Abstract
Proliferation and differentiation were assessed in a series of human colon carcinoma cell lines in response to a mineral-rich extract derived from the red marine algae, Lithothamnion calcareum. The extract contains 12% Ca2+, 1% Mg2+, and detectable amounts of 72 trace elements, but essentially no organic material. The red algae extract was as effective as inorganic Ca2+ alone in suppressing growth and inducing differentiation of colon carcinoma cells that are responsive to a physiological level of extracellular Ca2+ (1.4 mM). However, with cells that are resistant to Ca2+ alone, the extract was still able to reduce proliferation and stimulate differentiation.
Keywords: Calcium, colon cancer, Lithothamnion calcareum, red algae extract
1. Introduction
Several epidemiological studies have demonstrated a role for Ca2+ in colon cancer chemoprevention [1–5]. Dietary studies in animals are supportive. Several studies have shown that dietary Ca2+-supplementation, alone or in conjunction with vitamin D, reduces colonic epithelial cell abnormalities including areas of hyperplasia and formation of aberrant crypts and raised tubular polyps in healthy animals on a high-fat diet or in carcinogen-exposed animals [6– 12]. Cell culture studies provide insight into how Ca2+ may exert its chemopreventive activity. In the presence of Ca2+ there is a modulation of several proteins that are associated with the proliferation response. Among these are cyclin D1, P27 (Kip1), P21 (WAF1), c-fos, c-myc, c-jun, and members of the TGF-β family [13,14]. In addition, Ca2+ is critical for E-cadherin production and membrane localization in epithelial cells, and fosters sequestration of β-catenin at the cell surface along with E-cadherin [15–17]. Since nuclear β-catenin is a transcriptional activator for Wnt pathway (growth-stimulating) signaling [18–21], sequestration at the cell surface could be expected to down-regulate signaling events that lead to proliferation.
Although dietary calcium is an important contributor to health of the colonic mucosa, the degree of Ca2+-induced protection against colon cancer can be described as “modest.” Some studies, in fact, have failed to demonstrate any statistically significant protection [22,23]. Identifying additional dietary materials that could be used for colon cancer chemoprevention would have obvious value. In the present study we show that a mineral-rich extract of the red marine algae, Lithothamnion calcareum, [24] is effective in suppressing proliferation of human colon cancer cell lines in vitro, including cells that are resistant to physiological levels of extracellular Ca2+. The effectiveness of the mineral-rich extract in suppressing growth of Ca2+- resistant as well as Ca2+-sensitive colon carcinoma cells provides a rationale for assessing the effectiveness of this GRAS (generally-regarded as safe) natural product as a colon cancer chemopreventative.
2. Materials and methods
2.1. Red marine algae extract
A mineral-rich extract, derived from the red marine algae, Lithothamnion calcareum, was obtained as a gift from Marigot, Limited (Cork, IR). The red algae is harvested from the Atlantic waters off the southwest coast of Ireland and northwest coast of Iceland [24]. The mineralized fronds are separated from extraneous materials, sterilized, dried and milled under ISO and HACCP certification. The mineral extract contains 12% calcium, 1% magnesium, and measurable levels of 72 other trace minerals, including manganese, selenium, copper and zinc. Because the elements accumulated in the algal fronds represent minerals in seawater, there is little variation from batch to batch. The mineral extract is sold as a food supplement under the name Aquamin® (GRAS 000028) and is used in various products for human consumption in Europe, Asia, Australia and North America.
At 12% elemental calcium, the concentration of ionized Ca2+ in a solution of 2.5 mg/ml (highest amount used), was calculated to be approximately 6.7 mM. However, a small amount of precipitate was always seen when the extract was solubilized in culture medium. Taking this into account, we estimated the level of available Ca2+ in the algae extract to be 5.6 mM at 2.5 mg/ml. Low passage human dermal (neonatal foreskin) fibroblasts were used in a Ca2+-dependent survival assay to validate the Ca2+ concentration in the red algae extract. These cells do not survive in culture medium with an extracellular Ca2+ concentration below 0.15 mM [25]. Consistent with these past results, cells did not survive in a Ca2+-free, spinner-modified version of Dulbecco’s minimal essential medium supplemented with 5% dialyzed fetal bovine serum (SMEM-dFBS) alone, but survived when the Ca2+ level (calcium chloride) was raised to a concentration of 0.15 mM. When the algae extract was used in place of calcium chloride, survival was seen at concentrations of approximately 60–70 µg/ml.
