Abstract
Objective
To evaluate risk factors of sub-clinical atherosclerosis in a pediatric SLE population.
Methods
A prospective multicenter cohort of 221 patients underwent baseline measurements of carotid intima medial thickening (CIMT) as part of the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) trial. SLE disease measures, medications, and traditional risk factors for atherosclerosis were assessed. A standardized protocol was used to assess thickness of the bilateral common carotids and mean maximal IMT of 12 segments. Univariable analysis identified potential associations with CIMT that were examined in multivariable linear regression modeling.
Results
Based on mean-mean common or mean-max CIMT as the dependent variable, univariable analysis showed significant associations with increased CIMT: increasing age, longer SLE duration, minority status, higher BMI, male sex, increased creatinine clearance, higher Lp(a), proteinuria, azathioprine use, and prednisone dose. Azathioprine use (P=0.005 for mean-mean common; P=0.102 for mean-max model) and male sex (P< 0.001) were both associated with increases in mean-max CIMT. Moderate dose prednisone (0.15–0.4 mg/kg/day) was associated with decreases in mean-max CIMT (P=0.024) while high or low dose prednisone was associated with mean-mean common CIMT (P=0.021) or mean-max CIMT (P=0.064), respectively. BMI (P<0.001) and creatinine clearance (P=0.031), remained associated with increased mean-mean common CIMT, while increasing age (P<0.001) and increasing Lp(a) (P=0.005) were associated with increased mean-max CIMT.
Conclusion
Traditional as well as non-traditional risk factors are associated with increased CIMT in pediatric SLE patients in this cohort. Azathioprine treatment was associated with increased CIMT. The relationship of CIMT with prednisone dose may not be linear.
Over the last 3 decades, lupus-related mortality has decreased in all categories except cardiovascular disease (1). The lack of improvement in cardiovascular morbidity and mortality in systemic lupus erythematosus (SLE) may reflect improved survival as patients with lupus now live long enough to develop cardiovascular disease; however, women under 40 years of age with SLE are at high risk of developing myocardial infarction (MI) with risks ranging up to 50 times higher than control populations (2). The unique convergence of immune and vascular pathology in SLE places young women at unusually high risk of cardiovascular disease (3)—a risk that is not well addressed by current treatment or prevention regimens.
Atherosclerosis begins in childhood even in the absence of SLE. Autopsy studies of trauma victims commonly showed fatty streaks in the arteries of children (4). Children and adolescents with SLE are particularly at risk as they age given their life-long burden of exposure to multisystem inflammatory disease with high atherogenic potential. As a result, prevention of long-term cardiovascular complications in pediatric onset SLE presents a particularly attractive target for intervention with the possibility of significantly improving quality of life and increasing survival over many decades.
Several investigators have demonstrated atherosclerotic heart disease in asymptomatic patients with SLE across the age range using noninvasive imaging techniques such as computerized tomography, carotid ultrasound and arterial flow-mediated dilatation (3, 5–8). These studies have identified a variety of factors contributing to the development of subclinical atherosclerosis, including traditional risk factors (obesity, smoking, glucose intolerance, family history), medications, hypertension, increased homocysteine levels, and renal disease (9, 10), but also indicate that important additional factors exist in this population. The identity of lupus-specific factors is not well defined but likely relates to the immune pathogenesis of SLE (6, 11), implicating SLE itself as a potent independent risk factor. Although few published studies specifically address cardiovascular risk factors in pediatric SLE, the pathogenesis of atherosclerosis in children and adolescents with SLE is likely multifactorial as in adults. Investigators have shown that nephrotic range proteinuria in children with SLE is associated with carotid intima media thickening (CIMT) (5).
Since children and adolescents with SLE have fewer co-morbidities and traditional cardiovascular risk factors than adults with lupus, this population presents a unique opportunity to further understand the role of SLE in the pathogenesis of premature atherosclerosis. Currently there are no standard imaging procedures for assessment of preclinical atherosclerosis in young people; however, non-invasive techniques utilized in ongoing observational studies and clinical trials in a variety of disorders may result in clinically useful technology in the future. CIMT as measured by carotid ultrasound is a well studied surrogate marker of atherosclerosis which has been shown to predict cardiovascular events (strokes and myocardial infarction) (12). It has been used widely as an outcome measure in clinical trials including studies in children with increased risk of atherosclerosis due to familial hypercholesterolemia (13, 14). Although CIMT increases with age, the progression rate in healthy children and adolescents is negligible, 0.000 mm/year between 10–24 years of age in females and 10–19 years of age in males (15). In contrast, the progression rate as measured by CIMT in adult women with SLE is 0.004 mm/year (women <30 years of age) to 0.010mm/year (women >30 years of age) (Susan Manzi, personal communication) (16). Population studies show that increases in carotid wall thickening as small as 0.1 mm strongly correlate with increases in coronary and cerebral vascular events (17).
The ongoing Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) Trial is designed to prospectively assess the effect of atorvastatin on the progression of CIMT in children and young adults with SLE. While the final results of the APPLE Trial will not be available until 2010, the purpose of the current analysis is to investigate the association of traditional and non-traditional risk factors with a surrogate marker of early atherosclerosis (CIMT) in children and adolescents with SLE using APPLE baseline data.
METHODS
Participants
A prospective multicenter cohort of 221 children and adolescents with SLE from 21 sites in North America underwent ultrasound measurement of CIMT at enrollment as part of the APPLE Trial. The APPLE Trial is a double-blind randomized placebo controlled trial designed to determine the efficacy and safety of atorvastatin in preventing the progression of atherosclerosis in children and adolescents with SLE treated for 3 years. The primary endpoint is the rate of progression of mean CIMT in the common carotid artery over 3 years. Subjects are randomized to receive either placebo or atorvastatin (10 or 20 mg daily depending on weight). All participants are treated with folate, hydroxychloroquine, low dose aspirin, dietary recommendations, and risk factor counseling. Enrollment was completed in 2006 with study results expected in 2010.
All subjects met the American College of Rheumatology (ACR) 1997 revised diagnostic criteria for SLE. Additional inclusion requirements included weight ≥25 kg, outpatient status, age between 10 and 21 years, ability to complete self-report questionnaires in English or Spanish, willingness to comply with recommended diet, and willingness to use an approved birth control method. Patients were excluded from the study if they had active nephrotic syndrome, myositis, liver disease, renal insufficiency, or hypercholesterolemia warranting treatment (total cholesterol >350 mg/dL) at baseline. See Table 1 for the full inclusion and exclusion criteria for the APPLE Trial.
Table 1.
