Abstract
In exploring the cognitive reserve hypothesis in persons with substantial Alzheimer disease neuropathology, we aimed to determine the extent to which educational attainment and densities of diffuse plaques, neuritic plaques, and neurofibrillary tangles predict dementia. Participants were 1563 individuals aged 65 years or above who were assessed for dementia within 1 year of death. Generalized linear mixed models were used to examine whether education and density ratings of diffuse plaques and neuritic plaques, and neurofibrillary tangle stage were associated with a dementia diagnosis. Education interacted with densities of neuritic plaques to predict dementia. Tangle density independently predicted dementia, but did not interact with education. Diffuse plaque density was unrelated to dementia when adjusted for densities of neuritic plaques and tangles. Among individuals with Alzheimer disease neuropathology, educational attainment, as a surrogate of cognitive reserve, modifies the influence of neuritic, but not diffuse, plaque neuropathology on the expression of dementia.
Keywords: Alzheimer disease, cognitive reserve, education, neuropathology
The cognitive reserve hypothesis states that individuals with greater cognitive reserve are better able to cope with brain damage, including that associated with Alzheimer disease (AD) histopathology, thereby delaying the appearance of dementia symptoms.1 Educational experience is one proxy measure of individual differences in cognitive reserve, and is thought to reflect differences in the use of preexisting cognitive processing approaches or the recruitment of compensatory approaches.1 Support for the cognitive reserve hypothesis, and the use of educational experience as its proxy, comes from studies showing that individuals with greater education perform better on measures of cognitive functioning,2,3 are less likely to be demented,4 and are at less risk of developing AD.5
Additionally, in a sample composed of participants with a spectrum of AD lesions, education was found to interact with a composite measure of global AD neuropathology in predicting cognitive functioning.2 This interaction was taken to suggest that individuals with greater education require more pathology than participants with less education before reaching any particular level of cognitive impairment owing to better cognitive functioning throughout adult life, and that education also modifies the effect of neuropathology on cognition.2 When examined separately, densities of neuritic and diffuse plaques, but not neurofibrillary tangles, were found to interact with education in predicting cognitive functioning.2,3
Even among individuals with substantial levels of AD pathology participants with greater education, presumably reflecting greater cognitive reserve, are less likely to be clinically diagnosed with dementia in the year preceding death.6 The purpose of this study is to examine potential interaction effects of education with semiquantitative ratings of neuritic plaques, diffuse plaques, and tangles in predicting a dementia diagnosis among these individuals.
METHODS
Data were obtained from the 2005 National Alzheimer Coordinating Center (NACC) Minimum and Neuropathology data sets, which contain information for participants enrolled in Alzheimer’s Disease Centers (ADCs) supported by the National Institute on Aging (NIA). Participants who received any of the following autopsy diagnosis of AD were included in the sample: Khachaturian7 (AD Present or Absent), NIA-Reagan Institute8 (High, Intermediate, or Low likelihood of dementia being due to AD), and Consortium to establish a registry for Alzheimer Disease (CERAD)9 (Definite, Probable, or Possible AD). Study inclusion also required a semiquantitative assessment of AD pathology for each of the 3 signature lesions of AD: neuritic plaques, diffuse plaques, and neurofibrillary tangle stage.10
Although all participants met one of the inclusion diagnoses, not all participants had high-density ratings of each lesion type, because the neuropathology criteria vary as to the contribution of each lesion type in making the diagnoses. Khachaturian criteria rely on counts of senile plaques, including both neuritic and diffuse plaques, along with the density of neurofibrillary tangles in the neocortex for persons <75 years of age. Therefore, an older individual with frequent diffuse plaques, but few neuritic plaques could meet Khachaturian criteria for a diagnosis of AD Present. We included all CERAD and NIA/Reagan neuropathologic stages in our study criteria. Neurofibrillary tangles are not required for a CERAD diagnosis of AD, and these criteria allow the diagnosis of Possible AD for persons with sparse neuritic plaques. NIA/Reagan criteria allow the diagnosis of a “Low Likelihood” that dementia is due to AD when neuritic plaques are infrequent.
Other inclusion criteria were a clinical assessment for dementia within 1 year of death, age of 65 years or above at last assessment, and data on years of education.
