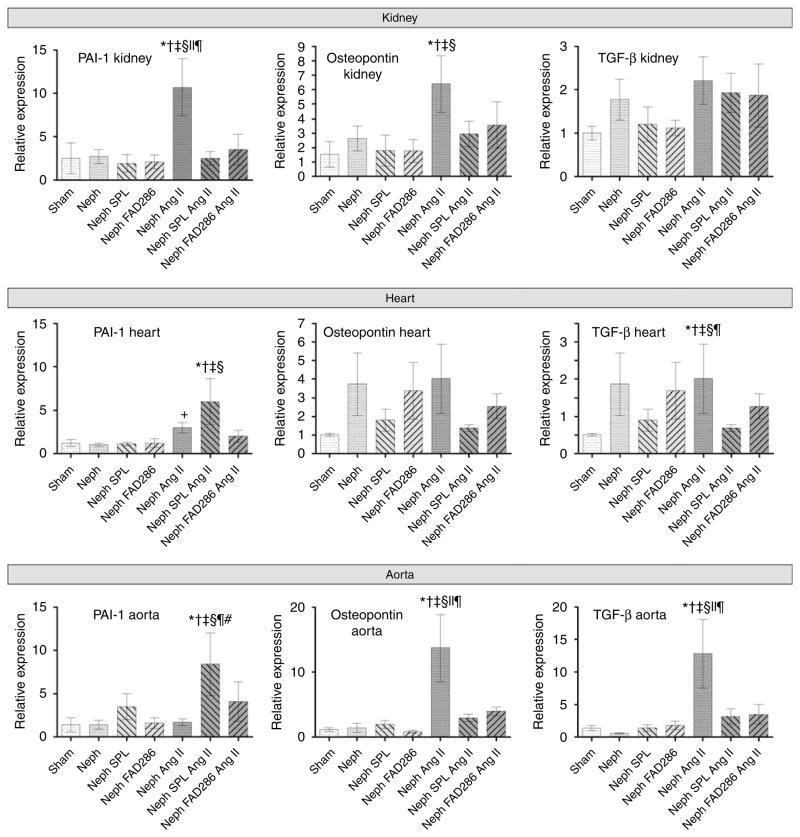
In the kidney, Ang II/salt increased osteopontin and PAI-1 mRNA expression, and tended to increase tumor growth factor-β (Figure 3). Either spironolactone or FAD286 decreased Ang II/salt-stimulated osteopontin and PAI-1 mRNA expression in the kidney. Ang II/salt increased osteopontin and tumor growth factor-β expression in the aorta (Figure 3), and spironolactone or FAD286 prevented these effects. In the heart, Ang II/salt significantly increased PAI-1 expression. However, during chronic Ang II/salt treatment, spironolactone enhanced PAI-1 mRNA expression in the heart and aorta and tumor growth factor-β1 mRNA expression in the heart. Cardiac PAI-1 mRNA expression correlated with the perivascular fibrosis score (r = 0.362, P = 0.023). By in situ hybridization PAI-1 mRNA expression localized predominantly to the vasculature of the heart and to the aortic adventitia (not shown). By western blot, PAI-1 protein was increased 10% in the heart of Ang II/salt-treated mice (P < 0.001 versus nephrectomy alone), but not in the heart of Ang II/salt + spironolactone or Ang II/salt + FAD286 mice. By immunohistochemistry, PAI-1 staining in the myocytes and vascular smooth muscle cells (VSMCs) was significantly increased in Ang II/salt-treated animals (P = 0.04) but there was no difference among Ang II/salt, Ang II/salt + spironolactone, and Ang II/salt + FAD286 (average score 2.1±0.9, 3.1±0.9, 3.6±1.5, and 3.5±0.6 in uninephrectomy alone, uninephrectomy + Ang II/salt, uninephrectomy + Ang II/salt + spironolactone, and uninephrectomy + Ang II/salt + FAD, respectively).
Figure 3. Effect of treatment on expression of PAI-1, osteopontin, and tumor growth factor β (TGF-β) mRNA in the kidney, heart, and aorta.
