Abstract
Proper prenatal and postnatal nutrition is essential for optimal brain development and function. The early use of event-related potentials enables neuroscientists to study the development of cognitive function from birth and to evaluate the role of specific nutrients in development. Perinatal iron deficiency occurs in severely affected infants of diabetic mothers. In animal models, severe perinatal iron deficiency targets the explicit memory system of the brain. Cross-sectional ERP studies have shown that infants of diabetic mothers have impairments in recognition memory from birth through 8 months of age. The purpose of this study was to evaluate longitudinal development of recognition memory using ERPs in infants of diabetic mothers compared with control infants. Infants of diabetic mothers were divided into high and low risk status based upon their birthweights and iron status and compared with healthy control infants. Infants were tested in the newborn period for auditory recognition memory, at 6 months for visual recognition memory and at 8 months for cross modal memory. ERPs were evaluated for developmental changes in the slow waves that are thought to reflect memory and the Nc component that is thought to reflect attention. The results of the study showed differences in development between the IDMs and control infants in the development of the slow waves over the left anterior temporal leads and age-related patterns of development in the NC component. These results are consistent with animal models showing that perinatal iron deficiency affects the development of the memory networks of the brain. This study highlights the value of using ERPs to translate basic science information obtained from animal models to the development of the human infant.
Introduction
Rapid growth and development of the brain begins in the fetal period and continues through early childhood (Nelson, 2002). Early brain developmental processes are known to be genetically driven but responsive to the environment (Nelson, In Press). Though not always conceptualized as such, nutrients supplied to the brain constitute part of the microenvironment of the nervous system. Many animal studies have documented that proper nutrition is essential for optimal brain development and function (Georgieff, 2001), but in the human infant, it is more difficult to establish the specific role of nutrients due to the multiplicity of interacting genetic, biologic, and other environmental factors during development. In the past, this problem was compounded by limitations in assessing cognitive development in early infancy. However, in the past 20 years there have been dramatic advances in the understanding of cognitive development in preverbal infants. These developments enable neuroscientists to study the development of cognitive functions such as language and memory from birth and to evaluate the role of nutrition and other risk factors in development.
In the context of nutritional insults to the developing brain, it is important to evaluate early cognitive functions as soon as possible because nutritional insults may occur both during the fetal and neonatal periods (Georgieff, 1990, 2001; Morley, 1994). In the fetus, maternal disease states and placental pathology are associated with poor growth and nutrition of the fetus (Gluckman, 2004; Rayburn, 1989), involving both general protein-energy nutrition as well as specific nutrients such as iron (deUngria, 2000; Georgieff, 1990). In infancy, medical illnesses such as prematurity are associated with periods of nutritional deprivation. In all of these situations, early diet may help to remediate these deficiencies and promote optimal brain function (Lucas, 1992). Therefore, early assessment of cognitive development may assist in understanding the immediate impact of nutritional deficiencies as well as allow for ongoing evaluation of the effectiveness of nutritional remediation.
Nutritional deficiencies may have different effects depending upon the stage of brain development of occurrence. For example, dietary iron deficiency is common in older infants and toddlers and it is known to affect myelin production and striate cortical development as well as motor function (Beard, 2003). Perinatal iron deficiency also occurs in infants of diabetic mothers (IDM) and infants with intrauterine growth restriction (Chockalingam, 1987). In the IDM, perinatal iron deficiency occurs as a result of deranged metabolism that is initiated with the mother's high blood sugar (glucose) levels. Excess glucose is transmitted to the fetus, resulting secretion of insulin. In the fetus, insulin increases the rate of metabolism and the consumption of oxygen. Elevated rates of fetal metabolism result in an increased demand for oxygen (which is obtained from the placenta and carried to the tissues by red blood cells). To compensate, the fetus will extract extra oxygen from the maternal blood by increasing the oxygen carrying protein, hemoglobin, and expanding the red blood cell mass. Unfortunately, expansion of the red blood cell mass requires additional iron that is only available by mobilizing fetal stores of iron that are in the liver, muscle, heart and brain (Chockalingam, 1987; Georgieff, 1990; Petry, 1992). In animal models of severe maternal diabetes, brain iron stores are depleted from portions of the developing explicit memory system (deUngria, 2000).
As the animal models indicated that perinatal iron deficiency would affect the explicit memory pathway, a longitudinal study was designed to evaluate this possibility in human infants through neurophysiologic evaluation of early development of explicit memory in infants of diabetic mothers compared with healthy newborn infants without histories of maternal diabetes. Explicit memory is dependent upon medial temporal lobe structures including the hippocampus (Broadbent, 2002; Nelson, In Press). The hippocampus and hippocampally-dependent memory system develop very early, with function apparent in the newborn infant (deRegnier, 2000, 2002; Seress, 2001). Examples of types of explicit memory include auditory and visual recognition memory and cross-modal memory (Nelson, In Press; Rose, 2001, 1983). These types of memory can be evaluated in infants using event-related potentials (ERPs), a neurophysiologic technique to study cognitive function that is in widespread use in infants, children, and adults. Our study used ERPs to evaluate memory development in infants of diabetic mothers because animal models suggested that these infants would be at risk for memory deficits. Though infants were enrolled into the study longitudinally, only cross-sectional results have been published thus far. These results will be reviewed briefly.