Inclusion and exclusion criteria for the APPLE trial
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • SLE diagnosed based on 1997 Revised ACR criteria | • drug-induced lupus |
| • weight ≥25 kg | • liver disease (ALT or AST >2x ULN) |
| • outpatient status | • myositis (CK >3x ULN) |
| • age between ≥10 and ≤21 years | • nephrotic syndrome (urinary protein >3 g/24 h or protein/creatinine ratio of >3.0 and serum albumin <2.3 g/dL) |
| • ability to complete self report questionnaires in English or Spanish | • hypercholesterolemia (total cholesterol >350 mg/dL) |
| • willingness to comply with recommended diet | • presence of xanthoma |
| • willingness to use approved birth control method | • familial hypercholesterolemia |
| • renal insufficiency (dialysis or creatinine > 2.5 mg/dL) | |
| • current use of cyclosporine or tacrolimus | |
| • pregnant or lactating | |
| • current use of oral contraceptives containing ≥50 mcg estradiol | |
| • life threatening non-SLE illness | |
| • current drug or alcohol abuse | |
| • inability to obtain adequate-quality CIMT images |
ULN – upper limit of normal
Clinical variables of interest
The APPLE baseline assessment included demographics, a history and physical examination performed by a pediatric rheumatologist, chart review, and subject and parent questionnaires. Variables of interest for this analysis were SLE clinical and laboratory parameters, non-lupus and lupus-related medical history, family history, current medication use, traditional risk factors for atherosclerosis, and SLE disease activity and damage. Race and ethnicity were self-reported. For the purposes of this analysis, subjects were categorized as minority if they were either Hispanic or non-Caucasian.
SLE disease activity and damage were assessed using the modified SELENA SLE Disease Activity Index (SLEDAI) (18) and Systemic Lupus International Collaborating Clinics/ACR Damage Index (SLICC) (19). For these analyses, the total SLEDAI score was used as a continuous variable. SLICC scores were analyzed as a dichotomous variable with subjects grouped as score of 0 (no damage) or score ≥1 (damage present). Additionally, SLE-specific laboratory testing was obtained. Proteinuria was defined as a spot urine protein/creatinine ratio > 0.5 or a timed urine with protein > 500 mg/24 hours. Creatinine clearance was calculated using the Schwartz formula (20).
Traditional risk factors for atherosclerosis, including hypertension, cardiovascular family history, smoking, diet, exercise, and body mass index (BMI) were assessed. A history of hypertension referred to reported current or historical hypertension. Similarly a history of nephrotic syndrome and history of nephritis referred to reported current or historical event. A family history of cardiovascular disease was defined as subject-reported cerebrovascular accident, myocardial infarction, angina or atherosclerosis in a parent or grandparent.
Oral glucocorticoid doses were weight-adjusted and recorded as prednisone equivalent mg/kg/day. Twelve hour fasting lipids, homocysteine, lipoprotein A (Lp(a)), and high sensitivity c-reactive protein (hsCRP) were processed centrally (PPD, Highland Heights, Kentucky). Total cholesterol, triglycerides and high density lipoprotein (HDL) were measured directly and low density lipoprotein (LDL) was calculated by the Friedwald equation (21). All other laboratory assessments were performed locally. HsCRP and Lp(a) were log transformed to achieve approximately normal distributions.
Measurement of carotid intima media thickening (CIMT)
Two CIMTs were obtained at baseline using a standardized ultrasound protocol similar to those from several previous clinical trials (22–24). Carotid ultrasound exams were performed using portable Siemens Cypress systems (Siemens AG, Munich, Germany) shared between clinical centers. All scanners were equipped with 7L3 transducers, a cardiology package with ECG tracing and specific APPLE presets in order to reduce variability across centers. Standardized longitudinal b-mode images were collected for three arterial segments defined relative to the tip of the flow divider (TFD) as the common carotid artery (CCA) (from 10 to 20 mm proximal to the tip of the flow divider), the carotid bifurcation (from the TFD to 10 mm proximal to the TFD) and the proximal 10 mm of the internal carotid artery (ICA). Near and far walls were imaged simultaneously in the CCA, but separately in the carotid bifurcation and ICA to improve the ability to align each wall horizontally in these segments. For each arterial segment, images were selected at 90, 120, 150 and 180 degrees marked on the Meijer's Arc® when the right side was scanned and at 270, 240, 210 and 180 degrees when the left side was scanned. Image selections were saved as 5 second digital clips and written to 640 MB magnetic optical disks for transfer to a central reading center (Ward A. Riley Ultrasound Center, Wake Forest University School of Medicine, Winston-Salem NC). All ultrasound scans were read by a single experienced reader using Image Pro software (Media Cybernetics, Inc. Bethesda, MD). For each image sequence, the single reader selected one frame for measurement when the heart was in systole (ECG tracing is on QRS complex). Leading (far wall) and trailing (near wall) edges of visualized blood-intima and media-adventitia boundaries were traced with a computer mouse-controlled caliper within a region of interest specified by the reader. Quality assurance procedures included central training and certification of all sonographers and the reader as well as regular site visits and performance reviews.
The combination of 3 arterial segments, two walls and two sides of the neck provides a set of 12 CIMT measurement sites, each imaged from 4 angles. For each measurement site, a maximum CIMT value defined as the largest of the 4 angle specific maximum CIMTs was calculated. The 12 maximum CIMT values were then averaged to determine the mean-max CIMT over near and far walls of the right and left CCA, carotid bifurcation and ICA. For each of the 4 measurement sites in the CCA, a mean CIMT value defined as the average of the 4 angle specific mean CIMTs was also calculated. The 4 mean CIMT values were then averaged to determine the mean-mean common CIMT. Per protocol, duplicate ultrasound exams were performed within a 4 week interval at baseline and prior to randomization for each participant. The values of mean-mean common CIMT and mean-max CIMT were averaged for these two exams to obtain stable baseline measures of CIMT. Both CIMT summary measures are recognized as noninvasive surrogate predictors of future cardiovascular events and widely used in clinical trials testing the efficacy of drugs in slowing or reversing atherosclerosis (25–28).
Statistical approaches
The characteristics of the study sample were summarized using descriptive statistics with dichotomous or ordinal data presented as percentages and continuous data as means, standard deviations (SD), and medians. Differences between groups were assessed with either the chi-squared test or the non-parametric wilcoxon test.
Using cross sectional baseline data from the APPLE Trial, we first investigated potential risk factors and their univariable relationship with each of the two baseline CIMT outcomes: 1) mean-mean common CIMT (mm) and 2) mean-max CIMT (mm). The potential risk factors included demographics, physical characteristics, renal function, markers of inflammation, medical history, lipid levels, current or recent medications, and disease indices. Linear regression models were fitted using one potential risk factor of CIMT at a time to select candidate variables for multivariable modeling. Residual plots of the regression models were used to assess the suitability of the linear model. Scatter plots with overlay of LOESS (locally weighted scatterplot smoothing) curves (29) were produced to examine the descriptive relationship between continuous risk factors and CIMT outcome variables. Histograms of CIMT values by levels of the categorical risk factors were used to examine the relationship between categorical variables and CIMT outcomes. For non-linear relationships between the continuous risk factors and the CIMT outcome, transformations or piecewise linear regression models were used to better characterize the univariable relationship.
