Abstract
The transcription factor SOX9 regulates cartilage extracellular matrix gene expression and is essential for chondrocyte differentiation. We previously showed that activation of p38 MAPK by cycloheximide in human chondrocytes leads to stabilization of SOX9 mRNA (Tew SR and Hardingham TE. J Biol Chem 281: 39471–39479, 2006). In this study we investigated whether regulation of p38 MAPK caused by changes in osmotic pressure could control SOX9 mRNA levels expression by a similar mechanism. Primary human articular chondrocytes isolated from osteoarthritic cartilage at passage 2-4 showed significantly raised SOX9 mRNA levels when exposed to hyperosmotic conditions for 5 h. The effect was strongest and most reproducible when actin stress fibers were disrupted by the Rho effector kinase inhibitor Y27632, or by culturing the cells within alginate beads. Freshly isolated chondrocytes, used within 24–48 h of isolation, did not contain actin stress fibers and upregulated SOX9 mRNA in response to hyperosmolarity in the presence and absence of Y27632. In these freshly isolated chondrocytes, hyperosmolarity led to an increase in the half-life of SOX9 mRNA, which was sensitive to the p38 MAPK inhibitor SB202190. SOX9 protein levels were increased by hyperosmotic culture over 24 h, and, in passaged chondrocytes, the activity of a COL2A1 enhancer driven luciferase assay was upregulated. However, in freshly isolated chondrocytes, COL2A1 mRNA levels were reduced by hyperosmotic conditions and the half-life was decreased. The results showed that the osmotic environment regulated both SOX9 and COL2A1 mRNA posttranscriptionally, but in fresh cells resulted in increased SOX9, but decreased COL2A1.
Keywords: osmolarity, p38 mitogen-activated protein kinase, mRNA stability, actin, COL2A1
the articular cartilage in synovial joints plays an essential role in absorbing mechanical stress and allowing smooth articulation during movement. Critical to this process is that the tissue's highly specialized extracellular matrix (ECM) is maintained and appropriately remodeled by the articular chondrocytes. The chondrocyte is characterized by the ability to synthesize the major components of this ECM, such as type II collagen and the proteoglycan aggrecan, and responds to external signals, such as mechanical loading and osmotic changes, by regulating their production (28). These stimuli are of interest because it is established that abnormal loading of cartilage either by disuse (27) or overuse (26) is detrimental to the tissue in vivo because the chondrocytes sense the mechanical forces on the tissue and adapt their ECM accordingly. Osmotic pressure may play a key role in this process because the flow of water out of joint cartilage during mechanical loading will lead to increased osmotic pressure within the tissue. This is due to the retention of the highly anionic proteoglycan and the necessary cationic counter ions. Hence, the osmotic pressure around the chondrocyte is changed directly by the loading on the tissue. The effects of osmolarity on chondrocyte ECM synthesis have been investigated in a number of studies with varied results. These studies have shown that chondrocyte proteoglycan synthesis can be decreased through the application of hyper- or hypo-osmotic conditions (25, 40, 41). Conversely, a recent study demonstrated that application of dynamic hypo-osmotic stresses resulted in increased expression of cartilage ECM genes (9). It has also been demonstrated that over 24 h culture, there is an increase in glycosaminoglycan synthesis by articular chondrocytes under hyperosmotic conditions (15). Interestingly, this latter study showed a requirement for p38 mitogen-activated protein kinase (MAPK) signaling, a transduction pathway known to be regulated by osmolarity in many organisms (30). Beyond this, the mechanisms controlling the response of chondrocytes to hyper- or hypo-osmolarity are poorly understood.
The expression of many genes encoding cartilage extracellular matrix proteins is controlled by the transcription factor SOX9 (10). The importance of SOX9 to the chondrocyte phenotype is underlined by the human genetic disease campomelic dysplasia, which is caused by SOX9 haplo-insufficiency (44). This results in a severe dwarfism syndrome, caused by inadequate cartilage generation during development. Regulation of SOX9 itself is only just beginning to be revealed and occurs at the transcriptional level by mechanisms involving long-range enhancer elements (3). We previously published data which showed that SOX9 mRNA levels could also be controlled by cycloheximide through p38 MAPK-dependent regulation of its mRNA stability (34). It is likely that this is regulated through protein interactions with a number of AU-rich elements (AREs) in the SOX9 mRNA 3′-untranslated region (3′-UTR; 34). In passaged chondrocytes, this process occurred most consistently when actin stress fibers were inhibited by culturing the cells in alginate beads or in the presence of compounds such as cytochalasin D, or the Rho effector kinase (ROCK) inhibitor Y27632. We wanted to expand on these results and identify physiologically relevant modulators of SOX9. In this study we hypothesized that medium osmolarity, a known regulator of the p38 MAPK pathway, could control SOX9 by a posttranscriptional mechanism. We investigated whether hyperosmotic culture conditions would regulate SOX9 mRNA in both dedifferentiated and freshly isolated human articular chondrocytes (HAC) and examined whether posttranscriptional control was occurring. Finally, we examined the effects of hyperosmotic conditions on the downstream SOX9 target gene COL2A1.
MATERIALS AND METHODS
Cell isolation and culture.
