Abstract
Melanoma, the most malignant form of human skin cancer, has a poor prognosis due to its strong metastatic ability. It was recently demonstrated that Epac, an effector molecule of cAMP, is involved in regulating cell migration; however, the role of Epac in melanoma cell migration remains unclear. We thus examined whether Epac regulates cell migration and metastasis of melanoma. Epac activation, by either specific agonist or overexpression of Epac, increased melanoma cell migration. Deletion of endogenous Epac with small interfering RNA decreased basal melanoma cell migration. These data suggested a major role of Epac in melanoma cell migration. Epac-induced cell migration was mediated by translocation of syndecan-2, a cell-surface heparan sulfate proteoglycan, to lipid rafts. This syndecan-2 translocation was regulated by tubulin polymerization via the Epac/phosphoinositol-3 kinase pathway. Epac-induced cell migration was also regulated by the production of heparan sulfate, a major extracellular matrix. Epac-induced heparan sulfate production was attributable to the increased expression of N-deacetylase/N-sulfotransferase-1 (NDST-1) accompanied by an increased NDST-1 translation rate. Finally, Epac overexpression enhanced lung colonization of melanoma cells in mice. Taken together, these data indicate that Epac regulates melanoma cell migration/metastasis mostly via syndecan-2 translocation and heparan sulfate production.
Keywords: metastasis, phosphoinositol-3 kinase, N-deacetylase/N-sulfotransferase-1
melanoma is a major cancer worldwide including the United States. The median life span of patients with advanced stage melanoma is less than a year because there are no effective therapies once the tumor has spread to vital organs (5). The process of tumor cell metastasis is conventionally understood as the migration of individual cells that detach from the primary tumor, enter lymphatic vessels or the bloodstream, attach to endothelial cells, and undergo transendothelial extravasation and proliferate in organs. Despite numerous efforts in the research field, the understanding and controlling of melanoma cell migration/metastasis has been unsuccessful.
In addition to the conventional cAMP signaling pathway through protein kinase A (PKA), a new, PKA-independent signaling pathway has been established with the discovery of the exchange protein directly activated by cAMP (Epac), a guanine nucleotide exchange factor (12). Two isoforms of Epac, Epac1 and Epac2, were shown to mediate cAMP signaling to activate a small-molecular-weight G protein Rap1 and to regulate cellular functions, including secretion, Ca2+ signaling, proliferation, and apoptosis (6). Several reports also indicate the involvement of Epac in regulating cell migration (16, 28); however, the molecular mechanisms that lead to increased cell migration through Epacs remain unknown.
Molecular events associated with cell migration are influenced by interactions between cell surface molecules and the extracellular matrix (ECM) components (4). Syndecan-2, a member of the cell surface heparan sulfate (HS) proteoglycans (HSPGs), regulates cell migration via binding to extracellular HS (19, 45). HS is a major ECM component and regulates multiple cellular functions including migration (14). These reports suggested that the coordination of syndecan-2 and HS plays a role in cell migration; however, little is known about the key molecule that regulates such coordination. In this report, we demonstrate that Epac increases melanoma cell migration using two human melanoma cell lines: SK-Mel-2, which was derived from regional metastasis, and SK-Mel-24, which was derived from lymph node metastasis; both cell lines show potent migratory ability (16, 38). We also demonstrated that syndecan-2 translocation and HS production are involved in Epac-induced cell migration. In vivo studies have shown that Epac increases melanoma metastasis to the lung in mice. We thus propose a mechanism of regulating melanoma cell migration/metastasis, which is related to HS and its core binding protein.
MATERIALS AND METHODS
Materials.