Behavioral studies have indicated that newborn infants can recognize the maternal voice and prefer it to a stranger's voice (DeCasper, 1980, 1986). In the first ERP study from our project, ERPs were recorded from infants of diabetic mothers and control infants tested with the maternal voice and a stranger's voice (deRegnier, 2000). This study showed significant differences between the ERPs from the maternal and strangers' voices in the control group but these differences were attenuated in the IDM group. Because of the link between maternal diabetes and iron metabolism, a follow up study evaluated a subset of IDMs who were also tested for iron stores (Sidappa, 2004). IDMs with normal iron studies were compared with IDMs suspected to have brain iron deficiency based on a biochemical marker of iron storage: the ferritin concentration. In this study, the IDMs with normal ferritin concentrations showed ERP differences between responses to the maternal and stranger's voices that were similar to the full term control group of the prior study. For the group suspected to have brain iron deficiency based on a low ferritin concentration (<35 ng/dl), there were no significant differences between the maternal and stranger's voices, suggesting that perinatal iron deficiency is related to the previously noted abnormalities in recognition memory.
The second test session of our longitudinal study recorded ERPs while 6-month-old infants viewed photos of the maternal face and a stranger's face (Nelson, 2000). The results of the study showed that while control infants showed significant differences between the maternal and stranger's faces, the IDM group showed no difference and therefore persistent evidence of abnormal memory development, particularly over the right hemisphere (the hemisphere involved with face recognition). In this cohort, there were insufficient numbers of infants with neonatal ferritin concentrations available to subgroup the infants on the basis of perinatal iron deficiency alone. Other less specific measures of high risk status were used, but no solid differences were found between those infants designated as high and low risk IDMs.
The iron status of the IDMs was followed in another subgroup of infants and by 8-9 months of age, iron deficiency had resolved (Georgieff, 2002). Noting this, we postulated two competing hypotheses: a) that with resolution of the iron deficiency and ongoing brain development, memory development would normalize in the IDM group, or b) iron deficiency occurring during a sensitive period of development (when hippocampally-based memory is coming on line; i.e., the first 6 months of life) would lead to persistent deficits. Further studies were done to evaluate these possibilities.
At eight months of age, cross modal memory was tested in the IDMs (Nelson, 2003). Cross modal memory refers to the ability to experience a stimulus in one sensory modality and to recognize that stimulus in another modality (Nelson, 1993; Rose, 1983). This is a more challenging test than simple uni-modal recognition as it requires both memory abilities and the ability to transfer memory from one sensory modality to another. In the ERP study, IDMs and controls palpated a small wooden shape without seeing it and subsequently were visually tested for recognition of that shape compared with a novel shape. In this study, differences were again seen between the IDMs and controls with differences in left hemisphere function in the IDMs. This was anticipated as object recognition is mediated in the left hemisphere. Because of the need for high signal to noise rations, we did not have a sufficient sample size to subgroup our infants, and thus were unable to assess high and low risk IDMs in this study.
Our study to this point has demonstrated differences in memory development between IDMs and control infants evaluated at separate time points through 8 months of age. However, it would be helpful to assess the longitudinal growth of memory abilities over time as this may lead to further insights into the developmental trajectory of the brain in infants with perinatal iron deficiency. The purpose of the present data analysis was to evaluate the growth of attention and memory in IDM and control infants using ERPs, combining the previously reported data with data from new subjects that were enrolled after publication of the initial studies, using a standardized procedure for data analysis at all three ages. A second purpose of the study was to evaluate differences in development between high and low risk IDMs that had not been possible previously due to insufficient numbers of high risk infants in the individual studies.
We therefore evaluated growth over time in high and low risk IDMs and controls by comparing changes in ERP components in identical time windows at all three ages, using newer statistical methods to evaluate growth in the ERP components. Growth in memory was assessed by evaluating the area of the late-onset slow wave over the same time period. Growth of attention was assessed by evaluating the change in the peak amplitude and latency of the Nc component from birth through 8 months of age. We postulated that patterns of growth in memory would differ for the IDMs and control infants over the first 8 postnatal months.
Methods
The study was approved by the Institutional Review Boards of the participating hospitals. Informed consent was obtained from each family.
Subjects
Subjects were healthy newborn infants and IDMs recruited either prenatally or in the first week of life to participate in a longitudinal study of the effects of maternal diabetes on infant memory development. Infants of gestational and pre-gestational diabetic mothers were both included. To provide appropriate control subjects for the IDMs (who may deliver prematurely), preterm infants born at or beyond 34 weeks gestation were included if they were healthy after birth. Infants failing their newborn hearing screening were excluded from the newborn ERP. Data from newly enrolled as well as previously studied infants were included.