Osteoarthritic (OA) human articular cartilage was obtained from patients undergoing total knee arthroplasty, with informed consent and full approval from the Cheshire Research Ethics Committee. HAC were isolated from macroscopically intact cartilage as described previously (36). Although these cells were from OA joints, we have previously shown that passaged primary human OA chondrocytes retain important chondrocyte properties comparable to chondrocytes from age-matched non-OA cartilage, such as their response to chondrogenic culture following SOX9 transduction (35) and at early passage the ability to form cartilage matrix in chondrogenic cultures (16). The chondrocytes were isolated and grown as monolayers in Dulbecco's modified Eagle's medium (DMEM; catalog no. 12430104, Invitrogen, Paisley, UK) containing 100 U/ml penicillin, 100 U/ml streptomycin, and 10% FBS (all from Invitrogen). Experiments were carried out with freshly isolated cells that were plated at high density (1 × 105 cells/cm2) and used within 48 h or with cells at the end of passages 2 to 4 (with a 1:2 split ratio). Alginate bead cultures were prepared with passaged HAC as described previously (20) and cultured for 3 days in DMEM + 10% FBS before being used in experiments. All experimental replicates were carried out using cells from different donors. A total of 19 donors were used during this study, with an age range of 52–81 yr and a mean age of 74 yr. The osmolarity of the basic culture media was ∼315 mosM, and to examine the effects of medium osmolarity on the cells, they were grown for 5 or 24 h in serum-free and antibiotic-free DMEM supplemented at a ratio of 3.7:1.3 with either water, 207 mM NaCl, or 527 mM NaCl solutions, respectively, yielding 270, 380, or 550 mosM media. The 380 mosM media were also adjusted to 550 mosM through the addition of sucrose (osmolarity assessed with a Loeser Microdigital-15 osmometer). All cultures were washed with Hanks' buffered saline solution (Invitrogen) and precultured in the 380 mosM media for 1 h before the beginning of experiments. The use of this range of osmolarities was based on a previous study which examined extracellular matrix regulation of bovine chondrocytes (15). The 380 mosM level is slightly higher than standard tissue culture media and was used as a control condition because it is within the osmolarity range observed in articular cartilage (41). The 5-h time period had previously been used to demonstrate cycloheximide-induced regulation of SOX9 in HAC (34) while 24-h cultures were used to detect sustained changes resulting from the effect of the osmotic environment. To examine actin organization in freshly isolated or passaged HAC, cells were allowed to adhere to glass coverslips overnight before they were fixed and stained with rhodamine-conjugated phalloidin (Invitrogen), and cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, obtained from Sigma, Poole, UK) as previously described (34). Where necessary, defined osmotic media were supplemented with 10 μM of the ROCK inhibitor Y27632 (Calbiochem, Nottingham, UK) or the p38 MAPK inhibitor SB202190 (Sigma) at 0.2 2 or 20 μM. In experiments with SB202190, it was dissolved in DMSO and diluted from a 1000× stock into osmotically defined media, and control cultures in the SB202190 experiment had a comparable addition of DMSO. This would result in an additional increase in the medium osmolarity of around 14 mosM above the defined 380 and 550 mosM levels. The contribution of SB202190 to the media osmolarity was very small. All inhibitors were added 1 h before the start of experiments during the preculture at 380 mosM.
Gene expression analysis.
Real-time polymerase chain reaction (PCR) was used to examine expression of SOX9 in the chondrocyte cultures. Total RNA was prepared from cell monolayers in 12-well culture plates using 0.5 ml Tri Reagent per well (Sigma). For alginate cultures, the beads were dissociated in Tri Reagent, the RNA was extracted into an aqueous phase by the addition of chloroform, and this was further purified using RNeasy spin columns (Qiagen, Crawley, UK). cDNA was synthesized from the RNA using murine-Maloney leukemia virus reverse transcriptase which was primed with random hexamer oligonucleotides (Promega, Southampton, UK) in a 25-μl reaction. The details of the Taqman real-time PCR analysis of SOX9 mRNA using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as a normalization factor have been described previously (34). Measurement of COL2A1 mRNA by real-time PCR was performed using a SYBR green PCR mastermix (Applied Biosystems, Warrington, UK), again with GAPDH as a normalization factor using previously described primers (22). The fitness of GAPDH as a valid normalization factor in these studies was demonstrated by examining cDNA from chondrocytes grown in 380 mosM or 550 mosM conditions using an Applied Biosystems Endogenous Control Plate (part no. 4396929) which contains 32 commonly used housekeeping genes. Cycle threshold values obtained from this plate were cross-compared using the GeNorm algorithm (42) (http://medgen.ugent.be/∼jvdesomp/genorm/) and GAPDH scored an M value of 0.035 which indicated its suitability as a normalization factor. To measure SOX9 mRNA turnover, freshly isolated or passaged HAC were grown in monolayers and treated under experimental conditions for 2 h before addition of the transcription inhibitor actinomycin D (1 μM, Sigma). mRNA decay was then measured by purifying total RNA at a number of time points 0–3 h after this. This RNA was reverse transcribed to produce cDNA which was examined by real-time PCR. SOX9 mRNA copy number in each sample was calculated using a calibration curve created from known dilutions of the pcDNA3SOX9-UT-FLAG vector, a kind gift from Prof. Benoit de Crombrugghe (Univ. of Texas M.D. Anderson Cancer Center, Houston, TX). Copy numbers were then normalized to input RNA concentrations which were measured using a Nanodrop ND-1000 spectrophotometer (Labtech, East Sussex, UK). Data were plotted on semi-log charts, and exponential regression lines were created using Microsoft Excel. The slope (m) of the regression lines were then used to calculate the mRNA half-life (t1/2) using the equation t1/2 = ln(2)/m. For data presentation, values averaged from multiple donors at each time point are plotted together in Figs. 3C and 5C. The half-lives reported in Table 1 and within the text are averages with standard deviations of values generated by regression for each donor, and these values were used in subsequent statistical analysis.
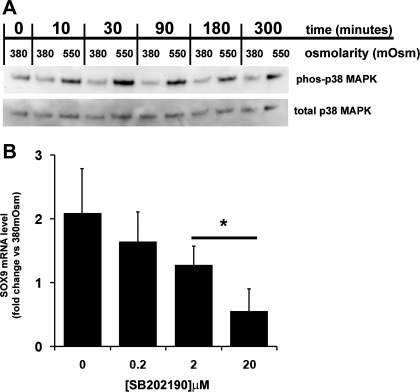
Fig. 3.
Involvement of p38 MAPK in hyperosmotic induction of SOX9 mRNA in freshly isolated HAC. A: Western blot analysis, using antibodies to phosphorylated and total p38 MAPK, of cell extracts from freshly isolated HAC which had been cultured in 380 or 550 mosM media for up to 5 h. B: real-time PCR analysis of SOX9 mRNA levels in freshly isolated HAC which have been grown under hyperosmotic (550 mosM) conditions in the presence of increasing concentrations of the p38 MAPK inhibitor SB202190 for 5 h. The inhibitor was added 1 h before osmolarity was changed. Data are presented as the fold change in expression compared with the same cells under 380 mosM conditions and without the inhibitor. Each data point represents the mean and SD of data from 3 donors. *P < 0.05 vs. 0 μM SB202190, mixed-effects linear regression.
