Abstract
Cytokine-induced tyrosine phosphorylation of the transcription factor STAT5 is required for its transcriptional activity. In this article we show that the small dual-specificity phosphatase VHR selectively dephosphorylates IFN-α- and β-activated, tyrosine-phosphorylated STAT5, leading to the subsequent inhibition of STAT5 function. Phosphorylation of VHR at Tyr138 was required for its phosphatase activity toward STAT5. In addition, the Src homology 2 domain of STAT5 was required for the effective dephosphorylation of STAT5 by VHR. The tyrosine kinase Tyk2, which mediates the phosphorylation of STAT5, was also responsible for the phosphorylation of VHR at Tyr138.
Signal transducers and activators of transcription, STAT, are a family of latent cytoplasmic transcription factors that are activated by many cytokines and growth factors (1). Upon ligand stimulation the receptor-associated Janus kinases selectively tyrosine phosphorylate STATs, which then dissociate from the receptor, dimerize, translocate to the nucleus, and bind to their respective enhancer elements (2). Activation of the Jak/STAT pathway is rapid and transient, with activated STAT molecules evident in the nucleus within minutes of cytokine stimulation but disappearing within a few hours (3). Several different levels at which negative regulation can be exerted over the Jak/STAT pathway have been demonstrated, including down-regulation of the receptor/ligand complex, induction of suppressors of cytokine signaling (SOCS), and dephosphorylation by nuclear tyrosine phosphatases (4). T cell protein tyrosine phosphatase (TcPTP)4 was demonstrated to be the nuclear tyrosine phosphatase for STAT1 (5). Similar to STAT1, STAT5 undergoes a rapid activation and deactivation cycle upon cytokine stimulation. The transient kinetics of STAT5 tyrosine phosphorylation following cytokine stimulation strongly suggest that tyrosine dephosphorylation is a critical mechanism in the down-regulation of STAT5 activity (6). SHP-2 and protein tyrosine phosphatase (PTP) 1B (PTP1B) have been reported to be involved in the cytosolic tyrosine dephosphorylation of STAT5 (7, 8). Aoki and Matusa (9) suggested that the nuclear tyrosine phosphatase TcPTP was catalytically competent with respect to the dephosphorylation of STAT5. However, studies in TcPTP−/− murine embryonic fibroblasts revealed that TcPTP-deficiency was of no consequence to STAT5 tyrosine dephosphorylation (5). Thus, the identity of the nuclear tyrosine phosphatase inactivating STAT5 remained elusive.
Materials and Methods
Cell culture
HEK293T, 2fTGH, HeLa, U1A, and U4A cells were maintained in DMEM with penicillin, streptomycin, and 10% FBS.
Reagents
IFNβ was a gift from Biogen Idec. Staurosporine was purchased from Sigma-Aldrich. Abs used were against FLAG (Sigma-Aldrich), hemagglutinin (HA) or MYC (Santa Cruz Biotechnology), phospho-STAT1(Tyr701) (New England Biolabs), phospho-STAT5(Tyr694/Tyr699) (Upstate Biotechnology), phospho-Tyr (Upstate Biotechnology), or phospho-STAT5(Ser726) (Upstate Biotechnology).
Cell extracts
Nuclear extracts were prepared by Dounce homogenizing cells in buffer A (20 mM HEPES (pH 7.9), 10 mM KCl, 1 mM MgCl2, 10% glycerol, and 0.1% Nonidet P-40) and the sedimentation of nuclei at 1000 rpm for 5 min. The supernatant was removed and collected as cytoplasmic extract, and nuclei were extracted with buffer A containing 300 mM NaCl to obtain nuclear extracts. To generate whole cell lysates, cells were lysed on the plates with buffer containing 20 mM HEPES (pH 7.4), 1% TX-100, 100 mM NaCl, 50 mM NaF, 10 mM β-glycerophosphate, 1 mM sodium vanadate, and 1 mM PMSF. Lysates were centrifuged at 13,000 × g for 5 min, and protein concentration was determined by the Lowry method (Bio-Rad protein assay).