Each infant's birthweight was plotted on a standard growth chart. Infants of diabetic mothers who had a birthweight more than 2 standard deviations above the mean were considered to be macrosomic (a marker for poor maternal diabetic control correlating with later outcome). Umbilical cord blood ferritin values were available for 79 of the infants. Ferritin levels less than 35 ng/dl are consistent with iron deficiency affecting the explicit memory structures within the developing brain (Sidappa, 2004). Therefore, IDMs with birthweights more than 2 standard deviations above the mean or with a cord blood ferritin level less than 35 ng/dl were considered to be high risk IDMs and the remaining IDMs were considered to be low risk.
ERP Recordings
The details of the recordings have been previously published (deRegnier, 2000; Nelson, 2003, 2000; Sidappa, 2004). Neonatal ERPS were recorded from Pz, Cz, Fz, T3 and T4 (Jasper, 1958) using Ag-AgCl electrodes. Six and 8 month ERPs were recorded from Oz, Pz, Cz, and Fz as well as T3/T4, T5/T6, and C3/C4. A ground electrode was placed on the forehead. Scalp electrodes in the newborn were referenced to the bilateral mastoids. In the 6 and 8 month old infant, a re-referencing program was available and the scalp electrodes were initially referenced to Cz and re-referenced off line to the average electrode. Eye electrodes were placed on the supra and infraorbital ridge of the right eye. Grass model 12 A5 amplifiers were used to record and filter the EEG and EOG signals as previously described.
The pre-stimulus baseline was 100 msec in all infants. The EEG was recorded for 2000 msec post-stimulus onset in the newborn and 1700 msec in the 6 and 8 month old infants. The inter-trial interval varied randomly between1900-2900 msec in the newborn, 500-1200 msec for 6 month infants and 500-1000 msec for 8 month old infants. Stimulus presentation and data collection, editing, averaging and analysis were performed with a personal computer. A-D resolution was 12 bit.
Experimental Design
Newborn Infants (Auditory Memory)
Infants were tested at 38-42 weeks post-menstrual age in the side-lying position in a behavioral stage of active sleep (Thoman, 1990). The maternal voice served as the familiar stimulus and a stranger's voice was the novel stimulus. Voices were recorded, digitized and edited to 80 dB SPL and 750 msec using SoundBlaster. The word, “baby”, was used as the stimulus. The stranger's voice was rotated for each infant and was the voice of the previously tested mother. The maternal voice alternated with the stranger's voice for 100 trials. Stimuli were presented to the right ear using an insert earphone, with the left ear occluded
Six-Month-Old Infants (Visual Memory)
Infants were tested at 6 months adjusted age using the maternal face as the familiar stimulus and a stranger's face as the novel stimulus. The maternal face was captured on videotape and digitized with Adobe Photoshop (V 3.0, Adobe Systems, Mountain View, CA). The stranger's face differed for each infant and was the face of a previous mother. Faces were presented to the infant at a distance of 40 cm and subtended a visual angle of 23 × 14 degrees (approximately two thirds life size). Infants were seated on the parent's lap, facing a screen that contained a computer monitor (13 inch screen) with side panels obscuring the infant's peripheral vision. A maximum of 100 stimuli were presented for 500 msec with equal probability between the maternal and stranger's face. If the infant grew fussy, the study was stopped. On average, each infant viewed 78 images. An experimenter sat behind a panel during the testing session, observing the infant. If the infant was not watching the screen, the observer would press a button relaying a signal to the computer to repeat the stimulus presentation until the infant was watching the presentation.
Eight-Month-Old Infants (Cross-Modal Memory)
Infants were tested at 8 months adjusted age using wooden geometric shapes as stimuli. Eleven red wooden shapes (such as a cross or a corkscrew) were digitally photographed under identical lighting conditions. Different shapes were randomly selected for each test session. Infants were seated on a parent's lap. The infant's hands were placed under a large apron. An experimenter introduced the infant to a single block by cupping her hands around the infant's hands and assisting the infant with manipulation of the block. The infant was required to manipulate the block for a 60 second tactile familiarization phase.
Similar to the 6 month test session, 8-month-old infants were seated in front of a computer monitor and the visual stimuli were presented 60 cm in front of the infant. Half of the stimuli were familiar (a digital image of the palpated object) and half were images of single novel object. A maximum of 100 stimuli were presented for 500 msec with an average of 75 images per infant.
Data Reduction
Data were digitized on line, stored on computer disks and edited for EEG artifacts by computer algorithm. Trials were excluded if the EEG signal exceeded the A-D values in any 50-msec time window. In the sleeping newborn, eye movement artifacts were unusual, and data from all channels were excluded if the EOG signal exceeded A-D values in any 100 msec time window. In the 6 and 8 month old infants who were actively viewing the stimuli, data were corrected for the influence of eye movements or blinks using a computerized algorithm (Gratton, 1983). If after this correction, the EOG signal exceeded 250 microvolts in any 50 msec time window, data at all channels were deleted for that trial.