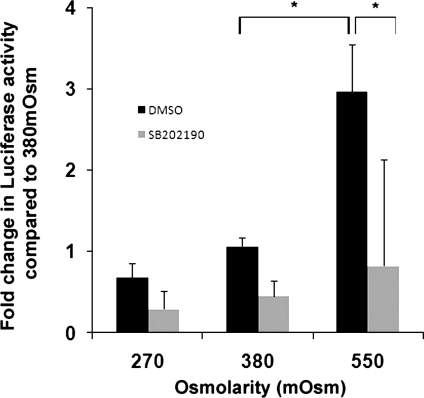
Fig. 5.
Activity of a COL2A1 promoter enhancer element in passaged HAC under different osmotic conditions. Passaged HAC were infected with a lentivirus containing a luciferase gene under the control of a 4 × 48-bp COL2A1 enhancer element. The cells were grown in media adjusted to 270, 380, or 550 mosM containing 10 μM Y27632 and either 20 μM SB202190 or DMSO as a carrier control for 24 h. Luciferase activity was then measured. Data are presented as the mean and SD of the fold change vs. HAC cultured at 380 mosM without Y27632 (*P < 0.01 one-way ANOVA and Bonferroni post hoc test; n = 4).
Table 1.
Half-life of SOX9 mRNA in freshly isolated human articular chondrocytes cultured in osmotically defined media with or without p38 MAPK inhibitor SB202190 (20 μM) and in the presence of actinomycin D (1 μM)
| Medium Osmolarity, mosM | Inhibitor(s) Present | No. of Donors Examined | SOX9 mRNA t1/2, h * |
|---|---|---|---|
| 380 | None | 6 | 1.2 (SD 0.2) |
| 380 | SB202190 | 2 | 1.5 (SD 0.4) |
| 550 | None | 3 | 5.4 (SD 2.2)† |
| 550 | SB202190 | 4 | 3.0 (SD 0.5) |
Values are means (SD).
Significant effect of 550 mosM on SOX9 mRNA half-life (t1/2) compared with 380 mosM (P < 0.01) tested by mixed-effects linear regression.
Western blot analysis of cell extracts.
The culture medium was removed and the cells were washed with Tris-buffered saline before being extracted in SDS sample buffer (62.5 mM Tris·HCl, pH 6.8, 2% wt/vol SDS, 10% glycerol, 0.01% wt/vol bromophenol blue) with the aid of a cell scraper. Samples were reduced by adding dithiothreitol to a final concentration of 50 mM, heated at 80°C for 10 min, and run on Novex 4–12% SDS-PAGE gels (Invitrogen). The separated proteins were then blotted onto nitrocellulose membranes before being probed with the following antibodies: anti-p38 MAPK no. 9212, anti-p38 MAPK phospho-Thr180/Tyr182 no. 9211 (both used at a 1:1,000 dilution and obtained from Cell Signaling Technologies, Danvers, MA), anti-SOX9 (used at 1:2,000 from Chemicon, Hampshire, UK) and anti-GAPDH-horseradish peroxidase (HRP)-conjugate (used at 1:10,000 from Sigma). Primary antibody (with the exception of anti-GAPDH) binding was localized using a HRP-conjugated goat anti-rabbit secondary antibody (Sigma) at 1:2,000, and all blots were developed using a Western Lightning Plus chemiluminescence kit (Perkin Elmer, Beaconsfield, UK).
Lentiviral-based luciferase reporter assay.
A COL2A1-specific promoter sequence driving the firefly luciferase gene was kindly provided by Prof. Benoit de Crombrugghe. The sequence is made up of 4 × 48-bp HMG-like enhancer elements upstream of the COL2A1 minimal promoter (−89 bp to +6 bp) and luciferase cDNA (19). The COL2A1-Luc sequence was excised from the parental plasmid pcDNA3.1-COL2A1-Luc and inserted into the K2-cytomegalovirus (CMV) lentivector (29) following prior removal of its own CMV promoter element. The lentivector was transfected into Producer 293T cells, and viral supernatant was harvested. This was used to transduce HAC at passage 2-3. These cells were then grown to passage 4, plated in multiwell plates grown in osmotically defined media. After 24 h, the cells were lysed using BrightGlo chemiluminescence reagent (Promega). The lysates were transferred to a black 96-well plate, and luminescence was recorded using a MicroLumat Plus luminometer (Berthold Technologies, Bad Wildbad, Germany).
Statistical analysis.
Because the cells from different donors showed significant biological variation, mixed-effects linear regression analysis was used to establish the statistical validity of results combined from different donors. This was applied to analyze the hyperosmotic effects on the expression of SOX9 in passaged and freshly isolated HAC and on the SOX9 mRNA half-life experiment. Significant changes in SOX9 expression following alginate culture and in the COL2A1 enhancer experiment were determined using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Analysis of COL2A1 gene expression and t1/2 data was performed using paired Student's t-test. These analyses were conducted using S-Plus, SPSS, and Excel software.
RESULTS
Hyperosmotic dependent increase in SOX9 mRNA in passaged HAC occurs in the absence of actin stress fibers.