For post hoc comparisons, *P < 0.05 versus sham, †P < 0.01 versus uninephrectomy (Neph), ‡P < 0.01 versus uninephrectomy + spironolactone (SPL), §P < 0.01 versus uninephrectomy + FAD286, ||P < 0.01 versus uninephrectomy + Ang II + spironolactone, ¶P < 0.01 versus uninephrectomy + Ang II + FAD286, #P < 0.01 versus uninephrectomy + Ang II.
Effect of spironolactone on Ang II and aldosterone-induced PAI-1 mRNA expression in VSMCs in vitro
Aldosterone (10−6 M) increased PAI-1 mRNA expression and enhanced the effect of Ang II (10−6 M) on PAI-1 expression in cultured rat VSMCs (Figure 4). Spironolactone (10−6 M) prevented Ang II-stimulated PAI-1 mRNA expression and attenuated the effect of Ang II + aldosterone on PAI-1 expression such that the PAI-1 mRNA expression was similar to that after treatment with aldosterone alone but still increased compared with control. Spironolactone had no effect on aldosterone-induced PAI-1 expression.
Figure 4. Effect of spironolactone (SPL, 1 μM) on PAI-1 expression in rat vascular smooth muscle cells treated with angiotensin II (Ang II, 1 μM), aldosterone (aldo, 1 μM) or the combination.
*P < 0.05 versus vehicle, †P < 0.05 versus Ang II alone, ‡P < 0.05 versus spironolactone, §P < 0.05 versus aldo + Ang II.
DISCUSSION
This study compared two different pharmacological strategies for negating the effect of aldosterone on Ang II/salt-induced end-organ damage–MR antagonism and aldosterone synthase inhibition. We hypothesized that MR antagonism would have a greater effect on Ang II/salt-induced end-organ damage if Ang II activated the MR through an aldosterone-independent mechanism. Conversely, we hypothesized that aldosterone synthase inhibition would have a greater effect on Ang II/salt-induced end-organ damage if aldosterone contributed to damage through an MR-independent mechanism. As reported earlier in separate studies of spironolactone and FAD286,1,6,7,9,10,20,21 however, both agents prevented Ang II/salt-induced renal injury, cardiac and aortic hypertrophy, and cardiac interstitial fibrosis through a blood pressure-independent mechanism. Neither spironolactone nor FAD286 prevented Ang II/salt-induced perivascular fibrosis at the doses administered.
Numerous earlier studies of MR antagonism have established that spironolactone or eplerenone decreases cardiac hypertrophy and interstitial fibrosis, aortic medial thickening, renal hypertrophy, tubulointerstitial fibrosis, and glomerular injury, as in this study.1,6,7,9,10,21 Likewise, Fiebeler et al.20 showed earlier that FAD286 decreased aldosterone concentrations, cardiac hypertrophy, albuminuria, and tubulointerstitial fibrosis in doubly transgenic rats overexpressing the human renin and angiotensin genes (dTGR). The authors did not report any effect of FAD286 on cardiac fibrosis or aortic remodeling in that study. Minnaard-Huiban et al. 22 have also reported that FAD286A, the R(+)-enantiomer fadrozole, and the MR antagonist canrenoate similarly reversed preexisting cardiac fibrosis in spontaneously hypertensive heart failure rats. To the best of our knowledge, however, this study is the first to compare the effects of pharmacological MR antagonism and aldosterone synthase inhibition on renal injury. Both spironolactone and FAD286 prevented Ang II/salt-induced albuminuria and glomerular injury. The equivalent effects of spironolactone and FAD286 on Ang II/salt-induced renal injury, cardiac interstitial fibrosis, and cardiac and aortic hypertrophy suggest that endogenous aldosterone induces these effects through an MR-dependent mechanism.
In contrast to the effect of spironolactone and FAD286 on cardiac hypertrophy and interstitial fibrosis, neither drug attenuated Ang II/salt-induced perivascular fibrosis in the heart at the doses studied. The effect of FAD286 on perivascular fibrosis has not been reported earlier. The lack of effect of spironolactone on perivascular coronary fibrosis, in contrast to interstitial fibrosis, may relate to dose. MR antagonism reduces perivascular fibrosis in the Ang II/salt model in part through a blood pressure-dependent mechanism. For example, Neves et al 10 reported that, in contrast to the MR-dependent effect of spironolactone on cardiac interstitial fibrosis, blood pressure reduction with either spironolactone or hydralazine decreased perivascular fibrosis in Ang II/salt-treated rats.