Individual averages were created for the familiar and novel stimuli for each infant at each age. At any given age, infants were excluded from further data analysis if the average did not contain at least 10 artifact-free trials for each electrode. To equalize the signal-to-noise ratio among stimulus types (e.g., familiar, novel), an equal number of trials was randomly selected for the average ERPs for the familiar and novel stimuli.
The individual average ERPs at each age were evaluated for two components of interest: the Nc and the slow waves. The Nc is a large negative component with a fronto-central maximum amplitude typically occurring at 350-800 msec after stimulus onset. It is thought to be an obligatory attentional component. It is typically assessed by evaluation of peak amplitudes and latencies. In contrast, the late negative and positive slow waves appear to be more directly related to memory. They are typically recorded over the midline but are often strongly lateralized. They may begin soon after stimulus onset but typically show maximal amplitudes late in the recording, after 800-1000 msec. Since slow waves do not have discrete peaks, they are best indexed by integrating the areas under the curve or using average amplitudes (Nelson, 1994; Nelson, 2001).
For this study, to equate study results from three different age groups and to allow for developmental changes, the two time windows chosen for analysis allowed the greatest variation in timing of the Nc component and reflected the most common time period for occurrence of the slow waves. For each infant's familiar and novel ERP waveforms, the peak amplitude and latency to peak was determined for the Nc component in the time window of 350-800 msec. To evaluate negative or positive slow wave activity, each infant's familiar ERP was subtracted from the novel ERP. The area under the curve for this difference wave was integrated in the time window of 800-1500 msec.
Statistical Analysis
Data were analyzed at the electrode sites in common for all 3 groups (Pz, Cz, Fz, T3, T4). Since we have previously reported results for individual ages in newborns, 6 month old and 8 month old infants, this data analysis focused on the longitudinal data. Growth curve analysis using SAS PROC MIXED version 9.1 was used to examine developmental trajectories and predictors of trajectories for the Nc peak amplitudes and latencies (assessing attentional development) as well as the area under the curve of the difference waves (assessing memory development). Since data were not available for all infants at all time points, restricted maximum likelihood estimation (REML) with an estimated degrees of freedom procedure was used as described by Kenwood and Rogers (Kenwood, 1997). This procedure was chosen as REML yields valid parameter estimates under the assumption of ignorable missing data which was assumed in this analysis (Diggle, 2002). Data are presented as the mean +/- one standard error.
Results
The longitudinal data analysis included 115 infants (70 control and 45 IDMs). Of the IDMs, 14 infants were considered to be high risk and 31 were considered to be low risk based upon their birthweights and cord blood ferritin levels. The gestational ages were similar between the three groups (control: 38.6 ± 0.3 wks; high-risk IDM: 38.5 ± 0.3 wks; low-risk IDM: 38.3 ± 0.3 wks). The low risk IDMs and control infants showed ferritin values that were within normal limits (140 ± 23 ng/dl and 138 ± 13 ng/dl respectively) whereas the ferritin concentration of the high risk IDMs was significantly lower (45 ± 16 ng/dl, p = 0.0 005), reflecting perinatal iron deficiency.
Artifact-free newborn data were available for 48 control infants and 31 IDM (10 high-risk, 21 low-risk). Artifact-free 6 month data were available for 36 control infants and 23 IDM (7 high-risk, 13 low-risk). Artifact-free 8 month data were available for 15 control infants and 13 IDM (6 high-risk, 7 low-risk).
For the Nc, significant unconditional results were found for both the peak and latency measurements at Fz (Table 1), with similar results at Cz and T3 (data not shown). Unconditional results do not have grouping variables or any other predictors of change in the model (i.e. the sample is treated as one group with no static covariates). Table 1 shows the specific tests for linear models of the Nc at Fz by peak, latency and condition (mother or stranger). The Nc peak amplitude became progressively more negative in a linear fashion over the first eight months at similar rates for the familiar and novel stimuli (Figure 1). Figure 2 shows that the Nc latency component shortened in a linear fashion over the first eight months for the novel condition but not the familiar condition. In the novel condition, the decrease in the latency was much more accelerated than the familiar condition, with a large difference between the familiar and novel stimuli in the newborn that narrowed at 6 and 8 months.
TABLE 1.
Tests Of Linear Models Of Nc At Fz By Peak, Latency, And Condition
| Response | Estimate | SE | Den. df | F | p-value |
|---|---|---|---|---|---|
| PEAK, FAMILIAR | |||||
| Intercept | -2.5611 | 1.2624 | 164 | 4.12 | 0.0441 |
| Linear* | -2.8171 | 0.2543 | 119 | 122.74 | <.0001 |
| PEAK, NOVEL | |||||
| Intercept | -4.3493 | 1.5117 | 164 | 8.28 | 0.0045 |
| Linear* | -2.7594 | 0.3128 | 139 | 77.83 | <.0001 |
| LATENCY, FAMILIAR | |||||
| Intercept | 562.4800 | 13.5449 | 164 | 1724.49 | <.0001 |
| Linear* | -4.3053 | 2.8435 | 141 | 2.29 | 0.1322 |
| LATENCY, NOVEL | |||||
| Intercept | 583.2500 | 14.5739 | 164 | 1601.61 | <.0001 |
| Linear* | -7.4217 | 2.9949 | 127 | 6.14 | 0.0145 |
indicates change in amplitude or latency measurement per month from the newborn to 8 month old infant. SE = standard error; Den. df = denominator degrees of freedom (all numerator df = 1).