Wortmannin, nocodazole, cycloheximide, and cyclodextrin were purchased from Sigma Aldrich. PD-98059 and LY-294002 reagents were purchased from EMD Biosciences. 8-(4-methoxyphenylthio)-2'-O-methyladenosine-3',5'-cAMP (8-pMeOPT-2-O-Me-cAMP) and N6-monobutyryl-cAMP (6-MB-cAMP) were purchased from Axxora. BD adeno-X expression system, BD-Adeno-X virus purification kit, and rapid titer kit were purchased from Clontech. MEM, FBS, trypsin-EDTA, Lipofectamine 2000, and penicillin-streptomycin were purchased from Invitrogen. Antibodies for phospho-glycogen synthetic kinase 3-β (GSK3β), GSK3β, phospho-Akt, and Akt were purchased from Cell Signaling. Antibodies for Epac1, Epac2, PKA (α-catalytic subunit), cAMP-response element-binding protein (CREB), phospho-CREB, and Rap1 were purchased from Santa Cruz. Anti-N-deacetylase/N-sulfotransferase-1 (NDST1) antibody was purchased from Abnova. Anti-syndecan-1 antibody was purchased from Invitrogen. Anti-anti-α-tubulin antibody was purchased from Abcam. Anti-HS antibody was purchased from Kamiya Biomedical.
Cell culture.
SK-Mel-2 and SK-Mel-24 (ATCC) cell lines were cultured in Eagle's MEM supplemented with 10% fetal bovine serum at 37°C-5% CO2. HEMA-LP human melanocytes (Cascade Biologics) were maintained in Medium 254 with Human Melanocyte Growth Supplement (Cascade Biologics).
Adenoviral overexpression.
Recombinant adenoviruses containing human LacZ, Epac1, or Epac2 were constructed (Adeno-X Expression System, Clontech). Human Epac1 and Epac2 cDNA were kindly provided by Dr. J. L. Bos (University Medical Center, Utrecht, The Netherlands). Adenovirus of PKA α-subunit was purchased from Vector Biolabs. The corresponding encoding sequence was cloned into pShuttle2 (Clontech) to obtain a mammalian expression cassette, which was then excised and ligated into BD Adeno-X Viral DNA. The recombinant vector was introduced into human embryonic kidney cells (HEK-293) to recover infectious adenovirus. Viruses were propagated in HEK-293 cells and purified by BD Adeno-X Virus Purification Kits. Viral titer was determined by Adeno-X Rapid Titer Kit (Clontech). As a control study, adenovirus vector harboring LacZ was used at the same multiplicity of infection. Cells were infected with adenovirus for 24 h and subjected to each experiment. In some experiments (for HS production and NDST-1 expression; see Fig. 5, B–H), cells were further incubated for 48 h in medium without adenovirus.
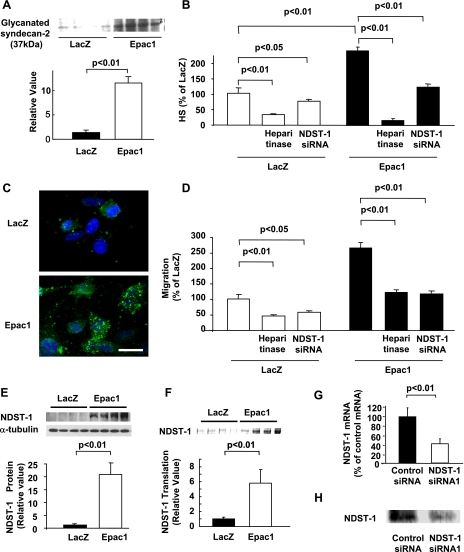
Fig. 5.
Epac increases cell migration by heparin sulfate (HS) production in SK-Mel-2. A: immunoblot showed that Epac1 overexpression increased glycanated form of syndecan-2. n = 4. B: HS ELISA showed that amount of HS was increased by Epac1 overexpression. Both heparitinase (0.08 U/ml) and NDST-1 siRNA decreased basal and Epac1 overexpression-induced HS amount. n = 4. C: immunocytochemistry showed that HS (green) was increased in cells overexpressing Epac1. Scale bar, 3 μm. D: migration assay showed that both heparitinase (0.08 U/ml) and NDST1 siRNA decreased basal and Epac1 overexpression-induced cell migration. n = 4. E: immunoblot showed that NDST-1 expression was increased by Epac1 overexpression. n = 4. F: autoradiography of [ 35S] methionine pulse labeling assay showed that NDST-1 protein translation was increased by Epac1 overexpression. n = 4. G: qPCR showed that NDST-1 siRNA decreased mRNA expression of NDST-1. n = 4. H: immunoblot showed that NDST-1 siRNA decreased protein expression of NDST-1.