Variables with p-value ≤0.2 in the univariable model were included in multivariable linear regression modeling. Several model building procedures were used including forward, backward, and stepwise regression to produce two final models, one for each of the two CIMT outcomes. Several methods were used to assess colinearity and stability of the final models, including assessment of the association between independent variables, colinearity diagnostics within regression framework, and Mallow's Cp values. All statistical inference was done within a linear regression model framework and a p-value ≤ 0.1 is considered statistically significant due to exploratory nature of the analyses. All calculations were performed using SAS version 8.2 (30).
RESULTS
Subject characteristics
A summary of baseline demographic and clinical SLE characteristics are shown in Table 2. Of the 221 enrolled subjects, the mean age was 15.7 years (range 10.1–21.7 years), 83% were female, and 65% were minority (Hispanic or non-Caucasian). Subjects had mean disease duration of 31 months (median 25 months, interquartile range 8 to 46 months) and had mildly active disease with an average SLEDAI score of 4.6. Most subjects had no damage as measured by the SLICC. Of the 178 subjects on prednisone at study baseline, the mean weight-adjusted prednisone dose is 0.19 mg/kg/day (median 015 mg/kg/day, and interquartile range 0.05 to 0.26 mg/kg/day). The two baseline (prerandomization) CIMTs were performed a median of 9 days apart (interquartile range 2, 21).
Table 2.
Baseline characteristics of the APPLE subjects.
| Variable | Mean (S.D.) or n (%) | Median | Total N |
|---|---|---|---|
| Age (years) | 15.7 (2.6) | 15.5 | 221 |
| Female | 184 (83.3%) | 221 | |
| Weight (kg) | 62.0 (17.2) | 58.7 | 221 |
| Body Mass Index | 24.4 (5.3) | 23.4 | 221 |
| Duration of Lupus (months) | 31.2 (28.5) | 25.0 | 220 |
| Hispanic | 54 (24.4%) | 221 | |
| Race*** | |||
| Caucasian | 114 (51.6%) | 221 | |
| African American | 59 (26.7%) | 221 | |
| Asian | 23 (10.4%) | 221 | |
| American Indian | 6 (2.7%) | 221 | |
| Native Hawaiian | 5 (2.3%) | 221 | |
| Other | 30 (13.6%) | 221 | |
| Minority (Hispanic Ethnicity or Non-Caucasian Race) | 144 (65.2%) | 221 | |
| Hx. Smoking (self-report) | 7 (3.2%) | 221 | |
| Prednisone Dose (mg/kg/day) | 0.19 (0.19) | 0.15 | 218 |
| Fam. Hx. Cardiovascular disease | 79 (37.4%) | 211 | |
| Creatinine Clearance (mL/min/m2) | 139.4 (33.0) | 134.1 | 216 |
| Proteinuria | 56 (25.5%) | 220 | |
| Hx. Renal Abnormalities | |||
| Hx. Hypertension | 73 (34.1%) | 214 | |
| Hx. Nephrotic syndrome | 38 (17.4%) | 219 | |
| Hx. Nephritis | 79 (36.1%) | 219 | |
| Other | 20 (10.5%) | 190 | |
| Current Medications | |||
| Hydroxychloroquine | 213 (96.4%) | 221 | |
| Corticosteroids | 181 (81.9%) | 221 | |
| Cyclophosphamide | 26 (11.8%) | 221 | |
| Mycophenolate | 53 (24.0%) | 221 | |
| Azathioprine | 30 (13.6%) | 221 | |
| Methotrexate | 29 (13.1%) | 221 | |
| ACE# inhibitors | 54 (24.4%) | 221 | |
| NSAIDs | 68 (30.8%) | 221 | |
| ASA | 147 (66.5%) | 221 | |
| Total Cholesterol (mg/dL) | 155.1 (38.0) | 148.0 | 211 |
| HDL Cholesterol (mg/dL) | 46.3 (12.8) | 44.0 | 211 |
| LDL Cholesterol (mg/dL) | 86.4 (31.4) | 78.5 | 210 |
| Triglycerides (mg/dL) | 114.0 (66.4) | 101.0 | 211 |
| Homocysteine (mcmol/L) | 7.5 (3.1) | 6.8 | 207 |
| Lp(a) (mg/dL) | 23.1 (26.8) | 13.5 | 206 |
| Log Lp(a) | 2.5 (1.2) | 2.6 | 206 |
| Mean-Maximal CIMT (mm) | 0.585 (0.052) | 0.579 | 221 |
| Mean-Mean Common CIMT (mm) | 0.468 (0.041) | 0.466 | 221 |
| HsCRP (mg/L) | 3.6 (13.9) | 0.7 | 202 |
| Log hsCRP | −0.27 (1.52) | −0.36 | 202 |
| ESR | 23.1 (22.9) | 16.0 | 148 |
| Anti-DS DNA antibody positive | 106 (48.0%) | 221 | |
| SLEDAI Total | 4.6 (4.2) | 4.0 | 221 |
| SLICC Damage Index >0 | 59 (26.7%) | 221 |
* Creatinine clearance calculated using Schwartz Formula as in methods
** Proteinuria defined as >500 mg/24 hours or spot protein:creatinine ratio of 0.5.
Some subjects self-reported more than one race.
Angiotensin converting enzyme
Univariable analysis
The average of the two baseline CIMT measurements rather than a single baseline measurement was used for analysis to increase precision. The two baseline scans were strongly correlated with Pearson correlation coefficients of 0.75 for mean-mean common CIMT and 0.76 for mean-max CIMT. Based on either mean-mean common or mean-max CIMT as the dependent variable, univariable analysis suggested the following risk factors to be significantly associated with increased CIMT: increasing age, longer duration of SLE, minority status, higher BMI, male sex, higher creatinine clearance, higher Lp(a), presence of proteinuria, current azathioprine use, and current weight-adjusted prednisone dose. The complete univariable modeling results are shown in Table 3.
Table 3.