Figure 1.
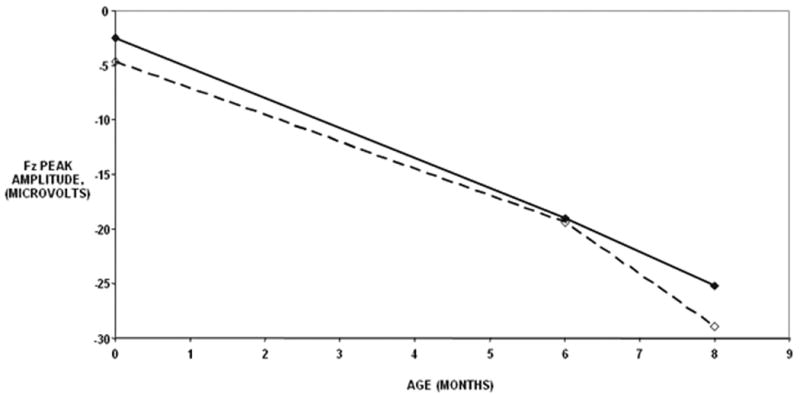
Change in Nc peak amplitude by age and stimulus type. Solid lines indicate the mean amplitudes for the familiar condition whereas the dashed lines indicate the mean amplitudes for the novel condition. The mean amplitudes decreased by 2.8 microvolts per month for the familiar and novel conditions between the newborn and 8 month ages.
Figure 2.
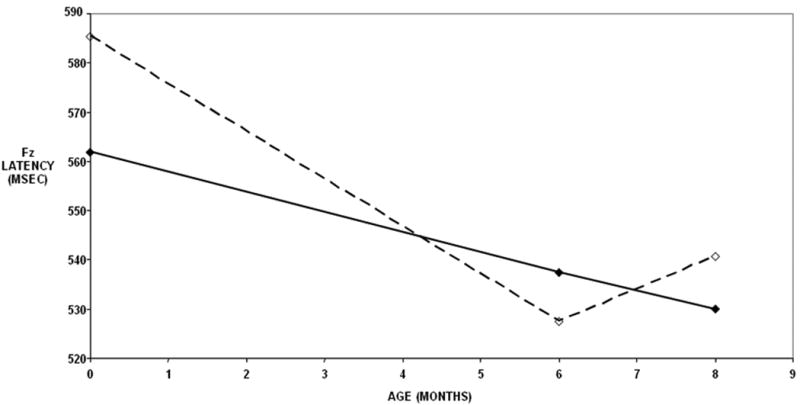
Change in the Nc latency by age and stimulus type. Solid lines indicate the mean latency for the familiar condition whereas the dashed lines indicate the mean latency for the novel condition. The mean latency for the novel stimulus decreased by 7.4 msec per month between the newborn and 8 month ages.
For the slow wave activity, significant differences between risk groups (control, low risk, high risk) were found for the difference wave (novel minus familiar) area under the curve at the T3 electrode only (omnibus group: F [6, 133] = 2.69, p = 0.017). Figure 3 shows the observed T3 difference wave areas under the curve for the groups by age and Table 2 shows the results of the specific tests. The groups did not differ in terms of neonatal slow waves. The low risk IDMs and control groups did not differ in terms of linear trend or non-linear (quadratic) trend. However the high risk group did significantly differ from the control and low risk groups in linear trend over the first 8 months, F [1, 111] = 7.79, p < 0.001, and in the non-linear trend, F [1, 111] = 9.59, p < 0.001. Figure 3 shows that the high risk group trend was concave-up, whereas the trend of the other two groups was concave down.
Figure 3.
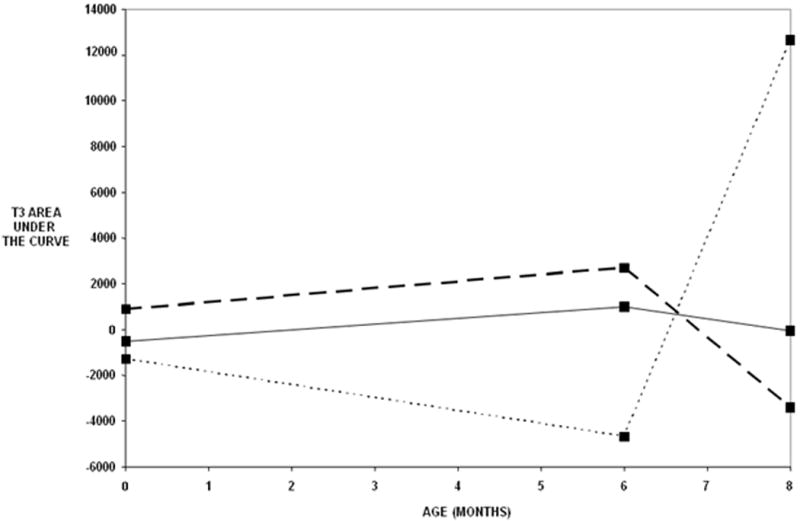
Observed T3 difference wave areas under curve (stranger – mother) by risk group and age. Solid lines indicate the mean difference wave areas for the control group. Long dashed lines indicate the mean areas for the low risk IDMs and the short dotted line indicates the mean areas for the high risk IDMs. See Table 2 for statistical computations.