Quantitative real-time PCR.
Quantitative real-time PCR (qPCR) was performed as we previously described (41). Total RNA was extracted using RNAeasy kit (QIAGEN), and then first-strand cDNA was synthesized using the Taqman RT reagents (Applied Biosystems). Real-time PCR was then carried out on a DNA Engine Opticon 2 system (MJ Research) using the SYBR Green qPCR kit (Bio-Rad). Three sets of predesigned primer mixes for each gene of interest were optimized. Specific oligonucleotide primers were used in this study: Epac1 [ Hs_RAPGEF3_1_SG (QT00003381, QIAGEN)] , Epac2 (Hs00199754-m1RAPGEF4, ABI), and NDST-1 [ Hs_NDST1_1SG Quantitect Primer Assay(QT01002638, QIAGEN)] .
Migration assay and invasion assay.
Migration assay was performed as we previously described by using the Boyden chambers (44) (pore size 8 μm, BD Biosciences). The upper chamber's polycarbonate insert film parts were coated by 75 μl fibronectin (50 μg/ml in PBS, BD Biosciences). Cultured cells were detached and the number of the cells was adjusted to 1 × 103 cells/μl of media. One-hundred microliters of the cell suspension were applied to the center of the upper chamber and then attached to the lower chamber. Thereafter, the cells were incubated in CO2 incubator at 37°C for 3 h unless specified. After fixation with 10% formalin neutral solution, cells were stained with Diff-Quick kit (Dade Behring). After mechanical removal of the cells on the upper surface of the membrane with a cotton swab, the cells that migrated onto the lower surface of the membrane were counted. Pictures were taken with a microscope followed by counting migrated cells with Image J software in randomly chosen 10 fields. Invasion assay was performed by using Boyden chambers as described above, except that Matrigel (BD Biosciences) was used as coating.
Time-lapse videomicroscopy.
Analysis of cell motility using time-lapse videomicroscopy was performed as we previously demonstrated (37). SK-Mel-2 cells overexpressing either LacZ or Epac1 were subjected to time-lapse video recording. Frames from the recording were digitized, and cell locations were identified at 30-min intervals by using either the centroids or nuclei. The speed of cell movement was determined for distances between their successive positions.
Western blot analysis.
Western blot analysis was performed as we described previously (20). Cells were lysed and sonicated in lysis buffer containing 25 mM Tris·HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1% Nonidet P-40, 1 mM dithiothreitol, 5% glycerol, phosphatase inhibitor (Sigma), protease inhibitor cocktail (Sigma), and 1 mM NaF. Equal amounts of protein (20–90 μg) were subjected to SDS-PAGE. After protein separation by electrophoresis, samples were transferred to Millipore Immobilon-P membrane, and immunoblotting with antibodies was performed. Signal intensities of the bands were quantified with Image J software (NIH).
Immunoprecipitation.
Immunoprecipitation was performed as previously described (34). Cells were lysed in RIPA buffer containing 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Immunoprecipitations were performed overnight at 4°C using antibodies with protein A-Sepharose. Samples were then subjected to Western blot analysis. For syndecan-2 immunoprecipitation, beads for immunoprecipitation were subjected to heparitinase treatment for 4 h at 37°C to separate syndecan-2 from HS chains.
Transfection with siRNA.
Epac1 small interfering RNA (siRNA) (Ambion), Epac1 siRNA (Ambion), syndecan-2 siRNA (Ambion), and NDST-1 siRNA (Qiagen) were transfected into subconfluent SK-Mel-2 cells using Lipofectamine 2000 (Invitrogen). A pool of double-stranded siRNAs containing equal parts of the following antisense sequences was used: 5′-AUCACUGUAUACCGGUUCC-3′ (Epac1), 5′-CUCUGGACUCUCUACAUCC-3′ (syndecan-2), and 5′-UUUAUUAGCAGUUAGUUCG-3′ (NDST-1). The corresponding nontargeting siRNA silencer negative control no. 2 siRNA (AM4613, Ambion) was used as a negative control. Twenty-four hours later, the medium containing siRNA was changed to fresh medium and incubated for 72 h. When siRNA transfection was combined with adenoviral infection, siRNA transfection followed the adenoviral infection.