Immunoprecipitation and immunoblotting
Cell lysates were incubated with isotype control or protein-specific Abs and protein G-Sepharose overnight at 4°C. After SDS-PAGE and transfer onto PVDF membrane, proteins were detected with the indicated primary Abs, HRP-conjugated secondary Abs and ECL.
Results and Discussion
To identify the nuclear tyrosine phosphatase responsible for STAT5 dephosphorylation, we screened HA-tagged tyrosine phosphatases with known or potential nuclear localization for their ability to dephosphorylate STAT5. In pulse-chase studies, most of the tyrosine phosphatases failed to affect the kinetics of STAT5 dephosphorylation (Fig. 1A, and data not shown). Indeed, the small dual-specificity phosphatase VHR was the only enzyme capable of altering the extent of IFNβ-induced STAT5 tyrosine phosphorylation in its wild-type (WT) or catalytically inactive forms (Fig. 1B). VHX, another member of the VH1 group of dual-specificity phosphatases, displayed no negative regulatory effects on STAT5 tyrosine phosphorylation, suggesting a high degree of specificity of these enzymes (Fig. 1C). Similar expression of all phosphatases was verified by anti-HA Western blotting (not shown).
FIGURE 1.
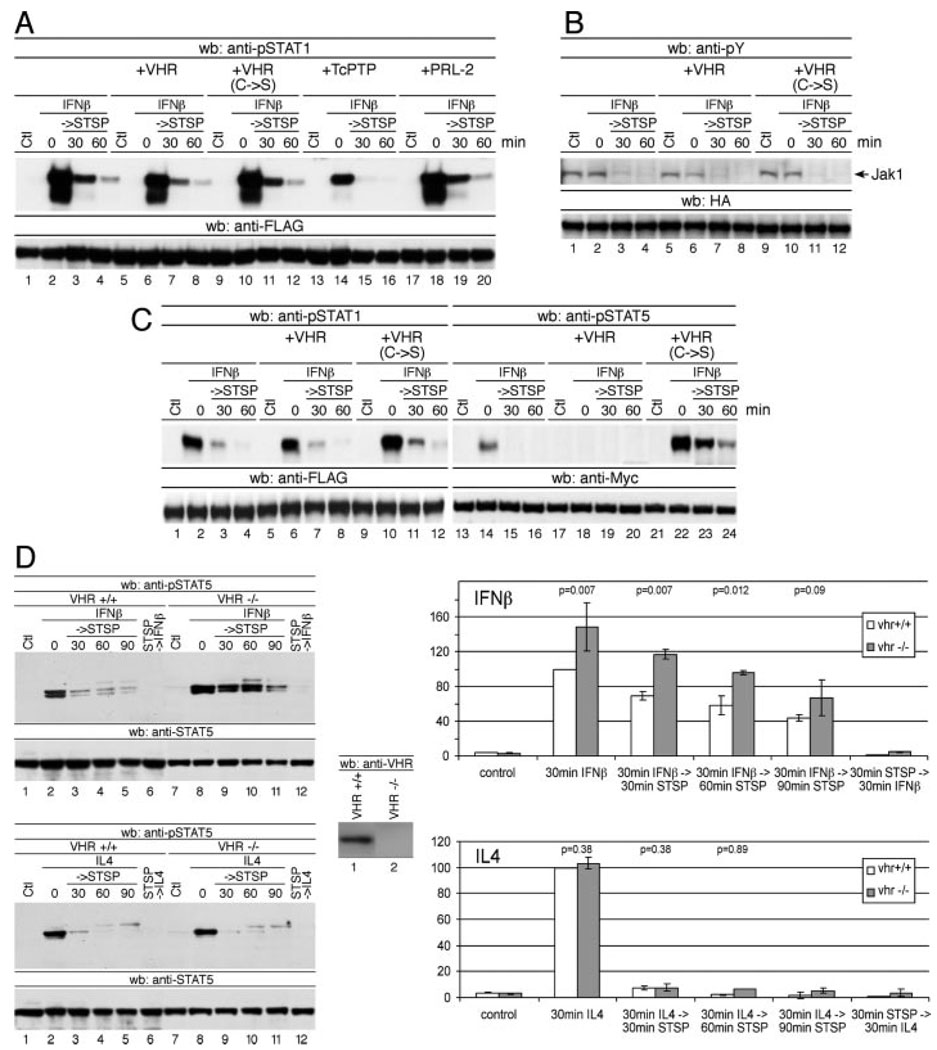
Identification of VHR as a STAT5 tyrosine phosphatase. A, 293T cells were transfected with plasmids encoding Myc-STAT5A and either empty vector or the respective WT or catalytically inactive phosphatases as indicated. Forty-eight hours after transfection, cells were left untreated (control (Ctl)) or stimulated with IFNβ (1000 U/ml) for 60 min before the addition of staurosporine (STSP) at 500 nM, and incubation was continued for the indicated time periods. Tyrosine phosphorylation of immunoprecipitated Myc-STAT5A was analyzed by using anti-phospho-STAT5 (upper panel) and anti-Myc Abs (lower panel). B and C, Same as in A, except that cells were transfected with Myc-STAT5 and empty vector or HA-tagged VHR or VHX. wb, Western blotting; LMPTP, low m.w. PTP, HePTP, hemopoietic PTP.