2.2. Colon carcinoma cells
Human colon carcinoma cell lines derived from five different tumors (CBS, Moser, Fet, HCT-116 and SW480) were used in the present investigation. A cloned subpopulation of the CBS line referred to as Ca2+-non-responsive variant-1 (NR-1) was also used. All of the cell lines were available from a previous study [26]. Cells were routinely maintained in monolayer culture using SMEM-dFBS and various amounts of the algal extract or calcium chloride as indicated in the Results Section. Growth was at 37°C in an atmosphere of 95% air and 5% CO2. Cells were subcultured by brief exposure to trypsin/ethylenediamine tetraacetic acid (EDTA) as needed.
2.3. Proliferation assay
Colon carcinoma cells (4×104 per well) were added to wells of a 24-well culture dish using SMEM-dFBS and allowed to attach overnight. The next day, cell counts were made to provide precise time-zero cell counts. Varying amounts of the algae extract or calcium chloride were added as indicated in the Results section. After incubation for three days, the cells were harvested by exposure to trypsin/EDTA and counted. Counting was done with an automated particle counter after verifying that the cells were in single cell suspension.
2.4. Cytotoxicity and apoptosis assays
To assess viability after treatment, the cells were incubated under the desired conditions for one day as above and then harvested and counted. Immediately following this, the cells were resuspended and again added to wells of a 24-well dish. All cells were added in the same culture medium, which consisted of DMEM with 10% non-dialyzed FBS. Cells that reattached were counted after six hours and compared to the number plated in each well. In parallel wells, cells were incubated for an additional 24 hours and then counted. Those cells that were able to reattach and proliferate were assumed to be viable.
Apoptosis was assessed by staining the cells with Annexin V-FITC and propidium iodide and analyzing stained cells via flow cytometry [27]. Briefly, cells were grown in SMEM-dFBS alone or in the same medium supplemented with either the algae extract or calcium chloride. After 48 hours, cells were washed twice with ice cold PBS and then resuspended in 1× binding buffer (BD Pharmingen, San Diego, CA) at a concentration of 1 × 106 cells/ml. 200 µl of the cell suspension was transferred to wells of a 96 well V bottom plate. Ten µl of Annexin V-FITC (BD Pharmingen, San Diego, CA) and five µl of propidium iodide (Invitrogen Molecular Probes, Carsbad, CA) were added to each well and incubated for 15 minutes in the dark. Samples were then analyzed by flow cytometry (LSR II, BD Biosciences, San Diego, CA). Data acquisition and analysis were done using BD FACSDiva software.
2.5. Preparation of cell lysates and immunoblot analysis
Cells were plated at 3×105 cells per well in 6-well tissue culture dishes and treated as described in the Results section. After three days of incubation, cells were washed and then lysed in 1× cell lysis buffer consisting of 20 mM Tris-HCl (pH 7.4), 2 mM sodium vanadate, 1.0 mM sodium fluoride, 100 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 25 µg/ml each of aprotinin, leupeptin and pepstatin, and 2 mM EDTA and EGTA. Lysis was performed by adding 200 µl of lysis buffer to each well and incubating on ice for 5 minutes. After incubation, cells were scraped and samples sonicated. Then the extracts were cleared by microcentrifugation at 14000 g for 15 minutes. Supernatants were collected and protein concentrations estimated using the BioRad DC protein assay kit (BioRad, Hercules, CA).
Western blotting for E-cadherin was carried out as described previously [28]. Briefly, samples were separated in SDS-PAGE under denaturing and reducing conditions and transferred to nitrocellulose membranes. After blocking with a 5% nonfat milk solution in Tris-buffered saline with 0.1% Tween (TTBS) at 4°C overnight, membranes were incubated for one hour at room temperature with the desired antibody, diluted 1:1000 in 5% nonfat milk/0.1% TTBS. Thereafter, the membranes were washed with TTBS and bound antibody detected using the Phototope-HRP Western blot detection kit (Cell Signaling Technology, Inc., Danvers, MA). A Kodak - 1000 X-OMAT processor was used to capture the positive images of the Western blots and these positive images were scanned and digitized. The digitized images were quantitated using NIH image analysis software. Membranes were also reprobed with Actin for normalization. The antibody to E-cadherin was from BD Biosciences (San Jose, CA) and antibody to actin was from Santa Cruz Biotech (Santa Cruz, CA).