We administered spironolactone at a dose of 5.8 mg/kg per d, whereas many earlier studies of the effect of spironolactone on cardiac fibrosis utilized doses of 20–30 mg/kg per d or higher. We chose this lower dose to avoid an antihypertensive effect. Earlier studies indicate that acute doses of spironolactone as low as 3 mg/kg inhibit aldosterone binding in tissue in vivo and that chronic doses of 4–5.8 mg/kg per d spironolactone decrease cardiac fibrosis or improve vascular function.23,24 In addition, the peak plasma concentration of spironolactone measured after administration of 5 mg/kg in the rat (560 ng/ml)25 is 8-fold higher than the peak plasma concentration (80 ng/ml) reported in humans after administration of 100 mg spironolactone. Importantly, the observation that spironolactone increased serum aldosterone concentrations at 8 weeks in Ang II/salt-treated rats and that spironolactone prevented Ang II/salt-induced cardiac hypertrophy and interstitial fibrosis, as well as renal injury, indicates that systemic MR blockade was achieved.
The lack of effect of spironolactone on cardiac perivascular fibrosis in Ang II/salt-treated rats could result from MR-independent effects of aldosterone. In this regard, Danser and co-workers have reported that aldosterone enhances Ang II-mediated coronary vasoconstriction through protein kinase C and extracellular signal-regulated kinase 1/2-dependent, but MR-independent, pathways.26 It is interesting to note that although spironolactone enhanced the effect of Ang II/salt on circulating aldosterone concentrations in this study, a beneficial effect of spironolactone on cardiac perivascular fibrosis in other studies has been associated with decreased or unchanged plasma or urinary aldosterone concentrations.1,8,27 Alternatively, non-specific effects of spironolactone, such as calcium channel blockade, could have contributed to the differential effects of spironolactone and FAD286.28,29
Spironolactone and FAD286 attenuated osteopontin mRNA expression in the kidneys and aortae of Ang II/salt-treated rats, in agreement with earlier studies indicating that aldosterone increases osteopontin expression through an MR-dependent mechanism.6,21 Likewise, both spironolactone and FAD286 decreased renal PAI-1 expression, just as aldosterone has been shown earlier to increase, and spironolactone to decrease, PAI-1 expression in the kidney.30–32 FAD286 also decreased PAI-1 mRNA expression in the heart in Ang II/salt-treated rats consistent with the data indicating that exogenous aldosterone increases PAI-1 expression in the heart in vivo.32 Spironolactone enhanced Ang II/salt-stimulated PAI-1 mRNA expression in the aorta and heart. Increased aldosterone at 8 weeks in Ang II/salt + spironolactone-treated rats may have contributed to this effect on aortic and cardiac mRNA expression. On the other hand, circulating PAI-1 antigen was increased at 4 weeks and not 8 weeks during Ang II/salt + spironolactone. Moreover, both spironolactone and FAD286 prevented the stimulatory effect of Ang II on PAI-1 protein expression in the heart, consistent with an earlier report of an inhibitory effect of spironolactone on cardiac PAI-1 expression in Ang II/L-NAME (NG-nitro-L-arginine methyl ester)-treated mice.33
To explore the possibility that ambient aldosterone concentrations influence the effect of MR blockade on Ang II/salt-stimulated PAI-1 mRNA expression in the vasculature, we examined the effect of high dose spironolactone on PAI-1 expression in cultured VSMCs. We chose to study the effect of spironolactone in VSMCs due to the vascular localization of cardiac PAI-1 expression by in situ hybridization, and because the effect of spironolactone on Ang II-stimulated PAI-1 expression has not been reported in this cell type. Ang II alone increased PAI-1 expression. Consistent with earlier studies showing that Ang II can activate the MR,16,34 spironolactone attenuated Ang II-stimulated PAI-1 expression suggesting that Ang II increases PAI-1 expression through an MR-dependent mechanism in VSMCs. In contrast, aldosterone increased PAI-1 expression through an MR-independent mechanism. The data suggest that in vivo, chronically elevated aldosterone concentrations contribute to increased perivascular PAI-1 mRNA expression during concurrent treatment with Ang II/salt and spironolactone.