The study sample was composed of data from individuals enrolled in 29 ADCs across the United States who died between 1986 and 2005. Because the autopsies were conducted locally for each ADC, the data reflect neuropathologic diagnoses made by multiple pathologists using a variety of tissue fixation, processing, staining, and immunohistochemical procedures. A 2003 survey of the neuropathology procedures used across ADCs indicates that the postmortem interval averaged between 0 and 4 hours for 10%, 5 to 8 hours for 35%, and 9 to 12 hours for 55%, of the 29 ADCs surveyed. Stains commonly used by the ADCs for identifying amyloid plaques encompassed: silver impregnation methods (69% of ADCs), Aβ immunohistochemistry (69%), thioflavin-S (34%), and Congo red (24%). A variety of techniques was also employed by the different centers in identifying neurofibrillary tangles: tau immunohistochemistry (79%), silver impregnation methods (76%), and thioflavin-S (34%).
The clinical diagnosis of dementia was made in accordance with standard criteria for dementia11,12 and the clinical diagnosis of AD in demented individuals also was based on standard criteria.13 The precise protocols used by clinicians at individual ADCs to obtain the information necessary to fulfill the criteria varied across sites, but all used key elements in common, such as the Mini-Mental State Examination.14
A generalized linear mixed model (logit link function) examined whether education and semiquantitative methods for staging neuritic plaques and diffuse plaques, and Braak and Braak10 neurofibrillary tangle stage were associated with a diagnosis of dementia (vs. no dementia) at the clinical assessment closest to death. Multiple preliminary models tested all possible 2-factor interactions between education and each of the neuropathology variables, and among the neuropathology variables themselves. Interaction effects that were significant in any of these preliminary models were included in the final model. The plaque and tangle variables were treated as categorical in the models. In the NACC dataset, plaque ratings (None, Sparse, Moderate, or Frequent) are based on the most severely affected cortical region. Owing to small numbers in some categories, the “None” and “Sparse” plaque categories and, similarly, the neurofibrillary tangle stage I and “No tangles” categories were combined into single categories in the analyses.
Because previous research examined interactions between education and each lesion type in separate models, rather than in a model combining all lesion types,2,3 we repeated the analyses in models including a single lesion type. Additionally, because educational categories, rather than years of education, is sometimes used as the variable of interest in cognitive reserve research,15 we reran the final model treating education as a categorical variable. All models adjusted for the effects of sex, race, age at death, and time from last clinical assessment to death. A random effect was included in the models to account for the effect of individual ADCs.
RESULTS
Of 8516 NACC participants who were evaluated for AD lesions using Khachaturian, CERAD, or NIA-Reagan Institute criteria, 1563 met inclusion criteria (Fig. 1). Compared with participants who did not meet study criteria, study participants were more likely to be female (52% vs. 48%, P=0.0012), white (96% vs. 95%, P=0.0447), and had more formal education (14.7 vs. 13.7 y, P<0.0001).
FIGURE 1.
Selection of the study sample.
Of those meeting study criteria, 743 (48%) were women and 1495 (96%) were white. Participants had a mean of 14.7 (SD=3.7, range=0 to 30) years of education, and a mean age at death of 82.6 (SD=7.6, range=65.2 to 111.1) years. The mean time between last clinical assessment and death was 0.48 (SD=0.29, range=0.003 to 1.00) years.
Of participants meeting inclusion criteria, 1278 (82%) were clinically diagnosed with dementia in the year preceding death. Preliminary modeling indicated that education interacted with neuritic plaque ratings, but not ratings of diffuse plaques or tangle stage, to predict dementia (Table 1). Other preliminary models (not shown) found no significant interactions among the AD lesions. Therefore, a term reflecting the interaction of education with neuritic plaque ratings was included in the final model.
TABLE 1.