Univariable relationships between clinical risk factors and CIMTs
| Mean-Mean Common CIMT (mm) | Mean-Max CIMT (mm) | |||
|---|---|---|---|---|
| Variable | Slope (S.E.) | P-Value | Slope (S.E.) | P-Value |
| Age (Years) | 0.001 (0.001) | 0.175 | 0.005 (0.001) | < 0.001 |
| Log hsCRP | 0.002 (0.002) | 0.237 | 0.0008 (0.002) | 0.752 |
| BMI | 0.001 (0.0005) | 0.005 | 0.0004 (0.0007) | 0.504 |
| SLEDAI Total | 0.0007 (0.0006) | 0.302 | −0.0002 (0.0008) | 0.849 |
| Positive anti-DS DNA antibody | 0.005 (0.005) | 0.368 | 0.002 (0.007) | 0.738 |
| Duration of Lupus (Months) | 0.0002 (0.00010) | 0.015 | 0.0004 (0.0001) | 0.001 |
| Creatinine Clearance* (mL/min/m2) | 0.004 (0.002) | 0.008 | 0.003 (0.002) | 0.134 |
| Proteinuria** | 0.016 (0.006) | 0.010 | 0.010 (0.008) | 0.236 |
| Hx. Nephrotic syndrome/Nephritis | 0.006 (0.006) | 0.276 | 0.007 (0.007) | 0.312 |
| Hx. Hypertension | 0.002 (0.006) | 0.681 | −0.002 (0.008) | 0.794 |
| Fam. Hx. of Cardiovascular Disease | −0.003 (0.006) | 0.568 | −0.002 (0.007) | 0.771 |
| LDL | −0.00006 (0.00009) | 0.508 | −0.00007 (0.0001) | 0.547 |
| HDL | −0.0002 (0.0002) | 0.372 | 0.0002 (0.0003) | 0.431 |
| Log Lp(a) | 0.004 (0.002) | 0.092 | 0.009 (0.003) | 0.002 |
| Homocysteine (mcmol/L) | 0.0010 (0.0009) | 0.281 | 0.00004 (0.001) | 0.975 |
| Female | −0.036 (0.007) | < 0.001 | −0.039 (0.009) | < 0.001 |
| SLICC Damage Index > 0 | 0.006 (0.006) | 0.325 | 0.012 (0.008) | 0.131 |
| Minority (Hispanic or non-Caucasian) | 0.011 (0.006) | 0.055 | 0.013 (0.007) | 0.072 |
| NSAIDs Current | 0.007 (0.006) | 0.235 | 0.003 (0.008) | 0.731 |
| Cyclophosphamide/Mycophenolate Current | 0.001 (0.006) | 0.800 | 0.001 (0.007) | 0.847 |
| Azathioprine Current | 0.017 (0.008) | 0.031 | 0.023 (0.010) | 0.022 |
| Methotrexate Current | 0.010 (0.008) | 0.209 | 0.005 (0.010) | 0.659 |
| ASA Current | −0.004 (0.006) | 0.492 | 0.007 (0.007) | 0.328 |
| ACE# Inhibitor Current | 0.010 (0.006) | 0.125 | 0.003 (0.008) | 0.724 |
| Prednisone (<0.15 mg/kg/day) | 0.086 (0.056) | 0.129 | 0.109 (0.072) | 0.132 |
| Prednisone (0.15–0.40 mg/kg/day) | −0.096 (0.043) | 0.027 | −0.150 (0.055) | 0.007 |
| Prednisone (>0.40 mg/kg/day) | 0.091 (0.041) | 0.027 | 0.052 (0.052) | 0.318 |
Creatinine clearance calculated using Schwartz Formula as in methods
Proteinuria defined as >500 mg/24 hours or spot protein:creatinine ratio of 0.5.
Angiotensin converting enzyme
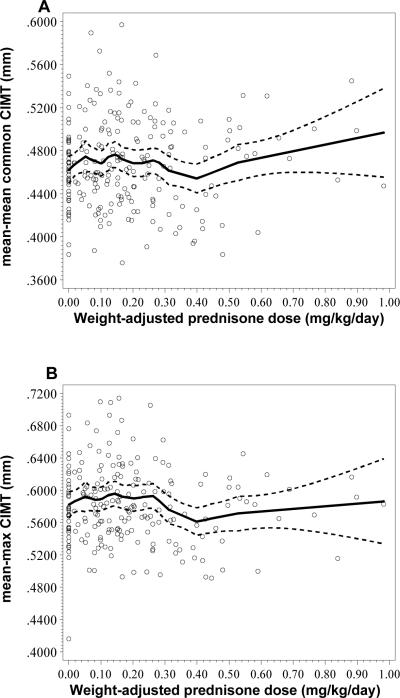
The relationship between weight-adjusted prednisone dose and both mean-mean common or mean-max CIMT was found to be non-monotonic with three distinct slopes. We identified three different slopes upon examining the relationship between prednisone and CIMT and therefore performed 3 piece-wise linear regressions using the following weight-adjusted prednisone dose ranges − 0.0 to 0.15 mg/kg/day, 0.15 to 0.4 mg/kg/day, and > 0.4 mg/kg/day. Descriptive relationships are shown in Figure 1 with LOESS curves overlaying the scatter plots of mean-mean common or mean-max CIMT vs. weight-adjusted prednisone dose.
Figure 1.
A. LOESS Curve showing mean-mean common CIMT vs. weight-adjusted prednisone dose (mg/kg/day) B. LOESS curve showing mean-max CIMT vs. Weight-adjusted prednisone dose (mg/kg/day).
Multivariable analysis
Different model building procedures yielded the same two final models. The results of the multivariable analysis for each mean-mean common CIMT and mean-max CIMT are shown in Table 4 including the adjusted slope estimates from the final linear regression models of the potential risk factors with the CIMT variable, corresponding standard errors, and p-values. Duration of SLE, minority status and presence of proteinuria are not present in the final models due to non-significant associations after adjustment of the other variables. Azathioprine use (P=0.005 for mean-mean common model and P=0.102 for mean-max model) and male sex (P< 0.001) both were associated with increases in mean-max CIMT. Moderate dose prednisone (0.15–0.4 mg/kg/day) was only associated with decreases in mean max CIMT (P=0.024) while high (>0.4 mg/kg/day) or low (<0.15 mg/kg/day) dose prednisone was associated with mean-mean common CIMT (P=0.021) or mean-max CIMT (P=0.064), respectively. BMI (P<0.001) and creatinine clearance (P=0.031), presumably related to hyperfiltration (31), remained associated only with increased mean-mean common CIMT, while increasing age (P<0.001) and increasing Lp(a) (P=0.005) were associated only with increased mean-max CIMT in the multivariable models. For the mean-mean common CIMT model, R2 = 0.22 and for the mean-max model, R2 = 0.20. Thus, the potential risk factors explained approximately 21% of variation in the CIMT variables. The multivariable models were stable and no important collinearity of variables was noted using regression diagnostics, including the Mallow's Cp values.
Table 4.
Multivariable adjusted relationships between clinical risk factors and CIMTs*
| Mean-Mean Common CIMT (mm) | Mean-Max CIMT (mm) | |||
|---|---|---|---|---|
| Variable Name | Slope (S.E.) | P-Value | Slope (S.E.) | P-Value |
| Age (Years) | NI | 0.004 (0.001) | < 0.001 | |
| BMI | 0.002 (0.0005) | < 0.001 | NI | |
| Creatinine Clearance (mL/min/m2) | 0.003 (0.002) | 0.031 | NI | |
| log of Lp(a) | NI | 0.008 (0.003) | 0.005 | |
| Female | −0.032 (0.007) | < 0.001 | −0.034 (0.009) | < 0.001 |
| Azathioprine Current | 0.021 (0.007) | 0.005 | 0.016 (0.010) | 0.102 |
| Prednisone (0.00–0.15 mg/kg/day) | 0.057 (0.051) | 0.262 | 0.128 (0.069) | 0.064 |
| Prednisone (0.15–0.40 mg/kg/day) | −0.022 (0.041) | 0.587 | −0.119 (0.052) | 0.024 |
| Prednisone (≥0.40 mg/kg/day) | 0.086 (0.037) | 0.021 | 0.049 (0.048) | 0.308 |
Only associations with p-value <= 0.1 in the final models are shown above.