In summary, activation of the renin–angiotensin–aldosterone system (RAAS) promotes cardiovascular and renal injury. Aldosterone may act through MR-independent effects, whereas MR activation by ligands other than aldosterone may contribute to end-organ damage. Data from this study indicate that during high salt intake and chronic elevation of Ang II, endogenous aldosterone and MR activation promote renal hypertrophy, glomerulosclerosis, tubulointerstitial fibrosis, vascular remodeling, and profibrotic gene expression in the kidney. Endogenous aldosterone also contributes to cardiac and aortic hypertrophy and cardiac interstitial fibrosis through the MR, whereas neither MR antagonism nor aldosterone synthase inhibition decreased perivascular fibrosis at the doses administered. MR antagonism and aldosterone synthase inhibition have similar protective effects.
MATERIALS AND METHODS
Rats
Male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN, USA) were housed in a temperature-controlled facility with a fixed light–dark cycle. All protocols and animal procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Experimental protocol
Eight-week-old rats were randomized to one of seven treatment groups: (1) sham-operated control rats provided 1% NaCl in the drinking water, vehicle by osmotic mini-pump (Alzet, Alza Corp., Palo Alto, CA, USA) and placebo chow; (2) uninephrectomized control rats given 1% NaCl, vehicle and placebo chow; (3) uninephrectomized rats given 1% NaCl, vehicle and spironolactone chow; (4) uninephrectomized control rats given 1% NaCl, vehicle, and FAD286 chow; (5) uninephrectomized rats given 1% NaCl, Ang II (1 μg/h) by mini-pump, and vehicle chow; (6) uninephrectomized rats given 1% NaCl, Ang II, and spironolactone chow; and (7) uninephrectomized rats given 1% NaCl, Ang II, and FAD286 chow. Drugs were formulated in standard rodent chow by TestDiet (Richmond, IN, USA) at concentrations calculated to provide doses of 5.8 mg/kg/d spironolactone and 4 mg/kg/d FAD286 HCl salt, based on studies published earlier indicating that these doses decreased end-organ damage.20,23
At randomization, uninephrectomy and mini-pump implantation were performed under isoflurane anesthesia. Pumps were explanted and replaced under anesthesia at 4 weeks. Blood pressure was measured before randomization and at every 2 weeks for 8 weeks using tail-cuff plethysmography (BP-2000 Blood Pressure Analysis System, Visitech Systems, Apex, NC, USA) in unanesthetized, trained rats pre-warmed for 30 min at 37 °C. In addition, carotid artery catheters were initially inserted for direct arterial blood pressure measurements at the time of pump implantation in a subset of animals within each group, but not in all rats, due to difficulty maintaining catheter patency. Blood (400 μl) was collected via the saphenous vein under isoflurane anesthesia before randomization and at 4 and 8 weeks. Fifty microliters of blood was collected directly into an iSTAT EC8 + cartridge (Heska Corp., Loveland, CO, USA) for determination of blood chemistries. The remaining blood was collected into chilled EDTA-coated capillary tubes (Microvette CB K2E, Sarstedt AG & Co., Numbrecht, Germany), centrifuged immediately at 6000 rpm for 5 min and plasma was stored at −80 °C. For PAI-1 concentrations, blood was collected into acidified citrate. Rats were housed in individual metabolic cages (Nalgene, Braintree Scientific, Braintree, MA, USA) at 0, 4, and 8 weeks for 24-h urine collection.
Animals were euthanized with pentobarbital and blood was collected through cardiac puncture. The renal artery was clamped and blood was drawn from the right ventricle. The base of the heart, the descending aorta, the adrenal glands, sections of kidney and liver, were fixed in 4% buffered paraformaldehyde overnight, and then processed and embedded in paraffin for histological evaluation. The remaining heart, aorta, kidney and liver, were snap frozen in liquid nitrogen for mRNA analysis.