Table 2.
Results Of Growth Curve Analysis Examining Risk Group Differences In Linear And Non-Linear (Quadratic) Trends
| Effect | Estimate | SE | Den. df | F-value | p-value |
|---|---|---|---|---|---|
| Intercept | |||||
| Overall | -381.62 | 1288.95 | 157 | 0.09 | 0.7676 |
| Low risk vs. Control | 1242.98 | 2336.74 | 157 | 0.28 | 0.5955 |
| High risk vs. Control | -798.51 | 3105.70 | 157 | 0.07 | 0.7974 |
| Linear Trend | |||||
| Overall | 707.95 | 1352.56 | 119 | 0.27 | 0.6017 |
| Low risk vs. Control | 2131.59 | 2417.29 | 115 | 0.78 | 0.3797 |
| High risk vs. Control | -8467.81 | 3032.99 | 111 | 7.79 | 0.0062 |
| Quadratic Trend | |||||
| Overall | -79.22 | 190.28 | 122 | 0.17 | 0.6779 |
| Low risk vs. Control | -344.08 | 339.06 | 119 | 1.03 | 0.3123 |
| High risk vs. Control | 1263.67 | 408.03 | 111 | 9.59 | 0.0025 |
SE = standard error; Den. df = denominator degrees of freedom (all numerator df = 1).
Discussion
This study showed that growth of the left temporal slow wave associated with memory was affected in high-risk IDMs through 8 months of age, whereas the frontal Nc component associated with attention showed an age-related pattern of development without effects of maternal diabetes. The study also showed that abnormal findings were limited to high risk IDMs with macrosomia or decreased cord blood ferritin concentrations.
Cognitively-based slow waves develop over the first year of life (de Haan, 1997; deRegnier, 2000; Nelson, 1994; Nelson, 1991). The type of slow wave that is elicited in an ERP study is dependent upon the age of infant and the degree of experience with the stimuli. If a newborn infant is familiar with one of two stimuli, and the other is entirely novel, the novel stimulus will typically evoke a negative slow wave that is maximal over the fronto-central scalp but can also be recorded from distant electrode sites (deRegnier, 2000, 2002; Therien, 2004). This ERP pattern is thought to reflect novelty detection which is thought to be a function of the hippocampus (Nelson, 2001). With development over the first few months of life, the speed of encoding a novel stimulus improves (Fagan, 1974, 1990) and the novel stimuli must vary on each trial to elicit negative slow waves (Nelson, 1991). In these older infants, when novel stimuli are repeated from trial to trial, they become partially familiar and elicit the positive slow wave. The neural generators of the negative and positive slow waves have not been definitively established, but it is known that these waves are elicited in tasks of recognition memory that are thought to be dependent upon the hippocampus and other medial temporal lobe structures (deRegnier, In Press; Nelson, 1995; Squire, 2001). These structures are vulnerable to perinatal iron deficiency. Severe perinatal iron deficiency is associated with loss of brain iron in humans (Petry, 1992) and is likely to have occurred only in the high risk IDM group in this study. In animal models, severe fetal iron deficiency causes decreases in hippocampal iron content and decreased activity in iron-dependent enzyme systems in addition to permanent hippocampal dendritic structural changes (deUngria, 2000; Jorgenson, 2003). Neural plasticity is a hallmark of the human infant brain, but our data suggest that neural consequences of perinatal iron deficiency may be long lasting. We have previously demonstrated that perinatal iron deficiency corrects without specific therapy in IDMs by 9 months of age (Georgieff, 2002), but the ERPs suggest this does not lead to improvement in memory function as persistent differences in memory development are still seen in high risk IDMs using ERPs at 8 months of age. This has recently been corroborated using behavioral testing of toddlers which showed that delayed imitation skills (another type of explicit memory) were deficient in IDMs (DeBoer, 2005).
In our previous studies, the number of infants with available ferritin concentrations and the ratios of those who would be considered high or low risk did not always facilitate specific comparison of high and low risk IDM subgroups, but this longitudinal study shows that this is essential in evaluating the IDMs. Even in this study, the number of high risk infants was relatively low, possibly because mothers who would enroll prenatally in a longitudinal study would have been motivated to keep their blood glucose under control. This longitudinal study does show that low risk IDMs display memory development that is similar to control infants over the first 8 months of age.