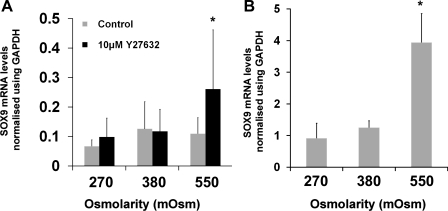
Initially, HAC which had been expanded in monolayer culture to passage 2-3 were used to determine the effect of medium osmolarity on SOX9 mRNA expression (Fig. 1A). Culture media with a normal osmolarity (380 mosM) was used as a control condition, with 270 mosM and 550 mosM media providing hypo- and hyperosmotic stimulation, respectively. In monolayer cultures after 5 h there was no significant difference between SOX9 mRNA levels at any osmolarity examined. However, when the chondrocytes were cultured with the ROCK inhibitor Y27632, SOX9 mRNA was upregulated by 2.4-fold in the 550 mosM cultures compared with 380 mosM cultures. Mixed-effects linear regression of the results showed that there was a specific interaction between 550 mosM conditions and the presence of Y27632 leading to the higher expression of SOX9 mRNA (P < 0.05). The effect of further modification of the cytoskeleton was investigated by culturing the chondrocytes in alginate beads, which leads to rounded cell morphology and a cortical actin distribution with no stress fibers (34, 43) (Fig. 1B). The passaged HAC were grown in alginate for 3 days before this experiment, and during this time, SOX9 mRNA levels increased markedly due to cellular redifferentiation caused by the rounded cell shape (34). A 5-h exposure to hyperosmotic culture was able to induce a further, significant (P < 0.01) 3.2-fold increase in the expression of SOX9 mRNA than was observed in 380 mosM cultures, and this was without a requirement for Y27632 treatment.
Fig. 1.
Effect of hyperosmolarity on SOX9 mRNA levels in passaged human articular chondrocytes (HAC). A: real-time PCR analysis of SOX9 mRNA levels in passaged HAC cultured under different osmotic conditions (given in mosM on x-axis) in the presence and absence of Y27632 (10 μM) for 5 h. Data presented were normalized using GAPDH expression and represent means and SDs from experiments conducted on cells from between 4 and 6 different donors. *Mixed-effects linear regression-interaction between hyperosmotic conditions and Y27632 (P < 0.05). B: real-time PCR analysis of SOX9 mRNA levels in passaged HAC cultured in alginate beads for 3 days in standard growth media before rinsing 3 times in serum-free DMEM and then cultured for 5 h in serum-free DMEM adjusted to 270, 380, or 550 mosM. Data are presented as means and SDs of expression values in HAC from 3 donors. *P < 0.05 vs. both 270 and 380 mosM, one-way ANOVA and Bonferroni post hoc test.
Freshly isolated HAC increase SOX9 mRNA in response to hyperosmotic conditions without requiring Y27632 treatment.
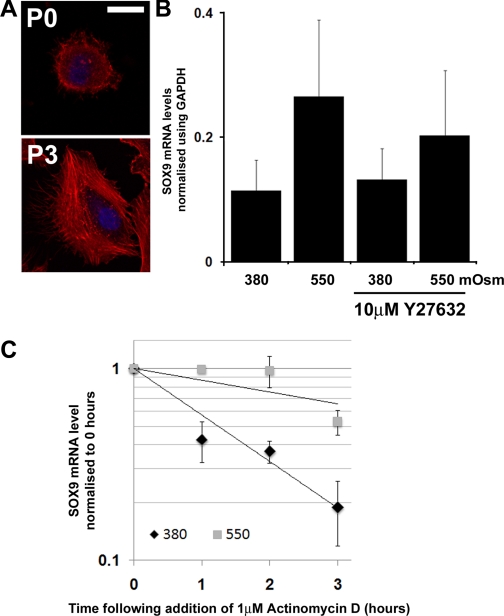
Passaged HAC provided a practical source of chondrocytes for experiments helped by the expansion in number during the culture process. However, there is a progressive change in their phenotype with increasing time in monolayer culture (5). Further experiments were therefore performed on primary HAC freshly isolated from tissue, to investigate whether the results were similar in cells before passage. Freshly isolated HAC have a diffuse actin distribution with some cortical localization in contrast to the extensive stress fiber network found in passage 3 cells (Fig. 2A), and we investigated their response to culture under hyperosmotic conditions. As before, we cultured the cells with/without the ROCK inhibitor Y27632. Cultures incubated for 5 h in 550 mosM medium showed a 2.3-fold increase in SOX9 mRNA compared with 380 mosM controls (Fig. 2B). Interestingly, the freshly isolated HAC did not require treatment with Y27632 to respond to hyperosmotic conditions, unlike the passaged HAC. Statistical analysis showed a significant effect of 550 mosM on the level of SOX9 mRNA in the freshly isolated chondrocytes (P < 0.001). To confirm that the effects on SOX9 mRNA were due to the osmotic environment and not caused by increased Na+ levels, we incubated freshly isolated HAC in NaCl-adjusted 380 mosM media for 1 h before replacing with the same media adjusted to 550 mosM by the addition of sucrose. After 5 h, when compared with cultures that had received media changes without sucrose, the levels of SOX9 mRNA had increased by 91% (fold change = 1.91 ± 0.33 SD, n = 4, P < 0.01 paired t-test). This confirmed that the cells were responding primarily to an increase in osmolarity.
Fig. 2.
Effect of hyperosmolarity on SOX9 mRNA levels in freshly isolated HAC. A: fluorescence micrographs showing distribution of actin (stained with rhodamine-conjugated phalloidin-red) in HAC when they are freshly isolated (P0) or after three passages in monolayer culture (P3). Cell nuclei are stained with DAPI (blue). Images are representative of the majority of cells in the cultures. Scale bar = 10 μM. B: real-time PCR analysis of SOX9 mRNA levels in freshly isolated HAC cultured at 380 or 550 mosM in the presence and absence of Y27632 (10 μM) for 5 h. Data represent means and SDs obtained from experiments with cells from 4 different donors. C: SOX9 mRNA decay in freshly isolated HAC cultured at different osmolarities. Within 48 h of extraction from tissue, HAC were cultured at 380 or 550 mosM for 2 h before addition of actinomycin D. RNA was then extracted in triplicate at 0, 1, 2, and 3 h for reverse transcription and analyzed by real-time PCR. Data represent means and SDs of the fold changes in SOX9 mRNA levels compared with time point 0 (n = 4).
Hyperosmolarity causes increased SOX9 mRNA t1/2 in freshly isolated HAC.