2.6. Confocal immunofluorescence microscopy
Immunostaining for E-cadherin was done as follows: briefly, cells were grown on uncoated Lab Tek II chamber slides in SMEM containing 5% dialyzed FBS or in the same medium supplemented with either the red algae extract or calcium chloride. After three days, cells were fixed with 4% formaldehyde for 20 minutes. After fixation, cells were washed 2× with wash buffer (0.05% Tween-20 in Dulbecco’s Phosphate Buffered Saline [DPBS]), followed by permeabilization with 0.1% Triton X-100 for 10 minutes. Cells were again washed and then exposed to a blocking solution consisting of 1% BSA in DPBS for 30 minutes. Next, cells were treated with antibody to E-cadherin in blocking solution for 1 hour. After three subsequent washing steps with DPBS (5 minutes each), cells were treated with Alexa Fluor 488-conjugated secondary antibody in blocking solution and incubated for 45 minutes (Invitrogen, Carslbad, CA). Following three additional washing steps, the cells were rinsed one time with water. Coverslips were mounted onto microscope slides with Prolong Anti-fade (Invitrogen). Stained cells were examined with a Zeiss LSM 510 confocal microscope using a 63× (C-Apochr) NA=1.2 water immersion objective lens. Laser excitation wavelengths included 364, 488 and 543 nm scanned in sequence by the line method.
2.7. Statistical evaluation
Data for most experiments were based on duplicate or triplicate samples. Data from several experiments were pooled and presented as means and standard errors. Since there were multiple groups in each experiment, data were analyzed by ANOVA followed by paired-group comparisons. Differences with either treatment (red algae extract or calcium chloride) were compared to values from controls (absence of either treatment) and to each other. Differences were accepted as significant at p<0.05.
3. Results
3.1. Effects of the mineral-rich red algae extract on proliferation of Ca2+-sensitive and Ca2+-resistant human colon carcinoma (CBS) cells
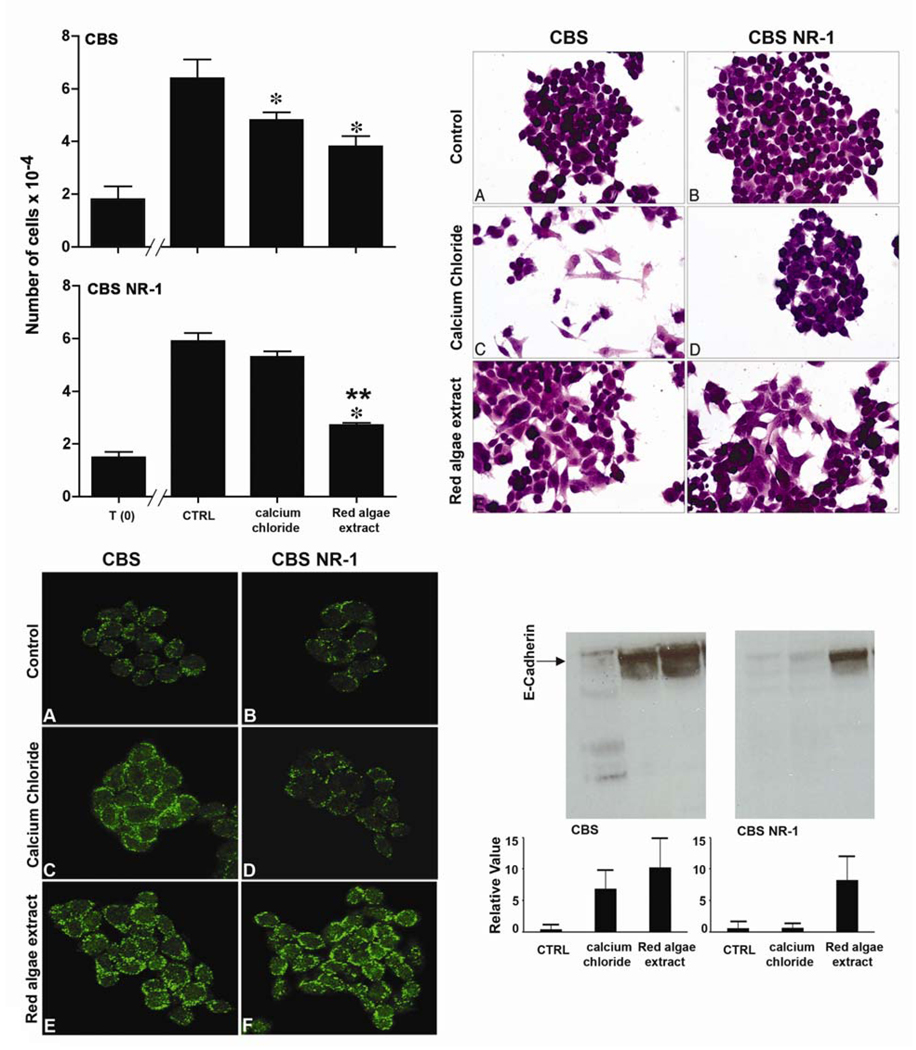
The upper-left panel of Figure 1 compares proliferation of parental CBS cells and Ca2+- resistant (NR-1) variant cells in SMEM-dFBS alone and in the same medium supplemented with the algae extract or with calcium chloride. The parental cells responded to a physiological level of calcium chloride (1.4 mM Ca2+) with growth reduction. Consistent with our past reports [28,29], the NR-1 variant cells did not respond to this level of Ca2+. That is, there was no reduction in growth. In contrast to these results, both the parental CBS cells and the NR-1 variant cells responded to the red algae extract. As part of our analysis, we assessed cytotoxicity and apoptosis under the same conditions. Growth suppression in neither cell line was accompanied by a significant increase in cell death with either the red algae extract or calcium chloride at Ca2+ concentrations up to 5.6 mM. Cell death, as determined in the stringent replating assay, was less than 7.5% in all cases and the percentage of apoptotic cells was less than 2.5% in all cases (Table 1). Furthermore, when cells were allowed to proliferate (under identical conditions) after exposure to the algae extract or to calcium chloride for one day, the rate of proliferation was similar (not shown).