Laboratory assays
Serum sodium, potassium, chloride, pH, HCO3, and blood urea nitrogen were measured by ion-selective electrode potentiometry. Blood urea nitrogen was first hydrolyzed to ammonium by the enzyme urease. Urine albumin was determined using an immuno-turbidimetric assay for albumin and colorimetric assay for creatinine (DCA2000R + Analyzer and Microalbumin/creatinine cartridges, Bayer Corp., Ekhart, IN, USA). Urine sodium and potassium were measured by flame photometry. Plasma aldosterone was measured using a commercially available radioimmunoassay (MP Biomedicals, Irvine, CA, USA), a primary antibody to aldosterone (NIDDK National Hormone and Peptide Program, Torrance, CA, USA), and a secondary anti-rabbit gamma globulin antibody (Linco Research, St Charles, MO, USA). Plasma corticosterone was measured using a commercially available radioimmunoassay kit (ImmuChem Double Antibody Corticosterone Kit, MP Biomedicals, Irvine, CA, USA). Active PAI-1 antigen was measured using a two-site ELISA method (ZYMUTEST, RK003A, DiaPharma, West Chester, OH, USA).
Histopathological analysis
Investigators unaware of treatment assignment assessed morphology. To evaluate left ventricular free wall thickness, and aortic adventitial and medial thickness, Masson’s trichrome-stained aortic and cardiac cross-sections were photographed on a Zeiss AxioScop 40 using MRGrab 1.0. and analyzed using ImageJ software (NIH, UK). Cardiac and renal interstitial fibrosis were scored from 0 to 4, with 0 indicating no fibrosis, 1 indicating 0–10%, 2 indicating 10–25%, 3 indicating 26–50%, and 4 indicating 51–75% involvement of tissue by fibrosis. Coronary arteries were graded as normal (0), or as showing mild perivascular fibrosis (1), hypertrophy with fibrosis and some collagen within the media (2), or extensive hypertrophy and perivascular fibrosis with periadvential mononuclear cell infiltrate (3). Renal arteries were graded as normal (0), or as showing mild (1), moderate (2), or severe hypertrophy (3). Glomerular sclerosis was graded on a scale of 0 to 4, with 0 indicating normal, 1 indicating 1–10% of glomeruli involved with sclerosis, 2 indicating 19–25% of glomeruli involved, 3 indicating 26–50%, and 4 indicating >50% of glomeruli sclerosed. PAI-1 immunohistochemistry was performed on heart and aortae. Staining of myocytes, VSMCs, and vascular endothelium was graded as no staining (0), trace/focal staining (1+), mild/focal staining (2+), or diffuse staining (3+). Immunohistochemistry and in situ hybridization were performed as described earlier.35
Quantitative real-time reverse transcriptase polymerase chain reaction
Total cardiac RNA was extracted using RNAWiz (Ambion, Austin, TX, USA) and Rneasy Midi Kit (Qiagen, Valencia, CA, USA). Total aortic and kidney RNA were extracted using Rneasy Mini Kit (Qiagen). Reverse transcription was performed using TaqMan Reverse Transcription Kit (Applied Biosystems, Branchburg, NJ, USA). Quantitative real-time polymerase chain reaction was performed on the iCycler iQ Multi-Color Real-Time PCR Detection System (BioRad, Hercules, CA, USA) using iQ SYBR Green Supermix (Biorad). Primers used were: PAI-1 (forward (TCCAC AAGTCTGATGGTAGC), reverse (GTTGCTCTTCCATTGTCTGA)); osteopontin (forward (CCAGCACACAAGCAGACGTT), reverse (TCAGTCCATAAGCCAAGCTATCAC)); tumor growth factor-β (forward (CGTGGAAATCAATGGGATCAG), reverse (GGCCATG AGGAGCAGGAA)); and glyceraldehyde-3-phosphate dehydrogenase (forward (CCTGCCAAGTATGATGACATCAA), reverse (AGCC CAGGATGCCCTTTAGT)). Experimental cycle threshold (Ct) values were normalized to glyceraldehyde-3-phosphate dehydrogenase measured on the same plate, and fold differences in gene expression were determined using the 2−ΔΔCt method.
Western blotting for PAI-1
Western blotting was performed as described earlier.36 The sample protein (125 μg) was loaded and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred into a 0.2-μm nitrocellulose membrane. PAI-1 was detected with a specific rabbit anti-mouse PAI-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:500 dilution.