NI – not included in the final models due to non-significant associations.
DISCUSSION
Analysis of the baseline data from the APPLE Trial, a racially and ethnically diverse multicenter prospective cohort of pediatric SLE patients, reveals that both traditional and non-traditional risk factors are associated with increased CIMT in children with SLE. Traditional risk factors predictive of increased CIMT in this cohort include increased BMI, male sex and increasing age. Perhaps more intriguing is the identification of nontraditional risk factors including treatment with azathioprine and weight-adjusted prednisone dose.
The frequent and chronic use of glucocorticoids in children and adolescents with SLE likely plays a role in the prevalence of dyslipidemia and atherosclerosis, but this has not been well characterized to date. Some studies in adult SLE suggest an association between prednisone use and both increased total cholesterol levels and CIMT (32, 33). In contrast, other studies in adults with SLE suggest that those with carotid plaque received less aggressive immunosuppressive therapy (including less prednisone and cyclophosphamide) than those without plaque, suggesting a potentially protective effect of immunosuppressive therapy on the development of atherosclerosis, possibly due to better control of underlying inflammatory disease (6, 10, 34). In the current study, moderate dose prednisone is associated with decreased mean-max CIMT, while higher or lower dose prednisone may predict increased mean-mean or mean-max CIMT, respectively. The demonstration of different relationships between prednisone dose and CIMT may suggest steroids are affecting more than one pathway in atherogenesis. At high doses, prednisone may increase CIMT by increasing traditional risk factors, such as cholesterol, LDL, and BMI. In contrast, with current understanding of the importance of inflammatory mechanisms in the pathogenesis of atherosclerosis (35), lower dose prednisone may be associated with higher CIMT due the presence of ongoing active inflammatory disease. Given the cross-sectional nature of the data, it was not possible to assess the association between cumulative steroid dose and CIMT. However, this will be assessed in longitudinal analysis of the APPLE Trial. The differences in CIMT based on steroid dose may provide insight into previous conflicting results concerning the impact of oral prednisone on cardiovascular risk and suggest that there may be a ”therapeutic window” concerning cardiovascular toxicity in this population.
Other medications used in the treatment of SLE may impact the development of atherosclerosis. For example, cyclosporine contributes to dyslipidemia (36, 37), while hydroxychloroquine improves lipid profiles and is associated with decreased cardiovascular events (38). Azathioprine, mycophenolate mofetil, and cyclophosphamide have not previously been shown to have significant impact on lipid profiles or atherogenesis (38). A surprising finding in this study was that azathioprine was associated with increased CIMT, unlike the other immunosuppressants. This appears to be an effect independent of other variables including SLE disease activity and history of nephritis and not explained by collinearity with other variables. To further understand the relationship between azathioprine and CIMT, the 30 subjects taking azathioprine were compared to the 190 patients not taking azathioprine. As noted in Table 5, subjects on azathioprine were on average one year older (mean age 16.7 years vs. 15.6 years; p=0.027), had slightly longer disease duration (median duration 31.5 months vs. 21.5 months; p=0.005), and were more like to have a SLICC score > 0 (43.3% vs. 24.1%; p =0.027). Upon removing azathioprine from multivariable modeling, duration of SLE became statistically significant but SLICC score did not, suggesting a possible confounding effect due to relationship between azathioprine use and disease duration. However, when both variables (azathioprine and disease duration) were included in the multivariable modeling, only azathioprine remained significant suggesting azathioprine has a stronger association with CIMT. In contrast, age was a significant risk factor for higher CIMT even when azathioprine was included in the multivariable model for mean-max CIMT, suggesting both age and azathioprine have independent effects on CIMT. Given the relative affordability of azathioprine, additional analyses to explore whether azathioprine use reflected socioeconomic status revealed no correlation between the use of azathioprine and household income or parental education (data not shown).
Table 5.
Comparison of subjects taking azathioprine (N=30) and not taking azathioprine (N=190).
| No Azathioprine | Azathioprine | ||||
|---|---|---|---|---|---|
| Variable | Mean (S.D.) or n (%) | Median | Mean (S.D.) or n (%) | Median | P-Value |
| Age (years) | 15.6 (2.7) | 15.5 | 16.7 (2.4) | 17.1 | 0.027 |
| Female | 158/191 (82.7%) | 26/30 (86.7%) | 0.591 | ||
| Weight (kg) | 61.7 (16.9) | 59.5 | 63.9 (19.0) | 58.0 | 0.914 |
| Body Mass Index | 24.3 (5.3) | 23.3 | 24.9 (5.8) | 24.1 | 0.690 |
| Duration of Lupus (Months) | 28.7 (25.9) | 21.5 | 47.0 (38.3) | 31.5 | 0.005 |
| Total Minority (Hispanic Ethnicity or Non-Caucasian Race) | 124/191 (64.9%) | 20/30 (66.7%) | 0.852 | ||
| Fam. Hx. CVD | 69/182 (37.9%) | 10/29 (34.5%) | 0.723 | ||
| History of Smoking | 6/191 (3.1%) | 1/30 (3.3%) | 1.000 | ||
| Prednisone Dose (mg/kg/day) | 0.19 (0.19) | 0.14 | 0.18 (0.17) | 0.15 | 0.870 |
| Creatinine Clearance (mL/min/m2)* | 140.0 (34.0) | 134.4 | 134.9 (25.4) | 133.2 | 0.604 |
| Proteinuria** | 45/191 (23.6%) | 11/29 (37.9%) | 0.098 | ||
| Renal abnormalities | |||||
| Hypertension | 65/184 (35.3%) | 8/30 (26.7%) | 0.354 | ||
| Nephrotic/Nephritic | 83/189 (43.9%) | 14/30 (46.7%) | 0.778 | ||
| Current Medications | |||||
| Hydroxychloroquine | 183/191 (95.8%) | 30/30 (100%) | 0.602 | ||
| Corticosteroids | 154/191 (80.6%) | 27/30 (90.0%) | 0.215 | ||
| Cyclophosphamide | 26/191 (13.6%) | 0/30 (0.0%) | 0.030 | ||
| Mycophenolate | 52/191 (27.2%) | 1/30 (3.3%) | 0.004 | ||
| Methotrexate | 27/191 (14.1%) | 2/30 (6.7%) | 0.385 | ||
| ACE# Inhibitor | 47/191 (24.6%) | 7/30 (23.3%) | 0.880 | ||
| ASA | 128/191 (67.0%) | 19/30 (63.3%) | 0.691 | ||
| NSAIDs | 59/191 (30.9%) | 9/30 (30.0%) | 0.922 | ||
| SLICC Damage Index > 0 | 46/191 (24.1%) | 13/30 (43.3%) | 0.027 | ||
| SLEDAI Positive anti-DS DNA | 94/191 (49.2%) | 12/30 (40.0%) | 0.348 | ||
| SLEDAI Total | 4.6 (4.2) | 4.0 | 4.7 (4.6) 4.0 | 0.985 | |
| ESR | 22.8 (23.3) | 15.0 | 24.9 (20.9) | 18.0 | 0.297 |
| Total Cholesterol (mg/dL) | 155.1 (38.4) | 148.0 | 155.6 (35.4) | 153.0 | 0.865 |