Fig. 1.
Effects of a red algae extract on proliferation and differentiation of parental human colon carcinoma (CBS) cells and Ca2+-resistant (NR-1) variant cells. Upper-left panel: Cells were treated with the mineral-rich red algae extract (2.5 mg/ml) or with calcium chloride (1.4mM) and cell numbers were determined after 72 hours of incubation. Values represent means and standard errors based on nine independent experiments with both cell types. Statistical significance of the differences was determined by ANOVA followed by paired group comparisons. *Indicates difference from control at p <0.05. **Indicates difference from calcium chloride alone at p <0.05. Upper right-hand panel: Morphology: Cells were stained with hematoxylin and eosin after 72 hours of incubation. Lower left-hand panel: Confocal immunofluorescence microscopy: E-cadherin was assessed after two days of treatment. Lower right-hand panel: Western blot for E-cadherin: Whole cell extracts made after 3 days of treatment with either the red algae extract or calcium chloride (1.4mM) were assessed.
Table I.
Comparison of the algae extract and calcium chloride for cytotoxicity and for induction of apoptosis in colon carcinoma cells
| Cell lines and conditions | Analysis | |
|---|---|---|
| % Dead (replating)1 |
% Apoptotic (Annexin V)2 |
|
| CBS | ||
| Control | 7.4 ± 0.7 | 1.1 |
| Ca2+ (1.4 mM) | 7.2 ± 1.1 | 1.1 |
| Ca2+ (5.6 mM) | 3.8 ± 0.4 | 1.9 |
| Algae extract (2.5 mg/ml) | 4.4 ± 0.5 | 2.0 |
| CBS NR-1 | ||
| Control | 5.5 ± 0.9 | 1.2 |
| Ca2+ (1.4 mM) | 5.1 ± 1.0 | 1.7 |
| Ca2+ (5.6 mM) | 4.5 ± 0.8 | 2.0 |
| Algae extract (2.5 mg/ml) | 6.9 ± 1.0 | 2.1 |
The replating assay assesses the capacity of the cells to reattach to the culture dish after exposure to the algae extract or calcium chloride. Cells were exposed to the red algae extract or to calcium chloride for one day and then assayed. Cells that reattached were assumed to be viable. Values shown are means and standard deviations based on duplicate samples in three independent experiments.
The Annexin V-FITC / propidium iodide is a fluorescence-based cytofluorimetric assay. Values shown are means of three separate experiments.
Figure 1 also demonstrates features of differentiation in the two CBS isolates in response to the red algae extract or calcium chloride. With the parental cells, both the mineral-rich extract and calcium chloride induced a change in cell shape from spherical to flattened (upper-right panels). Concomitantly, both treatments led to up-regulation and surface localization of E-cadherin (lower-left panels). Staining was more focal and more intense in the differentiating cells (compare staining pattern in C & E with pattern in A) The NR-1 variant cells responded to the high mineral extract (F) but not to calcium chloride (D). As shown in the lower-right panel, E-cadherin protein was increased (as indicated in western blot assays) in conjunction with altered cell surface expression. With the parent cells, up-regulation was seen with either agent. As expected, NR-1 variant cells responded to the red algae extract but not calcium chloride alone.