We have previously demonstrated that exposing HAC to stress by treating them with cycloheximide led to an increase of the t1/2 of the SOX9 mRNA (34). We therefore investigated the decay of the SOX9 mRNA in freshly isolated HAC following application of actinomycin D (RNA polymerase inhibitor). Decay curves generated using the mean values from all donors (Fig. 2C) demonstrated that culture in 550 mosM increased the t1/2 of SOX9 mRNA. To further quantify this, we calculated the t1/2 for each donor individually and performed mixed-effects linear regression analysis on the values (Table 1). At 380 mosM, the t1/2 of SOX9 mRNA was 1.2 h, but culture in 550 mosM medium raised the SOX9 mRNA t1/2 4.5-fold to 5.4 h. There was a significant effect of 550 mosM on the t1/2 of SOX9 mRNA in the freshly isolated chondrocytes (P < 0.01). These experiments showed that a rise in medium osmolarity increased the t1/2 of SOX9 mRNA in freshly isolated HAC.
p38 MAPK activity is required for hyperosmotic stimulation of SOX9 mRNA.
Since our previous work had shown that cycloheximide induces SOX9 mRNA stabilization in a p38 MAPK-dependent manner (34), we analyzed the effect of hyperosmotic stimulation on the activity of the p38 MAPK pathway in freshly isolated HAC. Growth media were removed and cells were cultured for 2 h in 380 mosM media. The media was again removed and replaced with more 380 mosM media or with media adjusted to 550 mosM. Western blot analysis using a phosphorylated p38 MAPK-specific antibody demonstrated an increase in p38 MAPK activation following transfer to 550 mosM medium but not when osmolarity was maintained at 380 mosM (Fig. 3A). Activation of the p38 MAPK pathway was rapid, occurring within 10 min, and peaking around 30 min. Real-time PCR analysis (Fig. 3B) showed that addition of the p38 MAPK inhibitor SB202190 to cultures of freshly isolated HAC prevented hyperosmotic induction of SOX9 mRNA in a dose-dependent manner (P < 0.05, one-way ANOVA), and mixed-effects linear regression analysis showed that reduction in SOX9 in 2 μM and 20 μM cultures when compared with 0 μM was significant (P < 0.05). We also investigated whether SB202190 affected the increase in SOX9 mRNA t1/2 caused by hyperosmotic conditions (Table 1), and the results showed that the magnitude of the increase was lower (2.5-fold) in 550 mosM compared with 380 mosM cultures. Comparing half-lives of 550 mosM cultures cultured with or without SB202190 using an unpaired Student's t-test showed that those containing the inhibitor were significantly decreased (P = 0.02). In two donors we also performed analysis of SOX9 t1/2 at 380 mosM in the presence of SB202190 and in each case observed values very similar to those seen under 380 mosM alone.
Regulation of COL2A1 by hyperosmotic conditions.
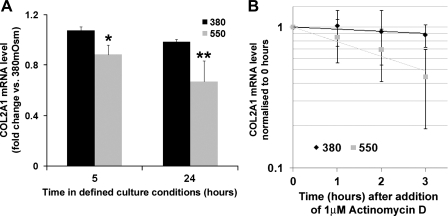
We were interested in the downstream consequences on HAC of hyperosmolarity and its effect on SOX9 mRNA regulation. Western blot analysis of freshly isolated chondrocytes cultured for 24 h in 550 mosM media showed increased SOX9 protein expression compared with 380 mosM controls (Fig. 4). To determine the effect of hyperosmolarity on SOX9 target genes, passaged chondrocytes were transfected with a lentivirus reporter construct containing an enhancer element from COL2A1 (the gene which encodes collagen type II α1 chain) upstream of luciferase (Fig. 5). This showed a significant 3-fold increase (P < 0.01) in activity after 24 h under 550 mosM conditions compared with 380 mosM. Lowering the osmolarity to 270 mosM caused a 0.64-fold, but nonsignificant, reduction. Inhibition of p38 MAPK in the cells with SB202190 led to an apparent reduction in the reporter activity at all osmolarities, but this was only significant (P < 0.01) at 550 mosM. Although high osmolarity increased the transcriptional activity of the COL2A1 reporter, determination of COL2A1 mRNA levels in freshly isolated chondrocytes showed small but significant reductions in COL2A1 levels at 550 mosM compared with 380 mosM at 5 h (18% reduction) and at 24 h (32% reduction) (Fig. 6A). This decrease in COL2A1 mRNA was surprising, given that hyperosmolarity increased levels of SOX9 mRNA and protein and elevated COL2A1 enhancer activity. We therefore decided to analyze the t1/2 of the COL2A1 mRNA in these two osmotic conditions (Fig. 6B). Following actinomycin D treatment, we found that COL2A1 mRNA decayed very slowly in chondrocytes cultured in 380 mosM media (t1/2 = 13.8 ± 4.6 SD h, n = 5), but that in hyperosmotic 550 mosM conditions the COL2A1 mRNA showed a much faster turnover, with a 3.6-fold decrease in the t1/2 to 3.9 ± 1.6 SD h (n = 4). This indicated that increasing the osmotic microenvironment of the chondrocytes greatly reduced COL2A1 mRNA t1/2 but could also enhance rates of COL2A1 transcription.
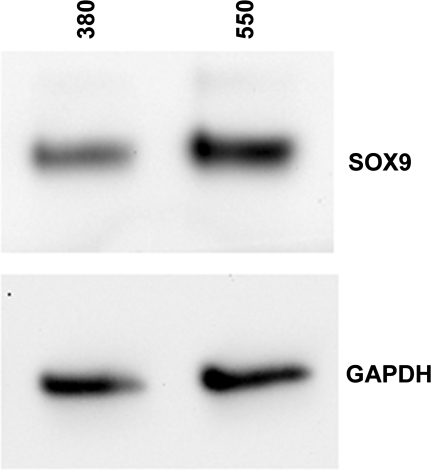
Fig. 4.
SOX9 protein levels in freshly isolated HAC under different osmotic conditions. Western blot analysis, using antibodies to SOX9 and GAPDH of cell extracts from freshly isolated HAC which had been cultured in 380 or 550 mosM media for 24 h.
Fig. 6.