| Triglycerides (mg/dL) | 115.3 (69.3) | 102.0 | 106.1 (44.9) | 97.0 | 0.888 |
| HDL Cholesterol (mg/dL) | 46.0 (12.4) | 44.0 | 48.2 (14.8) | 49.0 | 0.318 |
| LDL Cholesterol (mg/dL) | 86.4 (31.3) | 79.0 | 86.2 (32.3) | 78.0 | 0.924 |
| Homocysteine (mcmol/L) | 7.4 (3.2) | 6.8 | 7.6 (2.8) | 6.5 | 0.734 |
| Lp(a) (mg/dL) | 23.1 (27.6) | 14.0 | 23.0 (21.8) | 13.0 | 0.516 |
| log of Lp(a) | 2.5 (1.2) | 2.6 | 2.6 (1.1) | 2.6 | 0.516 |
| HS CRP (mg/L) | 3.35 (14.40) | 0.60 | 4.88 (10.83) | 0.80 | 0.165 |
| log of HS CRP | −0.34 (1.47) | −0.51 | 0.11 (1.77) | −0.22 | 0.165 |
| Mean-Maximal CIMT (mm) | 0.466 (0.040) | 0.462 | 0.483 (0.041) | 0.475 | 0.036 |
| Mean-Mean Common CIMT (mm) | 0.582 (0.050) | 0.578 | 0.605 (0.058) | 0.611 | 0.034 |
Creatinine clearance calculated using Schwartz Formula as in methods
Proteinuria defined as >500 mg/24 hours or spot protein:creatinine ratio of 0.5.
Angiotensin converting enzyme
While the potential pro-atherogenic effects of azathioprine are not known, an interesting in vitro observation using human umbilical vein endothelial cells showed that azathioprine and its active metabolite, 6-mercaptopurine, caused dose-dependent decreases in endothelial cell proliferation and altered endothelial nucleotide balance by reducing intracellular ATP and GTP (39). It is possible that azathioprine has a direct deleterious impact on endothelial cell function and the capacity for endothelial repair in SLE. In addition, in vitro studies suggest that azathioprine may increase homocysteine levels; however, we did not find a significant relationship between CIMT and homocysteine levels in this analysis (40). It is interesting to note that azathioprine was found to be a contributing predictor of vascular events in a racially/ethnically diverse cohort of adults with SLE in the multicenter LUMINA (Lupus in Minorities: Nature Versus Nurture) cohort (41). Additional study is necessary to further explore this potentially significant association.
Creatinine clearance may be increased in settings of glomerular hyperfiltration, such as proteinuria or hypertension (31, 42). Although patients with significant renal insufficiency or nephrotic syndrome were excluded from the APPLE Trial, many participants had proteinuria and hypertension. With univariable analysis, both proteinuria and creatinine clearance were associated with CIMT, but only creatinine clearance remained significant in the multivariable model. It is possible that creatinine clearance and proteinuria are correlated; however, longitudinal study of this cohort will be necessary to further evaluate this effect.
In this study, CIMT was assessed using two different approaches—mean-mean common CIMT and mean-max CIMT with a correlation of 0.78 between the two outcomes, suggesting that the two outcomes are correlated but are not perfectly linearly related. The two approaches differ in the segments of the carotid artery measured, as well as how the thickness is assessed (average vs. maximal thickness of the measured segments). Both have been used in prior clinical trials and review of the literature reveals no clear superiority of one method over the other in predicting atherosclerotic risk (43, 44). In multiple regression models, we identified factors (sex and medication use) that were significantly associated with both outcomes, and others that were significantly associated with only one outcome (age and Lp(a) were related to mean-max CIMT only, while BMI and creatinine clearance were related to mean-mean common CIMT only). Segment-specific risk factor associations have been reported previously in IMT studies and are generally considered to reflect the influence on focal thickening of local phenomenon such as sheer stress and arterial remodeling (45, 46). Interestingly, Espeland et al. (47) reported segment-specific risk factor associations in coronary artery disease cases, but not in controls of similar age. The observation that obesity is more closely related to common CIMT than to bifurcation or internal CIMT is consistent with prior reports in adults (46, 47). Associations between estimated creatinine clearance and common CIMT have previously been reported in children with chronic renal failure (48) and in young adults with myocardial infarction (49). Reports from children and adolescents with growth hormone deficiency (50) and from adult women with SLE and cardiovascular disease (51) have also documented significant increases for both Lp(a) and common CIMT relative to normal controls. Segment-specific differences in associations between CIMT and either creatinine clearance or Lp(a) have not been reported previously.
There are several limitations to this study. The sample size was only powered to detect difference in the primary outcome of the APPLE Trial – progression rate of CIMT. Thus it may not have sufficient power to identify all meaningful clinical associations of risk factors with baseline CIMT. However, this is the largest cohort of pediatric SLE patients ever examined for cardiovascular risk and the potential relationships reported here are hypothesis generating and can be confirmed by future studies. In addition, as in any clinic trial, there is an inherent selection bias such that the APPLE cohort may not be completely representative of pediatric lupus patients as a whole. For example, obese patients were more likely to be excluded due the inability to obtain high quality CIMT images. Patients with nephrotic range proteinuria, very elevated cholesterol or renal insufficiency, also excluded from the APPLE study, are known to have increased CIMT (5), These subsets of pediatric SLE patients may have increased CIMT and increased lipid abnormalities and different relationships between the risk factors and CIMT. Thus, their exclusion may affect the generalizability of the reported findings.
The presented models only explain approximately 21% of the variation in CIMT suggesting other measured or unmeasured risk factors not identified in the current study. Cross sectional analyses of the baseline data also limits the ability to draw conclusions about causation between risk factors and CIMT outcomes. Once the APPLE Trial is complete, longitudinal analysis will provide more meaningful assessment of the impact of glucocorticoids, azathioprine and other factors on CIMT. Unfortunately, the APPLE study does not include matched normal controls. Because of the inherent variation in CIMT measurement techniques, the absolute thickness can not be compared between studies. Therefore, there is no potential to compare the CIMT results in this cohort with a healthy population.