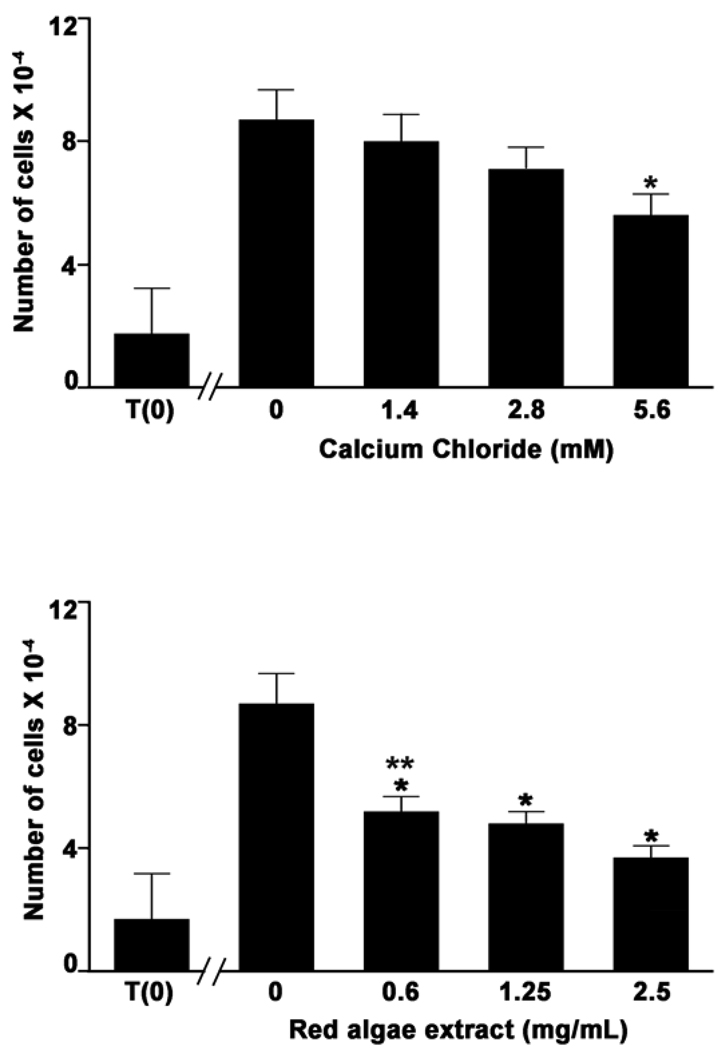
Based on these observations, we carried out more extensive concentration-dependent studies with NR-1 cells (Figure 2). Cell growth was inhibited by the algae extract at a concentration of 0.6 mg/ml (equivalent to approximately 1.4 mM Ca2+). In contrast, calcium chloride was ineffective at inhibiting the proliferation of NR-1 cells when used at concentrations below 5.6 mM (Figure 2).
Fig. 2.
Dose-responsive suppression of NR-1 proliferation with the red algae extract. Cell counts were made after 72 hours of incubation under the indicated conditions. Values represent means and standard errors based on six independent experiments. Statistical significance of the differences was determined by ANOVA followed by paired group comparisons. *Indicates difference from the control at p <0.05. **Indicates difference from the equivalent concentration of calcium chloride at p <0.05.
3.2. Effects of the mineral-rich red algae extract on proliferation of additional human colon carcinoma cell lines
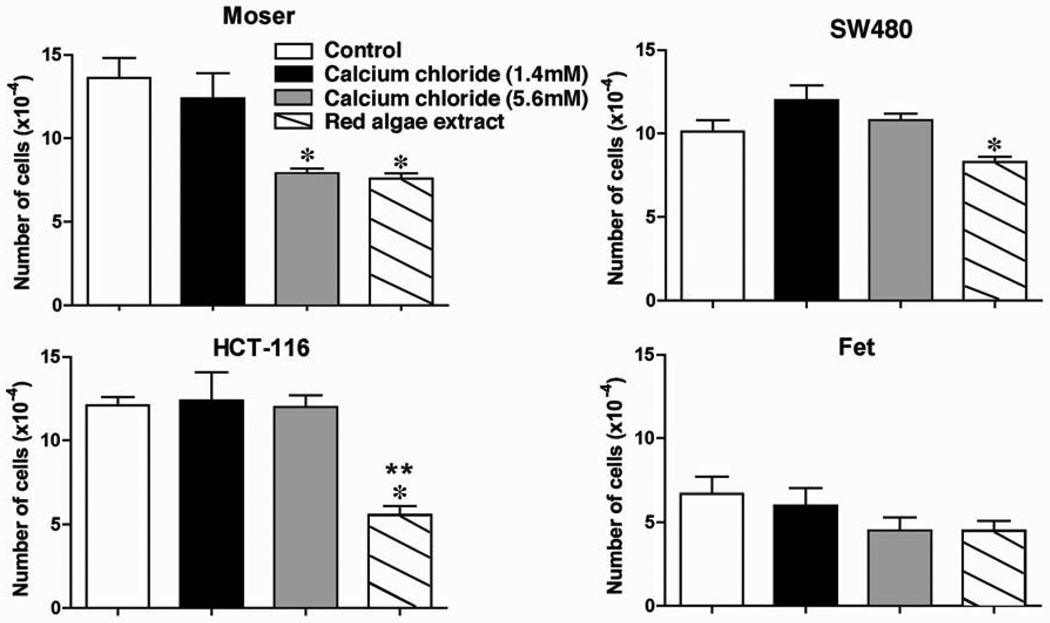
Figure 3 demonstrates growth-inhibitory effects of the algae extract with four additional human colon cancer cell lines. As controls for this experiment, both a physiological level of Ca2+ (1.4 mM calcium chloride) and the maximal amount deliverable in culture medium (5.6 mM) were used. As can be seen from the figure, statistically significant inhibition of proliferation was achieved in three of the lines with the algae extract. Of the three, the least sensitive line (SW480) demonstrated 28% growth inhibition. The most sensitive, HCT-116, demonstrated greater than 70% inhibition under the same conditions. Of the four lines, only Moser demonstrated a statistically-significant growth inhibition in response to calcium chloride (5.6 mM), while none of the lines were inhibited by calcium chloride at 1.4 mM Ca2+. Table II provides a summary of growth-inhibition findings with each of the cell lines (including parental and NR-1 CBS cells) and compares the algae extract to calcium chloride.