To test whether VHR bears a similar selectivity toward STAT5 as a TcPTP for STAT1, we examined the effects of VHR on STAT1 tyrosine dephosphorylation. In contrast to TcPTP, VHR appears to be unable to facilitate tyrosine dephosphorylation of STAT1 (Fig. 2A) or other STAT proteins (not shown). When both STAT1 and STAT5 were coexpressed in the presence or absence of VHR, only STAT5 displayed significantly altered tyrosine phosphorylation (Fig. 2B). We next wanted to exclude the possibility that VHR might affect STAT5 tyrosine phosphorylation indirectly via tyrosine dephosphorylation and inactivation of the upstream activators Jak1 and tyrosine kinase 2 (Tyk2). Substantiating our hypothesis, VHR did not affect phosphorylation of IFNα- and β-activated Jak1 (Fig. 2C) or Tyk2 (not shown). Similarly, phosphorylation of STAT5 on Ser726, known to be required for the optimal transcriptional activity of STAT5, was not affected by VHR (not shown). Lastly, nuclear colocalization of VHR and pSTAT5 was confirmed by immunofluorescence and confocal microscopy (not shown), supporting the notion that VHR acts directly on the nuclear STAT5 rather than targeting upstream activators.
FIGURE 2.
VHR selectively tyrosine dephosphorylates STAT5. A, Same as in Fig. 1A, except that FLAG-STAT1 was used instead of Myc-STAT5A. Immunoprecipitated FLAG-STAT1 was analyzed for its tyrosine phosphorylation using phospho-STAT1 Abs (upper panel). B, Same as in A, except that cells were transfected with both FLAG-STAT1 and Myc-STAT5A in addition to either empty vector or WT or C→S mutant VHR. Nuclear extracts were divided and subjected to immunoprecipitation using either anti-FLAG or anti-Myc Abs. Anti-FLAG and anti-Myc immunoprecipitates were analyzed by Western blotting (wb) using anti-phospho-STAT1 (lanes 1–12) and anti-phospho-STAT5 Abs (lanes 13–24), respectively. C, Cells were transiently transfected with the indicated VHR plasmids and HA-tagged Jak1 constructs. Extracts were prepared after 48 h and subjected to immunoprecipitation using anti-HA Abs. Immunoprecipitates were analyzed for phosphorylation using 4G10 anti-phosphotyrosine Abs. D, Splenic B cells were isolated from WT and VHR−/− mice and stimulated with IFNβ (1000 U/ml; upper panels) or IL-4 (lower panels) for 30 min before the addition of staurosporine (STSP; 500 nM), and incubation was continued for the indicated time periods. Tyrosine phosphorylation of STAT5 was analyzed by using anti-phospho-STAT5 Abs (bar graphs represent average ± SD of pSTAT5 normalized to total STAT5; n = 3).