In summary, traditional as well as non-traditional risk factors predict increased CIMT in pediatric patients with SLE. Of interest, treatment with azathioprine was associated with increased CIMT and the relationship with weight-adjusted oral prednisone dose may not be linear. Future longitudinal analysis of data from the APPLE Trial will further clarify the role of medications such as azathioprine and corticosteroids, as well as other potential risk factors of CIMT progression. Understanding risk factors in the development of atherosclerosis in children and adolescents with SLE will inform evidence-based treatment recommendations to avoid irreversible cardiovascular damage.
ACKNOWLEDGEMENTS
Duke University Medical Center—Esi Morgan Dewitt, MD, MSCE, C. Egla Rabinovich, MD, MPH, Janet Ellis, RT, RDMS, RVT, Janet Wootton, RN, RSCN; Stanford University School of Medicine—Peter Chira MD, MS, Joyce Hsu MD, MS, Tzielan Lee MD, Jan Perea BS, RDMS, RDCS; Schneider Children's Hospital—Beth Gottlieb, MD, Patricia Irigoyen, MD, Jennifer Luftig, MD, Shaz Siddiqi, MD, Zhen Ni, RDMS, RVT, RCDS, Marilynn Orlando, RN, BC, Eileen Pagano, RN, MS; Morgan Stanley Children's Hospital of New York Presbyterian—Andrew Eichenfield, MD, Lisa F. Imundo, MD, Philip Kahn, MD, Candido Batres, MD, Digna Cabral, BS; Hackensack University Medical Center—Kathleen A. Haines, MD, Suzanne C. Li, MD, PhD, Jennifer Weiss, MD, Mary Ellen Riordan, RN, Beena Vaidya, RCDS; University of California at San Francisco Medical Center—Michelle Mietus-Snyder, MD; Hospital for Sick Children—Lawrence Ng, BSc; Indiana University School of Medicine—Susan Ballinger, MD, Thomas Klausmeier, MD, Debra Hinchman, BS, RVT, Andrea Hudgins, CCRP; Texas Scottish Rite Hospital—Shirley Henry, RN, PNP, Shuzen Zhang, ARDMS, RVT; University Hospitals/Case Medical Center/Rainbow Babies and Children's Hospital—Elizabeth B. Brooks, MD, PhD, Stacy Miner, Nancy Szabo, and the Center for Drug Research at Rainbow Babies and Children's Hospital; Children's Hospital of Philadelphia—Libby Dorfeld, Sarajane Wiilson, BS; University of California, Los Angeles Medical Center—Tatiana Hernandez, BS, Jyotsna Vitale, BS, RT(M), RDMS, RVT; Children's Memorial Hospital, Northwestern—Angela Kress, Nicole Lowe, Falguni Patel; Seattle Children's Hospital and Regional Medical Center—Stephanie Hamilton, BSN; Medical University of South Carolina—Katie Caldwell, Diane Kamen, MD, MSCR; The University of Chicago—Becky Puplava, BS, Atanas Lonchev RVT, RDCS, RDMS; Nationwide Children's Hospital—Monica Bacani, RDMS; Cincinnati Children's Hospital Medical Center—Cynthia Rutherford, BS, CRCIII, Jamie Meyers-Eaton, BS, CRCIII; Creighton University Medical Center—Teresa Conway, RN, MS, CCRC, Lacey Frank, Lori Kuss, ASRT, RDMS, RDCS; Denver Children's Hospital—Hazel Senz, RN, BSN, CCRC; Mayo Clinic—Thomas Mason, MD, Jane Jaquith, CCRC, Diana E. Paepke-Tollefsrud, BS, RVT, RDMS, RT(R).
This work was supported by the NIH (NIAMS contract N01-AR-2-2265) and in part by The Duke Edna and Fred L. Mandel, Jr. Center for Hypertension and Atherosclerosis Research. Dr. Singer's participation was supported in part by grant UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. Dr. Schanberg has received consulting fees from Pfizer (less than $10,000). Pfizer contributed study drug to the APPLE trial.
REFERENCES
- 1.Bjornadal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964–95. J Rheumatol. 2004;31(4):713–9. [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr., Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 3.Westerweel PE, Luyten RK, Koomans HA, Derksen RH, Verhaar MC. Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum. 2007;56(5):1384–96. doi: 10.1002/art.22568. [DOI] [PubMed] [Google Scholar]
- 4.Berenson GS, Wattigney WA, Tracy RE, Newman WP, 3rd, Srinivasan SR, Webber LS, et al. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study) Am J Cardiol. 1992;70(9):851–8. doi: 10.1016/0002-9149(92)90726-f. [DOI] [PubMed] [Google Scholar]
- 5.Falaschi F, Ravelli A, Martignoni A, Migliavacca D, Sartori M, Pistorio A, et al. Nephrotic-range proteinuria, the major risk factor for early atherosclerosis in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2000;43(6):1405–9. doi: 10.1002/1529-0131(200006)43:6<1405::AID-ANR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2399–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez S, Garcia-Criado MA, Tassies D, Reverter JC, Cervera R, Gilabert MR, et al. Preclinical vascular disease in systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology (Oxford) 2005;44(6):756–61. doi: 10.1093/rheumatology/keh581. [DOI] [PubMed] [Google Scholar]
- 8.Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42(7):1309–11. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93(5):513–9. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 10.Roman MJ, Crow MK, Lockshin MD, Devereux RB, Paget SA, Sammaritano L, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56(10):3412–9. doi: 10.1002/art.22924. [DOI] [PubMed] [Google Scholar]
- 11.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, et al. Prevention Conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden: Writing Group III. Circulation. 2000;101(1):E16–22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 13.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. Jama. 2004;292(3):331–7. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 14.Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg AS, van der Graaf A, de Groot E, et al. Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation. 2007;116(6):664–8. doi: 10.1161/CIRCULATIONAHA.106.671016. [DOI] [PubMed] [Google Scholar]
- 15.Sass C, Herbeth B, Chapet O, Siest G, Visvikis S, Zannad F. Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the Stanislas cohort: effect of age, sex, anthropometry and blood pressure. J Hypertens. 1998;16(11):1593–602. doi: 10.1097/00004872-199816110-00005. [DOI] [PubMed] [Google Scholar]
- 16.Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Pratt JE, Tracy RP, Kuller LH, et al. Comparison of risk factors for vascular disease in the carotid artery and aorta in women with systemic lupus erythematosus. Arthritis Rheum. 2004;50(1):151–9. doi: 10.1002/art.11418. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 18.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142(12 Pt 1):953–62. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GJH, G.B., Edelmann DM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259. [PubMed] [Google Scholar]
- 21.Friedwald WT, Levy RI, Frederickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of preparative centrifuge. Clin Chem. 1972;18:499–502. 1972, 499—502. [PubMed] [Google Scholar]