Measurement of PAI-1mRNA expression in cultured rat vascular smooth muscle cells
Vascular smooth muscle cells were isolated from aortic explants from Wistar-Kyoto rats (Charles River Laboratories, Wilmington, MA, USA) and cultured in low glucose Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1.0% penicillin and streptomycin, as described earlier.37 After 24 h, cells were treated with vehicle, Ang II, or aldosterone, in the presence or absence of spironolactone. The final concentration of all active reagents was 10−6 M. Stock solutions of reagents (10−4 M) containing 1.4% ethanol and 0.1% dimethylsulfoxide were prepared and diluted 1:100 for each experiment. Media containing the same concentrations of ethanol and dimethylsulfoxide was used as control. Twenty-four hours later, complementary DNA was isolated using uMacs One-Step cDNA kit (Miltenyi Biotec Inc., Auburn, CA, USA). PAI-1 mRNA expression was measured by reverse transcriptase-PCR as described above.
Statistics
Data are presented as means±s.e. of the mean. The effect of treatment on SBP was determined using repeated-measures General Linear Model, in which the within-subject variable was time and the between-subject variable was treatment. Comparisons of hormone concentrations, injury scores, and tissue expression were made using one-way ANOVA (analysis of variance). Results were confirmed using non-parametric methods. A P-value of <0.05 was considered significant.
Acknowledgments
This work was supported by NIH Grants HL067308, HL077389, HL060906, DK056942, and DK059637. ESK was supported by a Sarnoff fellowship. FAD286 was a gift from Novartis. The authors are grateful to Jane Griffin and to Ellen Donnert for their excellent technical assistance in processing mouse tissues.
Footnotes
DISCLOSURE
Dr Brown serves as a consultant on the Novartis ARB/RAS Vascular Advisory Board.
References
- 1.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol. 1993;25:563–575. doi: 10.1006/jmcc.1993.1066. [DOI] [PubMed] [Google Scholar]
- 2.Young M, Fullerton M, Dilley R, et al. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest. 1994;93:2578–2583. doi: 10.1172/JCI117269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benetos A, Lacolley P, Safar ME. Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1997;17:1152–1156. doi: 10.1161/01.atv.17.6.1152. [DOI] [PubMed] [Google Scholar]
- 5.Rocha R, Rudolph AE, Frierdich GE, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–H1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 6.Blasi ER, Rocha R, Rudolph AE, et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 7.Rocha R, Chander PN, Khanna K, et al. Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension. 1998;31:451–458. doi: 10.1161/01.hyp.31.1.451. [DOI] [PubMed] [Google Scholar]
- 8.Fiebeler A, Schmidt F, Muller DN, et al. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension. 2007;37:787–793. doi: 10.1161/01.hyp.37.2.787. [DOI] [PubMed] [Google Scholar]
- 9.Young M, Funder JW. Eplerenone, but not steroid withdrawal, reverses cardiac fibrosis in deoxycorticosterone/salt-treated rats. Endocrinology. 2004;145:3153–3157. doi: 10.1210/en.2004-0005. [DOI] [PubMed] [Google Scholar]
- 10.Neves MF, Amiri F, Virdis A, et al. Role of aldosterone in angiotensin II-induced cardiac and aortic inflammation, fibrosis, and hypertrophy. Can J Physiol Pharmacol. 2005;83:999–1006. doi: 10.1139/y05-068. [DOI] [PubMed] [Google Scholar]
- 11.Struthers AD. Aldosterone escape during ACE inhibitor therapy in chronic heart failure. Eur Heart J. 1995;16(Suppl N):103–106. doi: 10.1093/eurheartj/16.suppl_n.103. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 13.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 14.Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005;18:44–49. doi: 10.1016/j.amjhyper.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Mihailidou AS, Mardini M, Funder JW. Rapid, nongenomic effects of aldosterone in the heart mediated by epsilon protein kinase C. Endocrinology. 2004;145:773–780. doi: 10.1210/en.2003-1137. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 17.Wehling M, Neylon CB, Fullerton M, et al. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ Res. 1995;76:973–979. doi: 10.1161/01.res.76.6.973. [DOI] [PubMed] [Google Scholar]