To verify that STAT5 is an authentic substrate for endogenous VHR, we used cells derived from animals with a targeted disruption of the VHR gene (F. Cerignoli, A. Alonso, and T. Mustelin, manuscript in preparation). Pulse-chase experiments on WT and VHR−/− splenic B cells revealed increased levels of STAT5 tyrosine phosphorylation upon IFNβ stimulation in VHR−/− cells (Fig. 2D, upper panels). Intriguingly, the difference was only evident when IFNβ was used for stimulation of cells, whereas no difference in STAT5 phosphorylation could be detected after IL-4 administration (Fig. 2D, lower panels). It is interesting to note that the loss of VHR again mainly affected the magnitude of STAT5 tyrosine phosphorylation, whereas the kinetics remained essentially unchanged. This speaks against a negative feedback regulation of VHR but is compatible with the activation of VHR by upstream signaling events.
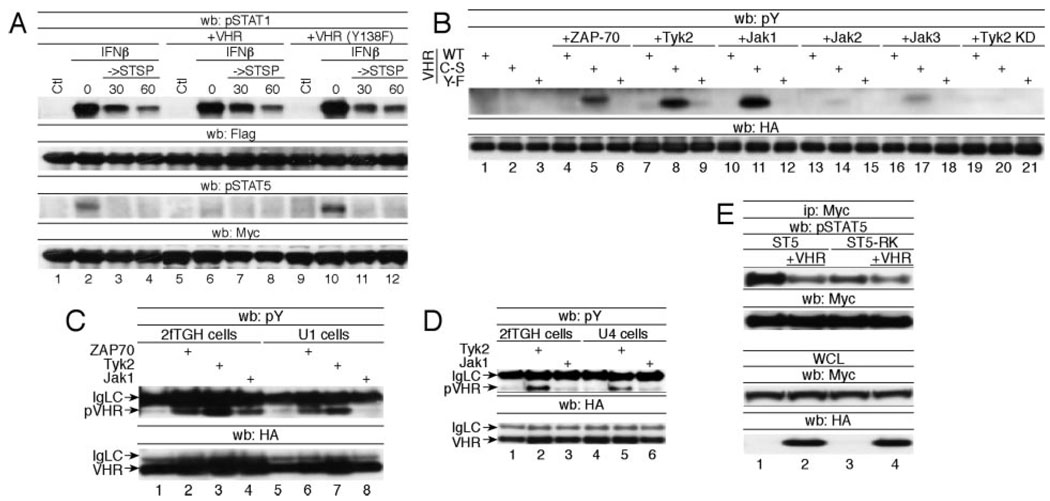
VHR belongs to the group of dual-specific phosphatases that inactivate the MAPKs ERK2 and JNK by dephosphorylating the phospho-Thr and phospho-Tyr residues in their respective activation loops (10–12). VHR, a predominantly nuclear enzyme, is phosphorylated at Tyr138 by ZAP70 upon TCR stimulation (13). Expression of VHR in ZAP70-deficient cells or expression of a VHR Y138F mutant causes no inhibition of the ERK1/2 pathway, indicating that Tyr138 phosphorylation is crucial for VHR to exert control over the MAPKs (13). To test whether phosphorylation of VHR at Tyr138 also plays a role in its phosphatase activity toward STAT5, we examined the dynamics of STAT5 tyrosine dephosphorylation in the presence of ectopically expressed VHR(Y→F). Like VHR(C→S), VHR(Y→F) acted in a fashion consistent with the function of a dominant negative mutant (Fig. 3A).
FIGURE 3.