Preliminary and Final General Mixed Models Testing the Lesion Variables Simultaneously*
| Preliminary Model |
Final Model |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Education, y | 0.85 | 0.75-0.98 | <0.0001 | 0.74 | 0.67-0.82 | <0.0001 |
| Neuritic plaques | 0.0037 | 0.0065 | ||||
| Frequent | 0.28 | 0.16-5.02 | 0.97 | 0.10-9.29 | ||
| Moderate | 0.03 | 0.002-0.29 | 0.07 | 0.01-0.51 | ||
| None or sparse | Ref | – | Ref | – | ||
| Neuritic plaques × education | 0.0053 | 0.0113 | ||||
| Frequent × education | 1.15 | 0.97-1.38 | 1.07 | 0.93-1.22 | ||
| Moderate × educcation | 1.28 | 1.10-1.49 | 1.20 | 1.06-1.36 | ||
| None or sparse × education | Ref | – | Ref | – | ||
| Diffuse plaques | 0.3667 | 0.6424 | ||||
| Frequent | 4.18 | 0.42-41.96 | 1.02 | 0.60-1.73 | ||
| Moderate | 1.24 | 0.10-15.83 | 0.82 | 0.45-1.47 | ||
| None or sparse | Ref | – | Ref | – | ||
| Diffuse plaques × education | 0.4114 | |||||
| Frequent × education | 0.92 | 0.79-1.06 | ||||
| Moderate × education | 0.97 | 0.83-1.14 | ||||
| None or sparse × education | Ref | – | ||||
| Braak stage | 0.0404 | <0.0001 | ||||
| VI | 203.73 | 4.14-10023.72 | 16.04 | 6.70-38.41 | ||
| V | 45.67 | 2.25-926.74 | 8.48 | 4.06-17.71 | ||
| IV | 26.26 | 1.60-430.90 | 1.80 | 0.92-3.54 | ||
| III | 13.51 | 0.83-220.59 | 1.10 | 0.56-2.15 | ||
| II | 103.45 | 3.22-3328.20 | 1.72 | 0.86-3.45 | ||
| No tangles or I | Ref | – | Ref | – | ||
| Braak stage by education | 0.1924 | |||||
| VI × education | 0.85 | 0.66-1.08 | ||||
| V × education | 0.89 | 0.74-1.08 | ||||
| IV × education | 0.84 | 0.70-1.00 | ||||
| III × education | 0.85 | 0.71-1.01 | ||||
| II × education | 0.77 | 0.63-0.95 | ||||
| No tangles or I × education | Ref | – | ||||
| Age at death, y | 0.96 | 0.94-0.99 | 0.0048 | 0.96 | 0.94-0.99 | 0.0058 |
| Time to death, y | 0.69 | 0.37-1.27 | 0.2332 | 0.68 | 0.37-1.24 | 0.2093 |
| Male sex | 0.90 | 0.62-1.31 | 0.5702 | 0.89 | 0.61-1.29 | 0.5356 |
| Race | 6.29 | 0.74-53.61 | 0.0924 | 6.41 | 0.77-52.91 | 0.0843 |
OR indicates odds ratios for variables involved in interaction terms are not directly interpretable.
In this model (Table 1), dementia was associated with younger age at death (P=0.0058) and higher neurofibrillary tangle stage (P<0.0001). There was no relationship between diffuse plaque ratings and dementia (P=0.64). The final model confirmed a significant interaction between education and neuritic plaques (P=0.0113). Because odds ratios for variables involved in interaction terms are not directly interpretable, the adjusted odds ratios for the association of years of education with dementia were calculated for each neuritic plaque rating. Adjusted odds ratios (95% confidence intervals) were 0.79 (0.72 to 0.87) for Frequent, 0.89 (0.82 to 0.96) for Moderate, and 0.74 (0.67 to 0.82) for “None or Sparse” neuritic plaques. Given the overlapping confidence intervals of the odds ratios, we ran the same model after collapsing the Frequent and Moderate groups, and compared the effect of education in predicting dementia in this group to the Sparse or None group, to clarify whether there is a nonincreasing effect in predicting dementia as the severity of neuritic plaque pathology increases. This test was statistically significant (2-sided P=0.028), indicating that the effect of education is weaker among individuals with more severe pathology.