In this study, we did not find effects of maternal diabetes on the development of the Nc component recorded over the midline and anterior temporal electrode sites. In our previous cross-sectional studies, no consistent changes in the Nc have emerged (Nelson, 2003, 2000). The Nc component has been associated predominantly with attention (Richards, 2003) rather than memory, though there are clearly links between attention and memory, both in behavioral studies and within the neuroanatomic pathways. Behaviorally, infants show greater attention to novel stimuli (Fagan, 1990). Neuroanatomically, the cingulate cortex has been implicated in infant attention and the generation of the Nc component (Reynolds, 2005) but the cingulate cortex is also part of the memory network of the brain (Wang, 2005; Yonelinas, 2005). This is relevant to the IDM model as the cingulate cortex is susceptible to perinatal iron deficiency (deUngria, 2000) and so it was possible that our studies may have found effects both on attention and memory. However, our study results for the Nc component do not provide strong evidence for any deleterious effects of high risk maternal diabetes on infant attention at this time.
This study also defined growth of the Nc component over the first 8 postnatal months. By analyzing the data longitudinally using growth curve analyses, a tiny neonatal Nc component was detected (Figure 1) in the ERP with a peak and latency that was linearly related to this same component in the 6 and 8 month old infants. The linear rate of decrease of the Nc amplitude and latency was striking given that the three age groups were tested using different paradigms and the fact that the infants were asleep for the newborn study. However, each paradigm was based upon behavioral evidence of memory capabilities for each age. The linear nature of the change in the amplitude and latency suggests that the tasks were equally difficult or engaging at each age and appropriate for a developmental study. Interestingly, the decrease in the latency was statistically significant only for the novel stimulus and the rate of decline in latency for that component was faster than for the familiar stimulus. This may be a correlate of the improvement in the speed of encoding that has been documented in behavioral studies (Fagan, 1974, 1990).
In summary, the results of this study showed that infant ERPs can be used both to understand neural correlates of cognitive development over time and to translate knowledge gained from animal models of nutritional deficiencies into information regarding the human brain and cognitive development of very young infants. Prior to this project, there were no human studies specifically of memory development in the infant of diabetic mother. Our longitudinal ERP study showed that the animal model was predictive of the human situation, with clinical evidence of brain iron depletion correlating with memory abilities. This has now been confirmed using behavioral tests of explicit memory development (delayed elicited imitation) (DeBoer, 2005). Thus, differences in memory development between IDMs and control infants appear to be correlated with fetal iron nutrition and metabolism. We are hopeful that these data can lead to therapeutic options for the IDM that can preserve memory function and toward this end, a trial of early iron therapy has recently been initiated. The use of the ERPs in very young infants has allowed this information to translate quickly from animal models into clinical trials. Thus, the ERP technique has been shown to be valuable in the evaluation of early nutritional insults in the human infant.
Acknowledgments
We wish to thank Neely Millr for her assistance with data analysis for this study. We thank the parents and infants for their ongoing participation in this study. The study was funded by National Institutes of Health grants NS32755 and HD29421.
References
- Beard JL, Connor JR. Iron status and neural functioning. Annual Review of Nutrition. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Clark RE, Zola S, Squire LR. The medial temporal lobe and memory. In: Squire LR, Schacter DL, editors. Neuropsychology of Memory. Third. New York: Guilford Press; 2002. pp. 3–23. [Google Scholar]
- Chockalingam U, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK. Cord transferrin and ferritin values in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J Pediatr. 1987;111:283–286. doi: 10.1016/s0022-3476(87)80088-4. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother's face by 6-month-old infants: A neurobehavioral study. Child Development. 1997;73:187–210. [PubMed] [Google Scholar]
- DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA. Explicit memory performance in infants of diabetic mothers at 1 year of age. Developmental Medicine and Child Neurology. 2005;47:525–531. doi: 10.1017/s0012162205001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: newborns prefer their mothers' voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborns' perception of speech sounds. Infant Behavior and Development. 1986;6:19–25. [Google Scholar]
- deRegnier R. Auditory Recognition Memory in Infancy. In: deHaan M, editor. Infant EEG and Event-Related Potentials. East Sussex, England: Psychology Press; In Press. [Google Scholar]
- deRegnier R, Nelson CA, Thomas K, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. Journal of Pediatrics. 2000;137:777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- deRegnier R, Wewerka S, Georgieff MK, Mattia F, Nelson CA. Influences of post-conceptional age and postnatal experience on the development of auditory recognition memory in the newborn infant. Developmental Psychobiology. 2002;41:216–225. doi: 10.1002/dev.10070. [DOI] [PubMed] [Google Scholar]