Requirement for VHR-Tyr138 phosphorylation as a STAT5 phosphatase. A, 293T cells were transiently transfected with FLAG-STAT1 or Myc-STAT5A and either empty vector or WT VHR, VHR(C→S), or VHR(Y→F). A pulse-chase experiment was performed as described in Fig. 1A. Tyrosine phosphorylation and efficiency of immunoprecipitation of FLAG-STAT1 and Myc-STAT5A were analyzed using the indicated Abs. B, Cells were transfected with the indicated HA-tagged VHR and kinase constructs and phosphorylation of immunoprecipitated VHR was determined by anti-phosphotyrosine (pY) Western blotting (wb). C, 2fTGH cells and their Tyk2-deficient counterparts (U1) were transfected with HA-VHR(C→S) and the indicated tyrosine kinase. Anti-HA immunoprecipitated VHR was analyzed for its tyrosine phosphorylation (upper panel). D, Same as C, except that Jak1-deficient U4 cells were used for transfection. E, Myc-STAT5A or STAT5A(R→K) were cotransfected with Tyk2 and HA-VHR as indicated. Phosphorylation of immunoprecipitated (Ip) STAT5 was determined by anti-phospho-STAT5 (pSTAT5) immunoblotting. IgLC, Ig light chain; STSP, staurosporine; WCL, whole cell lysate.
In contrast to the other Jak kinase family members, Tyk2 shares a significant degree of homology with Syk/ZAP70 in the kinase domain (Ref. 14 and our unpublished observations). As ZAP70 was previously identified as a tyrosine kinase for VHR, it was conceivable that Tyk2 could act in a similar function. To test this hypothesis, we examined whether the Janus kinase family members Jak1, Jak2, Jak3, and Tyk2 could tyrosine phosphorylate VHR. Because VHR is known to auto-dephosphorylate, we also included the catalytically inactive phosphatase in these experiments. As shown in Fig. 3B, Tyk2 and Jak1 were as efficient as ZAP70 in tyrosine phosphorylating VHR, whereas Jak2 and Jak3 displayed no activity toward the phosphatase. The Tyk2-mediated tyrosine phosphorylation of VHR was prevented by the additional mutation of Tyr138 in VHR. Furthermore, tyrosine phosphorylation of VHR required the kinase activity of Tyk2, because a kinase-dead mutant of Tyk2 failed to phosphorylate VHR. It is interesting to note that tyrosine phosphorylation was detected only in the catalytically inactive VHR but not in the WT phosphatase when coexpressed with the different kinases (Fig. 3B). However, tyrosine phosphorylation of WT VHR, but not of VHR(Y138F), was restored when the experiment was performed in the presence of the tyrosine phosphatase inhibitor orthovanadate (not shown), suggesting auto-dephosphorylation or trans-dephosphorylation of WT VHR.
It was surprising to find that Jak1 also induced VHR tyrosine phosphorylation, as the kinase bears little resemblance to Syk/ZAP70. Because 293T cells express endogenous Tyk2, we reasoned that Jak1 might drive Tyk2 activation through transphosphorylation. To test the hypothesis that phosphorylation of VHR by Jak1 may actually be mediated by Tyk2, we examined the ability of Jak1 to tyrosine phosphorylate VHR in the Tyk2-deficient U1A cell line (15). ZAP70 and Tyk2 still retained their ability to phosphorylate VHR (Fig. 3C) in U1A cells. In contrast, Jak1 could phosphorylate VHR only in the parental 2fTGH cell line but not in U1A cells despite the presence of overexpressed Jak1. When Jak1-deficient UA4 cells were used for the experiment (16), Tyk2-mediated tyrosine phosphorylation of VHR occurred just as readily as in WT 2fTGH cells (Fig. 3D), indicating that Jak1 was not required for the phosphorylation of VHR by Tyk2. This finding can also explain the fact that altered STAT5 phosphorylation in VHR−/− B cells was only observed after IFNβ but not after IL-4 stimulation, as only IFNβ is known to activate Tyk2.
Tyrosine phosphorylation of VHR does not effect its catalytic activity in vitro (13) and is therefore likely to exert a regulatory role by mediating protein-protein interactions. Thus, we hypothesized that if tyrosine-phosphorylated VHR can act as a binding partner for the STAT5 Src homology 2 (SH2) domain, it may act on the STAT5 dimer and displace one tyrosinephosphorylated STAT5 molecule from the SH2 domain of the other and subsequently dephosphorylate STAT5 on Tyr694.
Indeed, we found that tyrosine dephosphorylation of STAT5 by VHR required the SH2 domain of STAT5, because STAT5(R→K) harboring a defective SH2 domain was significantly less susceptible to dephosphorylation by VHR (Fig. 3E).