The model was repeated after trichotomizing education into the following categories: 0 to 12 years (n=523, 34%), 13 to 16 years (n=620, 40%), and 17 or more years (n=420, 27%). Results were similar to those found in the previous analysis, which showed significant associations between dementia and younger age at death (P=0.0092), and higher neurofibrillary tangle stage (P<0.0001), and indicating that the relationship between education and dementia varies with neuritic plaque stage (P=0.0386). Figure 2 shows the proportion of participants in each education category who were demented for each neuritic plaque rating.
FIGURE 2.
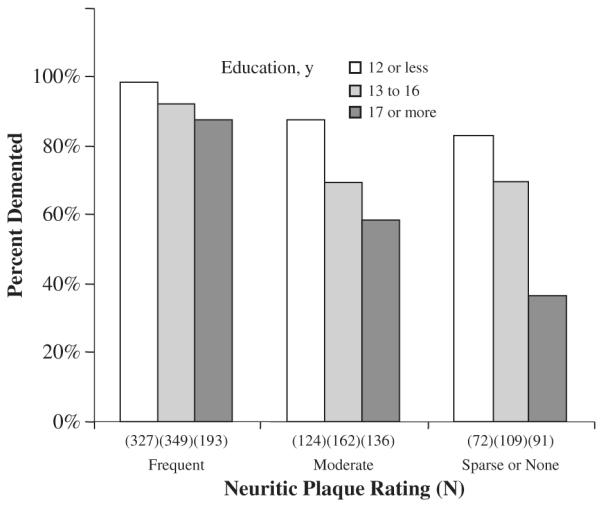
Proportion of participants diagnosed as clinically demented within 1 year of death at each neuritic plaque rating, categorized by years of education. Ns indicates the total number of participants, both demented and nondemented, with each combination of education category and neuritic plaque rating.
When the AD brain lesions were tested in separate models, fewer years of education was associated with a dementia diagnosis in all models (P<0.0001). Diffuse plaque ratings were predictive of dementia when their influence was considered alone (P<0.0001), but did not interact with education (P=0.18) in the preliminary model. As in the previous analyses, a significant interaction between neuritic plaque densities and education was found (P=0.0455), and tangle stage was associated with dementia (P<0.0001), but did not interact with education (P=0.29).
DISCUSSION
Among NACC participants, years of education and ratings of neuritic plaques and neurofibrillary tangles, but not diffuse plaques, predict the presence of dementia in the year preceding death. Further, an interaction effect was found between education and neuritic plaques, indicating that the relationship between education and dementia varies significantly with the amount of neuritic plaques present.
In a previous study, we used both NIA-Reagan Institute and CERAD neuropathologic diagnostic criteria to stage AD lesions in the brain, and found no interaction between education and diagnostic stage on the likelihood of dementia.6 The significant interaction found here between education and neuritic plaques, together with the lack of an interaction effect between education and neurofibrillary tangles, suggests that among individuals with extensive AD pathology, education modifies the effect of neuritic plaques, but not diffuse plaques, on the expression of dementia.
As is illustrated in Figure 2, the effect of increasing neuritic plaque rating on the likelihood of dementia is most dramatic for persons with more education, and likely reflects education-group differences in the threshold of neuritic plaques required to affect cognition. Among individuals with sparse or no neuritic plaques, 83% of participants with 12 or less years of education are already demented, in comparison to only 36% of individuals with 17 or more years of education. Presumably, as the frequency of neuritic plaques increases, more and more highly-educated participants reach the threshold for dementia. At the highest neuritic plaque level, most participants, regardless of education, have surpassed the threshold and become demented. The interaction thus seems to be a ceiling effect. This finding is consistent with the cognitive reserve hypothesis that individuals with greater cognitive reserve (as reflected by the length of the formal educational experience in the present study) are better able to cope with brain pathology and remain cognitively intact for a longer period.1
However, past work examining the effect of education on severity of cognitive impairment among persons with a broad spectrum of AD pathology, in addition to suggesting that individuals with greater education require more pathology than participants with less education before reaching any particular level of cognitive impairment, also indicated that each additional year of education lessens the impact of a unit of neuritic plaques.2 Our use of a sample composed of participants with a restricted range of neuropathology, most of whom were demented, is the likely reason that the interaction of neuritic plaques with education shown here seems to be a ceiling effect. Taken together, these results suggest that the relationship between education, neuritic plaques, and cognitive functioning in AD may differ depending on the degree of advancement of the disease.