Effect of hyperosmolarity on total levels and stability of COL2A1 mRNA in freshly isolated HAC. A: real-time PCR analysis of COL2A1 mRNA levels in freshly isolated HAC cultured at 380 or 550 mosM for 5 or 24 h. Each donor's samples were measured twice, and for each donor and each condition, all data were normalized to the first 380 mosM value recorded (*P < 0.05, n = 3; **P < 0.01, n = 4). B: real-time PCR analysis of COL2A1 mRNA decay. Freshly isolated chondrocytes were cultured at 380 or 550 mosM for 2 h before addition of actinomycin D. RNA was then extracted in triplicate at 0, 1, 2, and 3 h for reverse transcription and analyzed by real-time PCR. Data represent means and SDs of the fold changes in COL2A1 mRNA levels compared with time point 0 (n = 4).
DISCUSSION
Mechanisms that regulate the expression of SOX9 in chondrocytes are of great interest given the critical role that this transcription factor has in the control of chondrocyte phenotype. We have shown that increased levels of SOX9 expression in chondrocytes improve their ability to form cartilaginous ECM (35), and this is an important consideration in strategies for cartilage regeneration. More recently, we demonstrated that p38 MAPK signaling in HAC leads to stabilization of SOX9 mRNA (34). In the latter study, cycloheximide was shown to stabilize SOX9 mRNA through p38 MAPK activation, which was useful experimentally but had limited physiological relevance to articular chondrocyte biology. The purpose of the present study was to determine whether increases in medium osmolarity, a physiological stress which is known to activate p38 MAPK signaling, was able to regulate SOX9 mRNA through comparable mechanisms. Starting with the primary chondrocytes passaged in monolayer, we identified an effect of hyperosmolarity on SOX9 mRNA. Further examination of this effect of hyperosmolarity showed that it was also present in freshly isolated cells, which have retained their chondrocyte phenotype. The results showed that in these articular chondrocytes, hyperosmolarity induces higher levels of SOX9 mRNA and also increases SOX9 mRNA half-life, both of which were dependent on p38 MAPK signaling.
The twofold increase in SOX9 mRNA levels, which we have observed when chondrocytes are cultured under hyperosmotic conditions, appears to be significant because the changes in SOX9 expression typically seen in articular chondrocytes are quite restricted. There is evidence for the physiological importance of modest changes in SOX9 activity from mouse genetic studies, which show that the level of SOX9 expression is critically important to the chondrocyte phenotype during development, as either the knock-out or the knock-in of a single SOX9 allele has a severe effect on chondrocyte differentiation and on cartilage development (2, 6). Furthermore, haplo-insufficiency of SOX9 in humans causes campomelic dysplasia involving multiple developmental abnormalities including those in cartilage (44). The similarity in the magnitude of the increase in SOX9 mRNA after hyperosmotic stimulation in both freshly isolated (high SOX9 expression) and passaged chondrocytes (low SOX9 expression) indicated that this mode of regulation was independent from the differentiation state of the cell. The scale of the increase in SOX9 mRNA t1/2 in our experiments is likely to be functionally significant, especially when it is compared with posttranscriptional regulation of other genes. For example, posttranscriptional control of tumor necrosis factor-α (TNF-α) expression has been extensively reported, and increased expression of TNF-α, caused by impaired control of its mRNA degradation, leads to just over twofold increase in mRNA half-life which, in mouse models, causes severe autoimmune disease (7, 8, 32).
The potential role of the cytoskeleton in controlling the response of passaged chondrocytes is intriguing. Alginate bead culture results in a cortical actin distribution, somewhat similar to that seen in chondrocytes in vivo (34, 43). In monolayer cultures, hyperosmotic conditions brought about a significant increase in the levels of SOX9 mRNA only when they were grown within alginate or were treated with Y27632, an inhibitor of the enzyme ROCK, which plays an important role in the formation of actin stress fibers (21). We previously observed the same requirement for inhibiting actin structures in passaged HAC when SOX9 mRNA levels were stimulated through cycloheximide treatment (34). The underlying molecular mechanism for this is unclear, and the fact that freshly isolated cells do not require Y27632 fits well with the absence of actin stress fibers in these cultures. However, there is a potential consequence during the progression of osteoarthritis when there are changes to chondrocyte cell shape and cytoskeletal organization (18) which may cause them to transduce osmotic signals differently.
It is worth noting that, despite decreasing SOX9 mRNA levels in hyperosmotic cultures to below those of the 380 mosM controls (Fig. 3B), inhibition of p38 MAPK only partially reduced the hyperosmotic increase in SOX9 mRNA t1/2 (Table 1). This implies that p38 MAPK is not the only regulatory process controlling the rate of SOX9 mRNA decay. The role of p38 MAPK in controlling cellular response to osmotic challenge is well established in yeast and involves activation of a subset of kinases through which p38 MAPK is finally phosphorylated (30). A similar signaling system appears to exist in mammalian cells and involves Rac-MEKK3-dependent signaling, directed through the localization of signaling components to actin-associated scaffold proteins (39). Previous work has demonstrated a role for p38 MAPK in transduction of hyperosmotic signals in fibroblasts and, similar to our study, inhibition of the pathway only partially prevented this effect (17). In chondrocytes, p38 MAPK appears to have an important role in controlling cell phenotype during development. Mice engineered to have constitutive activation of the p38 MAPK pathway in cartilaginous tissues display a severe dwarfism caused by delayed chondrocyte hypertrophy, which is very similar to that exhibited by mice overexpressing SOX9 (2, 47). Inhibition of p38 MAPK also prevents hypertrophic differentiation of chondrocytes in vitro (31). In mature chondrocytes it has been demonstrated that cytokines such as IL-1β act through the p38 MAPK pathway to activate cartilage matrix degrading enzymes (23). Furthermore, there is also evidence that chondrocytes regulate extracellular matrix synthesis through p38 MAPK signaling. For instance, in ATDC5 cells, phosphorylation of p38 MAPK correlates strongly with increased aggrecan gene expression (45), while in bovine articular chondrocytes, increased glycosaminoglycan synthesis is caused by hyperosmotic stimulation and this can be prevented by the application of a p38 MAPK inhibitor (15). Studies using rat nucleus pulposus cells, which form an extracellular matrix similar to that of chondrocytes, have shown that exposure to hyperosmotic conditions results in increased expression of the transcription factor TonEBP and a subsequent transactivation of its target genes including aggrecan (37). This increased transactivation was sensitive to inhibition of p38 MAPK signaling (38). The present study adds to these findings by identifying hyperosmolarity as a potential physiological stimulus for the p38 MAPK-mediated posttranscriptional regulation of SOX9 mRNA we previously described (34). Hyperosmolarity was also able to promote activation of gene expression through a COL2A1 enhancer element, a known target of SOX9, which could be inhibited using the p38 MAPK inhibitor SB202190. These findings reveal interesting parallels between the response of TonEBP and SOX9 to hyperosmotic stimulation that would merit further investigation, particularly given that TonEBP is subject to posttranscriptional control in cells from mouse kidney following hyperosmotic stimulation (6).