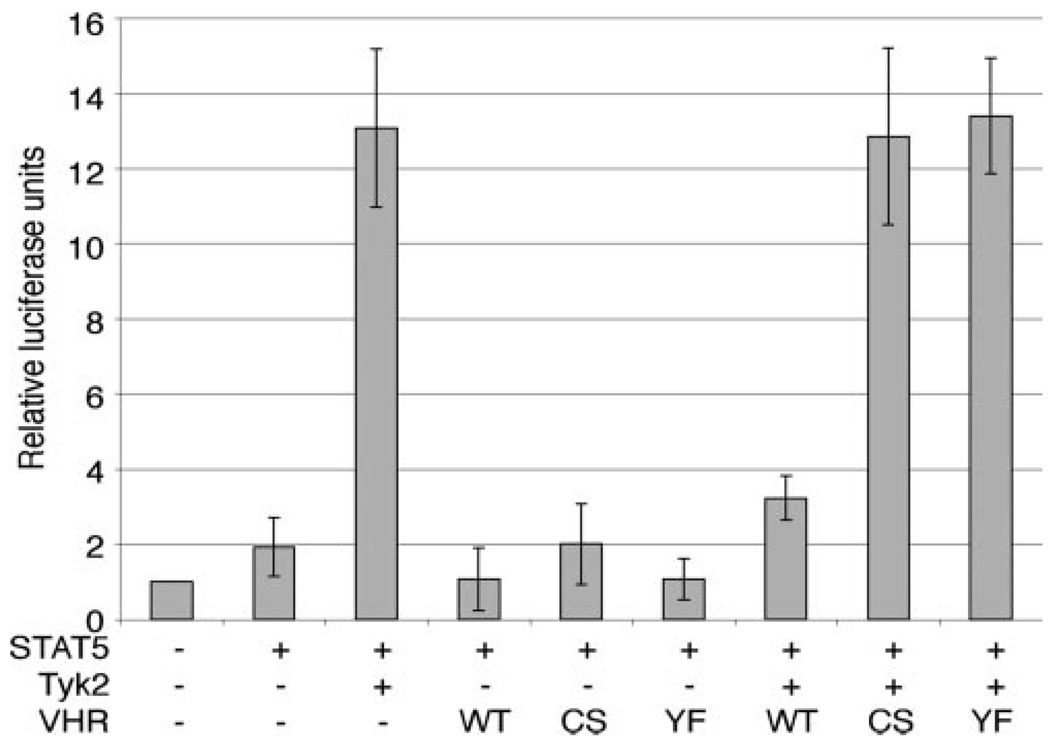
Lastly, we assessed the effects of VHR on the transcriptional induction of the β-casein gene promoter, a well known target of activated STAT5 (Fig. 4). STAT5 tyrosine phosphorylation and the luciferase reporter were fully induced by coexpression of STAT5 and Tyk2. VHR coexpression severely reduced Tyk2-induced, STAT5-mediated transcriptional activity to levels close to baseline values. The STAT5-mediated transcriptional induction appeared to be at maximum levels in this system, as the presence of the dominant-negative VHR(C→S) or VHR(Y→F) mutants did not further increase the reporter activity.
FIGURE 4.
Inhibition of STAT5-mediated transcription by VHR. 293T cells were transfected with the indicated plasmids and the STAT5-responsive β-casein-luciferase. CMV-driven β-galactosidase was included to normalize for transfection efficiency. Cells were lysed 48 h after transfection, and lysates were analyzed for luciferase and β-galactosidase activity. Average results of four independent experiments are shown. CS, C→S; YF, Y→F.
Tyrosine dephosphorylation of activated STATs is an important component of the negative regulation of cytokine signaling. STAT5 is the first transcription factor reported, in this study, to be a substrate for the small dual-specificity phosphatase VHR. It is of interest to note that Tyk2 plays dual roles by directing the tyrosine phosphorylation and activation of STAT5 as well as VHR, the negative regulator of STAT5. The direct coupling of Tyk2 to VHR activation offers a mechanism by which the Jak/STAT signaling pathway ties its own down-regulation intimately and mechanistically to the initial stimulus.