Fig. 3.
Effects of a red algae extract on growth of four different human colon carcinoma cell lines. Cell counts were made after 72 hours of incubation under the indicated conditions. Values represent means and standard errors based on 3 –6 independent experiments with each cell line. Statistical significance of the differences was determined by ANOVA followed by paired group comparisons. *Indicates difference from the respective control at p <0.05. **Indicates difference from 5.6 mM calcium chloride alone at p <0.05.
Table II.
Comparison of the algae extract and calcium chloride for ability to suppress growth of human colon carcinoma cells
| Cell line | Growth inhibition | ||
|---|---|---|---|
| Red algae extract | Calcium chloride | ||
| (mg/ml) | (mM Ca2+) | (mM Ca2+) | |
| CBS | 1.9 | (4.2) | 2.5 |
| CBS (NR-1) | 2.0 | (4.5) | > 5.6 |
| Moser | 1.9 | (4.2) | 5.2 |
| HCT-116 | 1.7 | (3.8) | > 5.6 |
| Fet | 2.5 | (5.6) | 5.6 |
| SW480 | > 2.5 | (> 5.6) | > 5.6 |
Inhibition values are presented as ED50, based on 3–9 different experiments with each cell line. The level of Ca2+ in each concentration of the red algae extract is shown in parentheses.
With each of the cell lines, growth inhibition was accompanied by expression of differentiation features (shape change from spherical to flattened) and by increased E-cadherin production (based on Western blot of whole cell lysates). With SW480, a change in cell shape and up-regulation of E-cadherin were observed with calcium chloride at 5.6 mM Ca2+ (not shown) even though significant growth inhibition was not achieved. Thus, in all cases where growth inhibition was observed, features associated with differentiation were also present. In contrast, features of differentiation could be seen even when growth inhibition was not achieved.
4. Discussion
Marine algae (especially members of the coralline family of red algae) [30,31] constitute a rich source of minerals that are accumulated from seawater over the life of the organism. The present study demonstrates that a mineral-rich material derived from the red algae, Lithothamnion calcareum, is capable of suppressing the growth of human colon cancer cell lines in vitro. The mineral-rich extract is effective in slowing proliferation and inducing differentiation under conditions in which cytotoxicity is not observed. The algae extract is as effective as calcium chloride alone in cells that are responsive to a physiological level of extracellular Ca2+. More importantly, however, the algae extract is an effective growth inhibitor with colon cancer cells that do not respond well to physiological levels of Ca2+ alone. The findings presented here raise several issues.
One issue, in light of these findings, is the nature of the elements in the algae extract that are responsible for inhibition of colon carcinoma cell growth. The data are consistent with the suggestion that Ca2+ is an important contributor to the algae extract’s effectiveness as a growth-suppressor. However, it is unlikely that the extract is simply a mixture of Ca2+ and other inert elements. Colon cancer cells that were resistant to calcium chloride supplementation (up to 5.6 mM Ca2+) responded to the algae extract with growth inhibition at concentrations as low 0.6 mg/ml (equivalent to approximately 1.4 mM Ca2+).
In addition to Ca2+, the algae extract also contains a high level of Mg2+. Recent studies have addressed the role of Mg2+ in colon cancer chemoprevention. While some studies have suggested no significant chemopreventive activity as a single variate, other studies have demonstrated a possible relationship between Mg2+ intake and reduced colon cancer incidence [33–35]. Recently it has been suggested that the Ca2+ : Mg2+ ratio is an important consideration [36]. By providing a source of Mg2+ as well as Ca2+, the algae extract provides what appears to be a more optimal ratio of Ca2+ to Mg2+, and this may have significant advantage over Ca2+ alone.