Our use of 550 mosM as a hyperosmotic condition is comparable to that used in other studies which have examined the effects of osmolarity on chondrocytes (15, 25). In recent years, magnetic resonance imaging (MRI) has been employed to examine the changes in cartilage thickness and water content in knee cartilage before and after exercise. Analysis of cartilage as a whole has shown that it is compressed by around 5% following running (11). However, studies where the distribution of fluid in different zones of the tissue have been examined following exercise show that there is a nonuniform loss of water within femoral cartilage with greatest loss toward the articular surface (24). So while the overall level of compression would suggest that the change in osmolarity experienced by chondrocytes during exercise might be modest, it is likely that the cells within the surface and intermediate zones will experience a disproportionately high increase in their environmental osmolarity. All of these studies involve MRI measurements carried out following exercise and we know little of the compression that the tissue is under at the moment of impact during exercise. Furthermore, the presence of lacunae around chondrocytes, rich in polyanions, will further elevate osmolarity close to the cells. Overall, there is still much that we do not know about the changing osmotic environment around the chondrocyte and the exact osmolarities that the cells experience in a given situation and at a given tissue depth. Because mechanical loading can lead to changes in osmolarity in articular cartilage, the regulation of the mRNA levels of SOX9 in response to the osmotic environment may form part of the mechanism of chondrocyte mechanotransduction. Our results suggest that the effect of an increase in osmotic pressure on chondrocytes would be to increase SOX9 mRNA stability and direct more SOX9 protein production. Following this rationale, the loss of ECM during degenerative joint diseases, such as in osteoarthritis, and the decrease in the osmolarity of the chondrocyte microenvironment could contribute to the decreased levels of SOX9 mRNA observed in chondrocytes within osteoarthritic cartilage (1, 14, 33). These experiments were carried out on primary human chondrocytes from osteoarthritic knee joints. Following isolation, they retain a rounded morphology for several days and express abundant levels of COL2A1, COL9A1, aggrecan, and cartilage oligomeric matrix protein (Tew SR and Clegg PD, unpublished observations, 2009) and form cartilage-like matrix in chondrogenic cultures (16) and compare well with age-matched control chondrocytes in matrix formation, when both were transduced with SOX9 (35). There is therefore no a priori reason to interpret the current findings as distinctive of osteoarthritic chondrocytes. However, due to the pathology in the joint from which the cartilage was isolated, we cannot rule out that the effect of osmolarity on these cells is linked to this pathology. Further study to compare mRNA regulation in response to altered osmolarity in chondrocytes from normal and osteoarthritic cartilage is clearly warranted.
We had initially predicted that osmotically driven increases in SOX9 may lead to the upregulation of extracellular matrix genes that it controls such as COL2A1. Our demonstration that hyperosmotic stimulation of chondrocytes could drive activity of a COL2A1 enhancer element [which SOX9 has been shown to bind to and regulate (48)] suggested that there was increased COL2A1 transcription. These results clearly revealed that the passaged chondrocytes expressing the reporter gene were able to transduce osmotic changes into the regulation of a COL2A1-specific enhancer element. It surprised us therefore that when we examined COL2A1 mRNA expression in freshly isolated chondrocytes, a more physiologically relevant system, exposure to hyperosmotic conditions gave not an increase but in fact a slight decrease in COL2A1 mRNA levels. This was interesting though because it has been established that collagen synthesis is downregulated by articular chondrocytes under hyperosmotic conditions (40, 41). It would appear that an increase in turnover of the COL2A1 mRNA may contribute to this reduction, demonstrating that regulation of COL2A1 mRNA can be a complex, multifactorial process relying not only on increased transcription but also on posttranscriptional control. The 3′-UTR of COL2A1 contains one AUUUA motif compared with eight in the SOX9 sequence and is classed a cluster 4 AU-rich element by the ARE database ARED (4). COL2A1 has been shown to be controlled posttranscriptionally in Stickler syndrome, but this was shown to be regulated by nonsense-mediated decay (12). There has been little study of the posttranscriptional response of COL2A1 to external stimuli which could control potential 3′-UTR regulatory elements. Changes in COL2A1 mRNA levels caused by interleukin-1 treatment or mechanical stimulation have previously been demonstrated, but these did not involve a posttranscriptional mechanism (13, 46). Our observation may therefore be the first description of the posttranscriptional control of COL2A1 and may provide an explanation for why earlier reports have demonstrated (relatively) lower levels of collagen production by chondrocytes cultured under hyperosmotic conditions likely to have increased SOX9 expression (40, 41).
In summary, we have shown that hyperosmotic conditions, which are known to affect articular chondrocyte ECM production, control the levels of SOX9 mRNA in primary human articular chondrocytes, through a posttranscriptional, p38 MAPK-dependent process. Furthermore, these conditions lead to posttranscriptional regulation of the SOX9 target gene COL2A1. This may form a part of the signaling processes through which chondrocytes can sense mechanical loads and maintain tissue homeostasis. Our findings suggest that optimal osmotic conditions for activation of chondrocyte collagen type II production are achieved by controlling steady-state levels through regulation of transactivation by factors, such as SOX9 combined with potent posttranscriptional control of gene expression.