- 18.Schneider M, Ulsenheimer A, Christ M, et al. Nongenomic effects of aldosterone on intracellular calcium in porcine endothelial cells. Am J Physiol. 1997;272:E616–E620. doi: 10.1152/ajpendo.1997.272.4.E616. [DOI] [PubMed] [Google Scholar]
- 19.Alzamora R, Marusic ET, Gonzalez M, et al. Nongenomic effect of aldosterone on Na+,K+-adenosine triphosphatase in arterial vessels. Endocrinology. 2003;144:1266–1272. doi: 10.1210/en.2002-220950. [DOI] [PubMed] [Google Scholar]
- 20.Fiebeler A, Nussberger J, Shagdarsuren E, et al. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
- 21.Rocha R, Martin-Berger CL, Yang P, et al. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002;143:4828–4836. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- 22.Minnaard-Huiban M, Emmen JM, Roumen L, et al. Fadrozole reverses cardiac fibrosis in spontaneously hypertensive heart failure rats: discordant enantioselectivity versus reduction of plasma aldosterone. Endocrinology. 2008;149:28–31. doi: 10.1210/en.2007-0584. [DOI] [PubMed] [Google Scholar]
- 23.Quaschning T, Ruschitzka F, Shaw S, et al. Aldosterone receptor antagonism normalizes vascular function in liquorice-induced hypertension. Hypertension. 2001;37:801–805. doi: 10.1161/01.hyp.37.2.801. [DOI] [PubMed] [Google Scholar]
- 24.Okada T, Nagai M, Taniguchi I, et al. Combined treatment with valsartan and spironolactone prevents cardiovascular remodeling in renovascular hypertensive rats. Int Heart J. 2006;47:783–793. doi: 10.1536/ihj.47.783. [DOI] [PubMed] [Google Scholar]
- 25.Karim A, Kook C, Zitzewitz DJ, et al. Species differences in the metabolism and disposition of spironolactone. Drug Metab Dispos. 1976;4:547–555. [PubMed] [Google Scholar]
- 26.Chai W, Garrelds IM, de Vries R, et al. Nongenomic effects of aldosterone in the human heart: interaction with angiotensin II. Hypertension. 2005;46:701–706. doi: 10.1161/01.HYP.0000182661.98259.4f. [DOI] [PubMed] [Google Scholar]
- 27.Nicoletti A, Heudes D, Hinglais N, et al. Left ventricular fibrosis in renovascular hypertensive rats. Effect of losartan and spironolactone. Hypertension. 1995;26:101–111. doi: 10.1161/01.hyp.26.1.101. [DOI] [PubMed] [Google Scholar]
- 28.Rossier MF, Lesouhaitier O, Perrier E, et al. Aldosterone regulation of T-type calcium channels. J Steroid Biochem Mol Biol. 2003;85:383–388. doi: 10.1016/s0960-0760(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 29.Mironneau J. Calcium channel antagonist effects of spironolactone, an aldosterone antagonist. Am J Cardiol. 1990;65:7K–8K. doi: 10.1016/0002-9149(90)91267-a. [DOI] [PubMed] [Google Scholar]
- 30.Brown NJ, Nakamura S, Ma L-J, et al. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int. 2000;58:1219–1227. doi: 10.1046/j.1523-1755.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 31.Fujisawa G, Okada K, Muto S, et al. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004;66:1493–1502. doi: 10.1111/j.1523-1755.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Weisberg A, Griffin JP, et al. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney Int. 2006;69:1064–1072. doi: 10.1038/sj.ki.5000201. [DOI] [PubMed] [Google Scholar]
- 33.Oestreicher EM, Martinez-Vasquez D, Stone JR, et al. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation. 2003;108:2517–2523. doi: 10.1161/01.CIR.0000097000.51723.6F. [DOI] [PubMed] [Google Scholar]
- 34.Mazak I, Fiebeler A, Muller DN, et al. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation. 2004;109:2792–2800. doi: 10.1161/01.CIR.0000131860.80444.AB. [DOI] [PubMed] [Google Scholar]
- 35.Brown NJ, Nakamura S, Ma L, et al. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int. 2000;58:1219–1227. doi: 10.1046/j.1523-1755.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 36.Brown NJ, Bradford J, Wang Z, et al. Modulation of angiotensin II and norepinephrine-induced plasminogen activator inhibitor-1 expression by AT1a receptor deficiency. Kidney Int. 2007;72:72–81. doi: 10.1038/sj.ki.5002268. [DOI] [PubMed] [Google Scholar]
- 37.Naftilan AJ, Pratt RE, Eldridge CS, et al. Angiotensin II induces c-fos expression in smooth muscle via transcriptional control. Hypertension. 1989;13:706–711. doi: 10.1161/01.hyp.13.6.706. [DOI] [PubMed] [Google Scholar]