In the primary analysis examining possible interaction effects between education and diffuse and neuritic plaques, and tangles, the effects of the 3 AD lesions on dementia were considered simultaneously, because these lesions coexist in the brain, at least at the time of the neuropathologic assessment. Diffuse plaque ratings were unrelated to a dementia diagnosis in that analysis, although we found diffuse plaque ratings to be predictive of dementia in models including diffuse plaques as the sole lesion variable. These results suggest that the predictive value of diffuse plaques among persons with substantial AD neuropathology decreases when adjusted for the level of neuritic plaques and tangles present in the brain.
As in our previous report,6 a small but significant effect of age was found, indicating that older participants were less likely to be demented. Others have reported that older adult age is associated with less severe dementia,16,17 probably because persons who develop the symptoms of AD at an advanced age will die of other causes before dementia symptoms reach a severe stage. Or, as we noted previously,2 this observation may be an artifact of the sample used, reflecting bias in participant referral to ADCs.
Use of a dataset composed of data from ADCs across the United States allows examination of associations between cognitive functioning and neuropathology in a very large number of individuals, and results are likely to be more generalizable to specialized clinical assessment for dementia as it is currently practiced across the United States than results generated from data at a single ADC. However, these advantages are accompanied by limitations. Compared with the general US population, study participants are more likely to be white, have more years of education on average, and generally live in particular geographic areas close to an ADC. Additionally, because participants both volunteered to take part in a research study, and agreed to brain donation, other characteristics of these participants may vary from those in the general population. For these reasons, caution should be used in generalizing these study results to the larger population of individuals with AD. Additionally, as noted earlier, data were not collected according to a single protocol. The sample used thus reflects diversity across the ADCs in participants, clinicians, neuropathologists, and assessment methods. However, we attempted to control for these differences by including ADC as a random variable in our analyses.
The reasons why plaque pathology, but not tangle pathology, interact with education in predicting cognitive functioning are currently unclear, although some have speculated that amyloid deposition in the form of plaques generally occurs at an earlier stage in AD, and that cognitive reserve may be helpful in coping with pathology at this earlier stage.3 Neocortical tangles, however, normally appear later in the disease process, and are likely to be accompanied by more severe neurodegeneration, which may overwhelm cognitive reserve.3 To aid in understanding how education and cognitive reserve impact the expression of dementia symptoms at different stages of AD pathogenesis, future research should complement the inclusion of the signature lesions of AD (diffuse plaques, neuritic plaques, and tangles), with measures of synaptic and neuronal loss which may correlate better with cognitive change. How these may interact with education in predicting cognitive change requires further investigation.
ACKNOWLEDGMENTS
The authors thank all NACC participants, NACC staff for their help in procuring and interpreting the data, and the individual ADCs who contributed information.
Supported by a National Alzheimer’s Coordinating Center Junior Investigator Award (U01-AG 016976); grants P50-AG05681 and P01-AG03991 from the National Institute on Aging of the National Institutes of Health, Bethesda, MD; the Buchanan Fund; and the Charles and Joanne Knight Alzheimer Research Initiative.
Footnotes
Some of the data described here were presented at the 10th International Conference on Alzheimer’s Disease and Related Disorders, Madrid, Spain, July 16, 2006.
REFERENCES
- 1.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 2.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DA, Schneider JA, Wilson RS, et al. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65:953–955. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education, and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay J, Laurin D, Vereault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 6.Roe CM, Xiong C, Miller JP, et al. Education and Alzheimer’s disease without dementia: support for the Cognitive Reserve Hypothesis. Neurology. 2007;68:223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- 7.Khachaturian S. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 8.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 suppl):S1–S2. [PubMed] [Google Scholar]
- 9.Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;4:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Publishing Inc; Washington, DC: 1987. Revised. [Google Scholar]
- 12.American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Publishing Inc; Washington, DC: 1994. [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Mortimer JA, Borenstein AR, Gosche KM, et al. Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol. 2005;18:218–223. doi: 10.1177/0891988705281869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 17.Prohovnik I, Perl DP, Davis KL, et al. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66:49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]