GRANTS
This work was funded by the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council, the Medical Research Council, and the Arthritis Research Campaign.
ACKNOWLEDGMENTS
The authors thank Richard Parkinson, M.D., and the staff at Clatterbridge Hospital, Wirral, Merseyside, for provision of human joint tissue.
REFERENCES
- 1.Aigner T, Gebhard PM, Schmid E, Bau B, Harley V, Poschl E. SOX9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol 22: 363–372, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 18: 1072–1087, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagheri-Fam S, Barrionuevo F, Dohrmann U, Gunther T, Schule R, Kemler R, Mallo M, Kanzler B, Scherer G. Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev Biol 291: 382–397, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bakheet T, Williams BR, Khabar KS. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res 34: D111–D114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 15: 1313–1321, 1978 [DOI] [PubMed] [Google Scholar]
- 6.Cai Q, Ferraris JD, Burg MB. High NaCl increases TonEBP/OREBP mRNA and protein by stabilizing its mRNA. Am J Physiol Renal Physiol 289: F803–F807, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science 281: 1001–1005, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Carrick DM, Lai WS, Blackshear PJ. The tandem CCCH zinc finger protein tristetraprolin and its relevance to cytokine mRNA turnover and arthritis. Arthritis Res Ther 6: 248–264, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol 291: C718–C725, 2006 [DOI] [PubMed] [Google Scholar]
- 10.de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol 19: 389–394, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Eckstein F, Lemberger B, Gratzke C, Hudelmaier M, Glaser C, Englmeier KH, Reiser M. In vivo cartilage deformation after different types of activity and its dependence on physical training status. Ann Rheum Dis 64: 291–295, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freddi S, Savarirayan R, Bateman JF. Molecular diagnosis of Stickler syndrome: a COL2A1 stop codon mutation screening strategy that is not compromised by mutant mRNA instability. Am J Med Genet 90: 398–406, 2000 [PubMed] [Google Scholar]
- 13.Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem 54: 85–99, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Haag J, Gebhard PM, Aigner T. SOX gene expression in human osteoarthritic cartilage. Pathobiology 75: 195–199, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hopewell B, Urban JP. Adaptation of articular chondrocytes to changes in osmolality. Biorheology 40: 73–77, 2003 [PubMed] [Google Scholar]
- 16.Katopodi T, Tew SR, Clegg PD, Hardingham TE. The influence of donor and hypoxic conditions on the assembly of cartilage matrix by osteoarthritic human articular chondrocytes on Hyalograft matrices. Biomaterials 30: 535–540, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Ko BC, Lam AK, Kapus A, Fan L, Chung SK, Chung SS. Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP). J Biol Chem 277: 46085–46092, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kouri JB, Jimenez SA, Quintero M, Chico A. Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthritis Cartilage 4: 111–125, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol 17: 2336–2346, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Tew SR, Russell AM, Gonzalez K, Hardingham TE, Hawkins RE. Transduction of human articular chondrocytes with adenoviral, retroviral and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng 10: 575–584, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Martin I, Jakob M, Schafer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage 9: 112–118, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum 43: 801–811, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, Smith MB. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology 234: 245–249, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Palmer GD, Chao Ph PH, Raia F, Mauck RL, Valhmu WB, Hung CT. Time-dependent aggrecan gene expression of articular chondrocytes in response to hyperosmotic loading. Osteoarthritis Cartilage 9: 761–770, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Palmoski MJ, Brandt KD. Effects of static and cyclic compressive loading on articular cartilage plugs in vitro. Arthritis Rheum 27: 675–681, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Palmoski MJ, Colyer RA, Brandt KD. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum 23: 325–334, 1980 [DOI] [PubMed] [Google Scholar]
- 28.Schulz RM, Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J 36: 539–568, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Seppen J, Rijnberg M, Cooreman MP, Oude Elferink RP. Lentiviral vectors for efficient transduction of isolated primary quiescent hepatocytes. J Hepatol 36: 459–465, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Sheikh-Hamad D, Gustin MC. MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am J Physiol Renal Physiol 287: F1102–F1110, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Stanton LA, Sabari S, Sampaio AV, Underhill TM, Beier F. p38 MAP kinase signalling is required for hypertrophic chondrocyte differentiation. Biochem J 378: 53–62, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4: 445–454, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Tew SR, Clegg PD, Brew CJ, Redmond CM, Hardingham TE. SOX9 transduction of a human chondrocytic cell line identifies novel genes regulated in primary human chondrocytes and in osteoarthritis. Arthritis Res Ther 9: R107, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tew SR, Hardingham TE. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J Biol Chem 281: 39471–39479, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Tew SR, Li Y, Pothacharoen P, Tweats LM, Hawkins RE, Hardingham TE. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage 13: 80–89, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Tew SR, Murdoch AD, Rauchenberg RP, Hardingham TE. Cellular methods in cartilage research: primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods 45: 2–9, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem 281: 25416–25424, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res 22: 965–974, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell'Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol 5: 1104–1110, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Urban JP, Bayliss MT. Regulation of proteoglycan synthesis rate in cartilage in vitro: influence of extracellular ionic composition. Biochim Biophys Acta 992: 59–65, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol 154: 262–270, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinall RL, Lo SH, Reddi AH. Regulation of articular chondrocyte phenotype by bone morphogenetic protein 7, interleukin 1, and cellular context is dependent on the cytoskeleton. Exp Cell Res 272: 32–44, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, and Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79: 1111–1120, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem 276: 14466–14473, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Xie J, Han ZY, Matsuda T. Mechanical compressive loading stimulates the activity of proximal region of human COL2A1 gene promoter in transfected chondrocytes. Biochem Biophys Res Commun 344: 1192–1199, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Zhang R, Murakami S, Coustry F, Wang Y, de Crombrugghe B. Constitutive activation of MKK6 in chondrocytes of transgenic mice inhibits proliferation and delays endochondral bone formation. Proc Natl Acad Sci USA 103: 365–370, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou G, Lefebvre V, Zhang Z, Eberspaecher H, de Crombrugghe B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem 273: 14989–14997, 1998 [DOI] [PubMed] [Google Scholar]