- 22.Bots ML, Evans GW, Riley W, Meijer R, McBride KH, Paskett ED, et al. The Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study: design and baseline characteristics. Control Clin Trials. 2003;24(6):752–75. doi: 10.1016/s0197-2456(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 23.Crouse JR, 3rd, Grobbee DE, O'Leary DH, Bots ML, Evans GW, Palmer MK, et al. Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin in subclinical atherosclerosis--the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther. 2004;18(3):231–8. doi: 10.1023/B:CARD.0000033645.55138.3d. [DOI] [PubMed] [Google Scholar]
- 24.Kastelein JJ, van Leuven SI, Evans GW, Riley WA, Revkin JH, Shear CL, et al. Designs of RADIANCE 1 and 2: carotid ultrasound studies comparing the effects of torcetrapib/atorvastatin with atorvastatin alone on atherosclerosis. Curr Med Res Opin. 2007;23(4):885–94. doi: 10.1185/030079907x182121. [DOI] [PubMed] [Google Scholar]
- 25.Bots ML, Evans GW, Riley W, McBride KH, Paskett ED, Helmond FA, et al. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima-media thickness: the Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. Eur Heart J. 2006;27(6):746–55. doi: 10.1093/eurheartj/ehi695. [DOI] [PubMed] [Google Scholar]
- 26.Crouse JR, 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. Jama. 2007;297(12):1344–53. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 27.Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356(16):1620–30. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 28.Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370(9582):153–60. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- 29.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- 30.SAS Institute Inc. SAS/STAT® User's Guide. Version 8 SAS Institute Inc.; Cary, NC: 1999. p. 3884. [Google Scholar]
- 31.Palatini P, Mormino P, Dorigatti F, Santonastaso M, Mos L, De Toni R, et al. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. 2006;70(3):578–84. doi: 10.1038/sj.ki.5001603. [DOI] [PubMed] [Google Scholar]
- 32.Doria A, Shoenfeld Y, Wu R, Gambari PF, Puato M, Ghirardello A, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62(11):1071–7. doi: 10.1136/ard.62.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am J Med. 1994;96(3):254–9. doi: 10.1016/0002-9343(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 34.Tyrrell PN, Beyene J, Benseler SM, Sarkissian T, Silverman ED. Predictors of Lipid Abnormalities in Children with New-Onset Systemic Lupus Erythematosus. J Rheumatol. 2007 [PubMed] [Google Scholar]
- 35.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 36.Stone NJ. Secondary causes of hyperlipidemia. Med Clin North Am. 1994;78(1):117–41. doi: 10.1016/s0025-7125(16)30179-1. [DOI] [PubMed] [Google Scholar]
- 37.Marcen R, Chahin J, Alarcon A, Bravo J. Conversion from cyclosporine microemulsion to tacrolimus in stable kidney transplant patients with hypercholesterolemia is related to an improvement in cardiovascular risk profile: a prospective study. Transplant Proc. 2006;38(8):2427–30. doi: 10.1016/j.transproceed.2006.08.070. [DOI] [PubMed] [Google Scholar]
- 38.Bessant R, Duncan R, Ambler G, Swanton J, Isenberg DA, Gordon C, et al. Prevalence of conventional and lupus-specific risk factors for cardiovascular disease in patients with systemic lupus erythematosus: A case-control study. Arthritis Rheum. 2006;55(6):892–9. doi: 10.1002/art.22343. [DOI] [PubMed] [Google Scholar]
- 39.Weigel G, Griesmacher A, DeAbreu RA, Wolner E, Mueller MM. Azathioprine and 6-mercaptopurine alter the nucleotide balance in endothelial cells. Thromb Res. 1999;94(2):87–94. doi: 10.1016/s0049-3848(98)00199-6. [DOI] [PubMed] [Google Scholar]
- 40.Stet EH, De Abreu RA, Bokkerink JP, Blom HJ, Lambooy LH, Vogels-Mentink TM, et al. Decrease in S-adenosylmethionine synthesis by 6-mercaptopurine and methylmercaptopurine ribonucleoside in Molt F4 human malignant lymphoblasts. Biochem J. 1994;304(Pt 1):163–8. doi: 10.1042/bj3040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toloza SM, Uribe AG, McGwin G, Jr., Alarcon GS, Fessler BJ, Bastian HM, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50(12):3947–57. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 42.Cottiero RA, Madaio MP, Levey AS. Glomerular filtration rate and urinary albumin excretion rate in systemic lupus erythematosus. Nephron. 1995;169:140–146. doi: 10.1159/000188429. [DOI] [PubMed] [Google Scholar]
- 43.Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34(12):2985–94. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 44.Taylor A, Shaw LJ, Fayad Z, O'Leary D, Brown BG, Nissen S, et al. Tracking atherosclerosis regression: a clinical tool in preventive cardiology. Atherosclerosis. 2005;180(1):1–10. doi: 10.1016/j.atherosclerosis.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Espeland MA, Evans GW, Wagenknecht LE, O'Leary DH, Zaccaro DJ, Crouse JR, et al. Site-specific progression of carotid artery intimal-medial thickness. Atherosclerosis. 2003;171(1):137–43. doi: 10.1016/j.atherosclerosis.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Schott LL, Wildman RP, Brockwell S, Simkin-Silverman LR, Kuller LH, Sutton-Tyrrell K. Segment-specific effects of cardiovascular risk factors on carotid artery intima-medial thickness in women at midlife. Arterioscler Thromb Vasc Biol. 2004;24(10):1951–6. doi: 10.1161/01.ATV.0000141119.02205.6b. [DOI] [PubMed] [Google Scholar]
- 47.Espeland MA, Tang R, Terry JG, Davis DH, Mercuri M, Crouse JR., 3rd Associations of risk factors with segment-specific intimal-medial thickness of the extracranial carotid artery. Stroke. 1999;30(5):1047–55. doi: 10.1161/01.str.30.5.1047. [DOI] [PubMed] [Google Scholar]
- 48.Litwin M, Wuhl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, et al. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol. 2005;16(5):1494–500. doi: 10.1681/ASN.2004110932. [DOI] [PubMed] [Google Scholar]
- 49.Erzen B, Sabovic M, Sebestjen M, Kaplan-Pavlovcic S, Poredos P. Correlation between glomerular filtration and markers of the atherosclerotic process in young patients with myocardial infarction. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.08.101. [DOI] [PubMed] [Google Scholar]
- 50.Szczepaniska Kostro J, Tolwinska J, Urban M, Gardziejczyk M, Glowinska B. Cardiac mass and function, carotid artery intima media thickness, homocysteine and lipoprotein levels in children and adolescents with growth hormone deficiency. J Pediatr Endocrinol Metab. 2004;17(10):1405–13. [PubMed] [Google Scholar]
- 51.Svenungsson E, Jensen-Urstad K, Heimburger M, Silveira A, Hamsten A, de Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104(16):1887–93. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]