- deUngria M, Rao R, Wobken JD, Luciana M, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase activity in selective regions of the brain. Pediatric Research. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. 2nd. New York: Oxford; 2002. [Google Scholar]
- Fagan JF. Infant recognition memory: the effects of length of familiarization and type of discrimination task. Child Development. 1974;45:351–356. doi: 10.1111/j.1467-8624.1974.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Fagan JF. The paired comparison paradigm and infant intelligence. Annals of the New York Academy of Sciences. 1990;608:337–357. doi: 10.1111/j.1749-6632.1990.tb48902.x. [DOI] [PubMed] [Google Scholar]
- Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, Ophoven JJ, Widness JA. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. Journal of Pediatrics. 1990;117:455–461. doi: 10.1016/s0022-3476(05)81097-2. [DOI] [PubMed] [Google Scholar]
- Georgieff MK, Rao R. The role of nutrition in cognitive development. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. pp. 45–58. [Google Scholar]
- Georgieff MK, Wewerka S, Nelson CA, deRegnier R. Iron status at 9 months in infants with low iron stores at birth. Journal of Pediatrics. 2002;141:405–409. doi: 10.1067/mpd.2002.127090. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Seminars in Fetal and Neonatal Medicine. 2004;9:419–425. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method of off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:371–375. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the international federation. Electroencephalography Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Developmental Neuroscience. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- Kenwood MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Lucas A, Morley R, Cole TJ, Lister G, Leeson-Payne C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet. 1992;339:261–264. doi: 10.1016/0140-6736(92)91329-7. [DOI] [PubMed] [Google Scholar]
- Morley R, Lucas A. Influence of early diet on outcome in preterm infants. Acta Pediatrica Supplement. 1994;405:123–126. doi: 10.1111/j.1651-2227.1994.tb13410.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G, Fischer K, editors. Human Behavior and the Developing Brain. New York: Guilford Press; 1994. pp. 269–313. [Google Scholar]
- Nelson CA. The ontogeny of human memory: A cognitive neuroscience perspective. Developmental Psychology. 1995;31:723–738. [Google Scholar]
- Nelson CA. Neural development and life-long plasticity. In: Lerner RM, Jacobs F, Wetlieb D, editors. Promoting Positive Child, Adolescent, and Family Development: Handbook of Program and Policy Interventions. Thousand Oaks, CA: Sage Publications; 2002. pp. 31–60. [Google Scholar]
- Nelson CA, Collins PF. An event-related potential and looking time analysis of infants' responses to familiar and novel events: implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, de Haan M, Thomas KM. Neural bases of cognitive development. In: Damon W, Lerner R, Kuhn D, Siegler R, editors. Handbook of Child Psychology, 6th Edition, Vol 2: Cognition, Perception and Language. New Jersey: John Wiley & Sons, Inc.; In Press. [Google Scholar]
- Nelson CA, Henschel M, Collins PF. Neural correlates of cross-modal recognition memory by 8-month-old infants. Brain and Cognition. 1993;29:411–420. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Monk CS. The use of event-related potentials in the study of cognitive development. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. pp. 125–136. [Google Scholar]
- Nelson CA, Wewerka S, Borscheid AJ, deRegnier R, Georgieff MK. Electrophysiologic evidence of impaired cross-modal recognition memory in 8-month-old infants of diabetic mothers. Journal of Pediatrics. 2003;142 doi: 10.1067/mpd.2003.210. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, deRegnier R, Georgieff MK. Neurocognitive sequelae of infants of diabetic mothers. Behavioral Neuroscience. 2000;114:950–956. [PubMed] [Google Scholar]
- Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. Journal of Pediatrics. 1992;121:109–115. doi: 10.1016/s0022-3476(05)82554-5. [DOI] [PubMed] [Google Scholar]
- Rayburn W, Sander C, Compton A. Histologic examination of the placenta in the growth-retarded fetus. American Journal of Perinatology. 1989;6:58–61. doi: 10.1055/s-2007-999546. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infants: an event-related potential and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Attention and recognition memory in the 1st year of life: a longitudinal study of preterm and full-term infants. Developmental Psychology. 2001;37:135–151. [PubMed] [Google Scholar]
- Rose SA, Gottfried AW, Bridger WH. Infants' cross-modal transfer from solid objects to their graphic representations. Child Development. 1983;54:686–694. [PubMed] [Google Scholar]
- Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson ECA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge MA: MIT Press; 2001. pp. 45–58. [Google Scholar]
- Sidappa A, Georgieff MK, Wewerka S, Worwa C, Nelson CA, deRegnier R. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatric Research. 2004;55:1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- Squire LR, Schmolck H, Stark SM. Impaired auditory recognition memory in amnesic patients with medial temporal lobe lesions. Learning and Memory. 2001;8:252–256. doi: 10.1101/lm.42001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien JM, Worwa CT, Mattia FR, deRegnier RO. Altered pathways for auditory discrimination and recognition memory in premature newborns. Developmental Medicine and Child Neurology. 2004;46:816–824. doi: 10.1017/s0012162204001434. [DOI] [PubMed] [Google Scholar]
- Thoman EB. Sleeping and waking states in infants: A functional perspective. Neuroscience and biobehavioral reviews. 1990;14:93–107. doi: 10.1016/s0149-7634(05)80165-4. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. Journal of Neuroscience. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