Footnotes
This work was supported in part by National Institutes of Health Grant R01 CA80105 (to M. D.).
Abbreviations used in this paper: TcPTP, T cell protein tyrosine phosphatase; HA, hem-agglutinin; PTP, protein tyrosine phosphatase; SH2, Src homology 2; Tyk2, tyrosine kinase 2; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Larner AC, David M, Feldman GM, Igarashi K, Hackett RH, Webb DAS, Sweitzer SM, Petricoin EF, III, Finbloom DS. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993;261:1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- 2.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 3.David M, Grimley PM, Finbloom DS, Larner AC. A nuclear tyrosine phosphatase downregulates interferon-induced gene expression. Mol. Cell. Biol. 1993;13:7515–7521. doi: 10.1128/mcb.13.12.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilton DJ. Negative regulators of cytokine signal transduction. Cell. Mol. Life Sci. 1999;55:1568–1577. doi: 10.1007/s000180050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ten Hoeve J, de Jesus Ibarra-Sanchez M, Fu Y, Zhu W, Tremblay M, David M, Shuai K. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol. Cell. Biol. 2001;22:5662–5668. doi: 10.1128/MCB.22.16.5662-5668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu CL, Burakoff SJ. Involvement of proteasomes in regulating Jak-STAT pathways upon interleukin-2 stimulation. J. Biol. Chem. 1997;272:14017–14020. doi: 10.1074/jbc.272.22.14017. [DOI] [PubMed] [Google Scholar]
- 7.Yu CL, Jin YJ, Burakoff SJ. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J. Biol. Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]
- 8.Aoki N, Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J. Biol. Chem. 2000;275:39718–39726. doi: 10.1074/jbc.M005615200. [DOI] [PubMed] [Google Scholar]
- 9.Aoki N, Matsuda T. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol. Endocrinol. 2002;16:58–69. doi: 10.1210/mend.16.1.0761. [DOI] [PubMed] [Google Scholar]
- 10.Alonso A, Saxena M, Williams S, Mustelin T. Inhibitory role for dual specificity phosphatase VHR in T cell antigen receptor and CD28-induced Erk and Jnk activation. J. Biol. Chem. 2001;276:4766–4771. doi: 10.1074/jbc.M006497200. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher MA, Todd JL, Rice AE, Tanner KG, Denu JM. Structural basis for the recognition of a bisphosphorylated MAP kinase peptide by human VHR protein Phosphatase. Biochemistry. 2002;41:3009–3017. doi: 10.1021/bi015799l. [DOI] [PubMed] [Google Scholar]
- 12.Todd JL, Tanner KG, Denu JM. Extracellular regulated kinases (ERK) 1 and ERK2 are authentic substrates for the dual-specificity protein-tyrosine phosphatase VHR. A novel role in down-regulating the ERK pathway. J. Biol. Chem. 1999;274:13271–13280. doi: 10.1074/jbc.274.19.13271. [DOI] [PubMed] [Google Scholar]
- 13.Alonso A, Rahmouni S, Williams S, van Stipdonk M, Jaroszewski L, Godzik A, Abraham RT, Schoenberger SP, Mustelin T. Tyrosine phosphorylation of VHR phosphatase by ZAP-70. Nat Immunol. 2003;4:44–48. doi: 10.1038/ni856. [DOI] [PubMed] [Google Scholar]
- 14.Su L, David M. Distinct mechanisms of STAT phosphorylation via the interferon-α/β receptor. Selective inhibition of STAT3 and STAT5 by piceatannol. J. Biol. Chem. 2000;275:12661–12666. doi: 10.1074/jbc.275.17.12661. [DOI] [PubMed] [Google Scholar]
- 15.Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon α/β signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 16.Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur AG, Barbieri G, Withuhn BA, Schindler C, et al. The protein tyrosine kinase JAK1 complements defects in the interferon-α/β and -γ signal transduction. Nature. 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]