The algae extract is more than just a mix of Ca2+ and Mg2+. It also contains measurable levels of 72 different trace elements accumulated from sea water over the lifespan of the algae. Some of these trace elements, such as manganese, selenium, zinc and copper, have been purported to promote health through one mechanism or another. In particular, these metals are critical components of cellular anti-oxidant enzymes [37]. In spite of this, only a few studies have attempted to obtain evidence relating levels of most trace elements with colon cancer rates [38]. How each of the moieties in the algae extract (either alone or in combination) contributes to tumor prevention will need to be investigated.
Are these findings relevant to human colon cancer? Similar to what is seen in individual tumor lines, reduced Ca2+-responsiveness in evolving colon tumor lesions could underlie the loss of growth control in these lesions. Our own past studies have shown that the extracellular calcium-sensing receptor (CaSR), a critical regulator of cellular interactions with extracellular Ca2+ [39], is lost from the surface of colon carcinoma cells in vivo [26,28,29,40]. We have shown that in the presence of a functional CaSR, Ca2+ stimulation leads to E-cadherin production and membrane inclusion. This leads to incorporation of β-catenin in the cell surface-cytoskeletal complex and prevents translocation to the nucleus and Wnt stimulation [28]. Since Wnt pathway signaling is a major inducer of proliferation in colon epithelial cells, the sequestration of β-catenin at the surface during differentiation could be expected to reduce proliferation without being cytotoxic. The lack of a functional CaSR could explain the failure of even high levels of extracellular Ca2+ to exert effective chemopreventive activity as noted in some epidemiological studies [22,23]. By virtue of its additional components along with a high level of Ca2+, the algae extract may function either by stimulating CaSR production and expression or by bypassing its requirement altogether. In any case, the expected result would be growth reduction without cytotoxicity. Whether the red algae extract can, in fact, be used under conditions needed for effective chemoprevention in the colon will require long-term studies, first in animals and then in humans. Currently we are in the midst of a 15-month study with rodents on a high fat, “Western style” diet that is known to promote adenoma formation in the animals [10–12]. All that can be said at this time is that a high-fat diet supplemented with the Lithothamnion calcareum mineral-rich extract at a level providing a 7-fold increase in Ca2+ (as well as the other elements in the extract) is well-tolerated by the animals. After 15 months on the diet, there is no detectable change in the appearance of the animals, no measurable effect on weight gain, and no other gross or metabolic abnormalities that can be attributed to the mineral-rich red algae extract (manuscript in preparation).
In summary, proliferation and differentiation were assessed in a series of human colon carcinoma cell lines in response to a mineral-rich extract derived from the red marine algae, Lithothamnion calcareum. The algae extract was as effective as inorganic Ca2+ alone in suppressing growth and inducing differentiation in colon carcinoma cells that are responsive to a physiological level of extracellular Ca2+. However, with cells that are resistant to calcium alone, the red algae extract was still able to reduce proliferation and stimulate differentiation. Based on the findings presented here, the use of the red algae extract in place of extracellular Ca2+ alone might be of value as a colon cancer chemopreventative.
Acknowledgement
The authors would like to thank Marigot, Inc. of Cork, Ireland as the source of the mineral-rich red algae extract.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
All named authors of the original research paper entitled “Growth-inhibitory effects of a mineralized extract from the red marine algae, Lithothamnion calcareum, on Ca2+-sensitive and Ca2+-resistant human colon carcinoma cells” express that there exist no financial and/or personal relationships with other people and/or organizations that could inappropriately influence their work.
References
- 1.Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States) Cancer Causes Control. 2000;11:459–466. doi: 10.1023/a:1008914108739. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C, Braga C, Negri E, Franceschi S, Russo A, Conti E, Falcini F, Giacosa A, Montella M, Decarli A. Intake of selected micronutrients and risk of colorectal cancer. Int J. Cancer. 1997;73:525–530. doi: 10.1002/(sici)1097-0215(19971114)73:4<525::aid-ijc12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Bond JH, Greenberg ER. Calcium supplements for the prevention of colorectal adenomas, Calcium Polyp Prevention Study Group. N. Engl J. Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 4.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, Heber D. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J. Natl. Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 5.Holt PR, Wolper C, Moss SF, Yang K, Lipkin M. Comparison of calcium supplementation or low-fat dairy foods on epithelial cell proliferation and differentiation. Nutr. Cancer. 2001;41:150–155. doi: 10.1080/01635581.2001.9680626. [DOI] [PubMed] [Google Scholar]
- 6.Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J. Nutr. 1993;123:144–152. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 7.Mokady E, Schwartz B, Shany S, Lamprecht SA. A protective role of dietary vitamin D3 in rat colon carcinogenesis. Nutr. Cancer. 2000;38:65–73. doi: 10.1207/S15327914NC381_10. [DOI] [PubMed] [Google Scholar]
- 8.Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991;51:5608–5613. [PubMed] [Google Scholar]
- 9.Comer PF, Clark TD, Glauert HP. Effect of dietary vitamin D3 (cholecalciferol) on colon carcinogenesis induced by 1,2-dimethylhydrazine in male Fischer 344 rats. Nutr. Cancer. 1993;19:113–124. doi: 10.1080/01635589309514242. [DOI] [PubMed] [Google Scholar]
- 10.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57B1/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 11.Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W, Velcich A, Lipkin M, Augenlicht L. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803–7810. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- 12.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57BI/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat. Rev. Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 15.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J. Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 19.Mariadason JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, Augenlicht LH. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 20.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 21.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 22.Kampman E, Giovannucci E, van ’t Veer P, Rimm E, Stampfer MJ, Colditz GA, Kok FJ, Willett WC. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am. J. Epidemiol. 1994;139:16–29. doi: 10.1093/oxfordjournals.aje.a116931. [DOI] [PubMed] [Google Scholar]
- 23.Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women, The Iowa Women’s Health Study. Am. J. Epidemiol. 1993;137:1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- 24.Frestedt JL, Walsh M, Kuskowski MA, Zenk JL. A natural mineral supplement provides relief from knee osteoarthritis symptoms: a randomized controlled pilot trial. Nutr. J. 2008;7:9. doi: 10.1186/1475-2891-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varani J, Shayevitz J, Perry D, Mitra RS, Nickoloff BJ, Voorhees JJ. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am. J. Pathol. 1990;136:1275–1281. [PMC free article] [PubMed] [Google Scholar]
- 26.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 27.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 28.Bhagavathula N, Hanosh AW, Nerusu KC, Appelman H, Chakrabarty S, Varani J. Regulation of E-cadherin and beta-catenin by Ca2+ in colon carcinoma is dependent on calcium-sensing receptor expression and function. Int. J. Cancer. 2007;121:1455–1462. doi: 10.1002/ijc.22858. [DOI] [PubMed] [Google Scholar]
- 29.Bhagavathula N, Kelley EA, Reddy M, Nerusu KC, Leonard C, Fay K, Chakrabarty S, Varani J. Upregulation of calcium-sensing receptor and mitogen-activated protein kinase signalling in the regulation of growth and differentiation in colon carcinoma Br. J. Cancer. 2005;93:1364–1371. doi: 10.1038/sj.bjc.6602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blunden G, Campbell SA, Smith JR, Guiry MD, Hession CC, Griffin RL. Chemical and physical characterization of calcified red algal deposits known as maërl. J. Appl. Phycol. 1997;9:11–17. [Google Scholar]
- 31.Blunden G, Farnham WF, Jephson N, Fenn RH, Plunkett BA. The composition of maerl from the Glenan Islands of southern Brittany. Bot. Mar. 1977;20:121–125. [Google Scholar]
- 32.Lin J, Cook NR, Lee IM, Manson JE, Zhang SM. Total magnesium intake and colorectal cancer incidence in women. Cancer Epidemiol. Biomarkers Prev. 2006;15:2006–2009. doi: 10.1158/1055-9965.EPI-06-0454. [DOI] [PubMed] [Google Scholar]
- 33.van den Brandt PA, Smits KM, Goldbohm RA, Weijenberg MP. Magnesium intake and colorectal can risk in the Netherlands Cohort Study. Br. J. Cancer. 2007;96:510–513. doi: 10.1038/sj.bjc.6603577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson SC, Bergkvist L, Wolk A. Magnesium intake in relation to risk of colorectal cancer in women. JAMA. 2005;293:86–89. doi: 10.1001/jama.293.1.86. [DOI] [PubMed] [Google Scholar]
- 35.Folsom AR, Hong CP. Magnesium intake and reduced risk of colon cancer in a prospective study of women. Am. J. Epidemiol. 2006;163:232–235. doi: 10.1093/aje/kwj037. [DOI] [PubMed] [Google Scholar]
- 36.Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, Smalley WE, Li M, Shyr Y, Zheng W. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 2007;86:743–751. doi: 10.1093/ajcn/86.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris ED. Regulation of antioxidant enzymes. FASEB J. 1992;6:2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- 38.Kikuchi H, Iwane S, Munakata A, Tamura K, Nakaji S, Sugawara K. Trace element levels in drinking water and the incidence of colorectal cancer. Tohoku J. Exp. Med. 1999;188:217–225. doi: 10.1620/tjem.188.217. [DOI] [PubMed] [Google Scholar]
- 39.Whitfield JF. Calcium, calcium-sensing receptor and colon cancer. Cancer Lett. 2009;275:9–16. doi: 10.1016/j.canlet.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3) Cancer Res. 2005;65:493–498. [PubMed] [Google Scholar]