HS ELISA.
HS content was determined as previously described with HS ELISA kit (Seikagaku) (36). Cells were collected and disrupted by sonication followed by centrifugation at 14,000 rpm for 10 min. The supernatants were collected and diluted six times and incubated for 18 h at 4°C in the plates coated with HS antibody (20 μg/diluted sample). The secondary reaction with horseradish peroxidase-conjugated streptavidin-biotinylated antibody was carried out for 1 h at room temperature. After color development and stop reaction, OD was measured at 350/630 nm.
Tubulin polymerization assay.
Tubulin polymerization assay was performed as we previously described (23). Cells were washed gently twice with 2 ml prewarmed PBS/wash. After the addition of 400 μl of microtubule-stabilizing buffer (MSB) containing 100 mM Tris·HCl (pH 6.75), 1 mM EGTA, 1 mM MgCl2, 2 M glycerol, 0.1% Triton-X 100, 200 μM PMSF, 10 U/ml egg white trypsin inhibitor (ETI), and 20 μg/ml leupeptin, the cells were incubated for 15 min at 37°C. The cells were then incubated again with 400 μl MSB containing 0.1% Triton-X for 15 min at 37°C. Eighty microliters of 72% trichloroacetic acid and 80 μl of 0.15% DOC were added to total 800 μl of the collected samples. The mixtures were incubated on ice for 10 min and centrifuged at 14,000 rpm for 15 min. The pellets were resuspended with 100 μl of 100 mM NaOH and subjected to SDS-PAGE as a monomeric tubulin fraction. The remaining cells were resuspended with 70 μl lysis buffer [ 50 mM Tris·HCl (pH 6.8), 1 mM EDTA, 1% SDS, 10% glycerol, and 1 mM PMSF] and homogenated using a sonicator (1 s, once) and subjected for SDS-PAGE as a polymeric tubulin fraction.
[ 35S] methionine pulse-labeling assay.
[ 35S] Methionine pulse-labeling assay was performed as previously described (3). The cells were incubated with 100 μCi [ 35S] methionine for 18 h at 37°C. The cells were then lysed with immunoprecipitation buffer (0.5% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, and 0.05 M Tris·HCl, pH 7.5) followed by immunoprecipitation with anti-NDST-1 antibody. The amount of radiolabeled NDST-1 precipitate was analyzed by SDS-PAGE followed by autoradiography overnight at 4°C.
Sucrose density gradient centrifugation.
Lipid rafts-enriched membrane fractions with sucrose density gradient centrifugation were prepared as we previously described (34). Briefly, cells were homogenated in 2 ml of 500 mM sodium carbonate (pH 11.0) with protease inhibitors (1 μg/ml leupeptin, 0.1 mM PMSF, and 50 U/ml ETI) and lysed by sonication. The lysate was then adjusted to 45% sucrose by mixing with 2 ml of 90% sucrose prepared in 25 mM 2-morpholinoethanesulfonic acid (MES) (pH 6.5) and 0.15 mM NaCl (MBS buffer) and placed at the bottom of 5% and 35% discontinuous sucrose gradient (in MBS buffer containing 100 mM sodium carbonate) for an overnight ultracentrifugation (260,000 g). Fractions were removed sequentially from the top and designated as fractions 1 through 13. Then fraction 6, the lipid rafts-enriched fraction, was subjected to immunoprecipitation or Western blot analysis. Flotillin, a lipid raft-associated protein, was used to confirm the existence of lipid rafts in these fractions.
Immunocytochemistry.
Immunocytochemistry was performed as we previously described (21). SK-Mel-2 cells cells on glass coverslips were fixed on glass coverslips, washed, and permeabilized with 0.02% Triton-X followed by incubation with primary and secondary antibodies for 30 min at room temperature. Alexa Fluor 488- and 594-conjugated goat anti-rabbit or anti-mouse antibodies (Molecular Probes) were used. The pictures were taken with a digital camera operated on a Nikon Eclipse TE200 or a confocal microscope (Zeiss Axiovert 100M). For mounting media, Prolong-Gold antifade with DAPI (Molecular Probes) was used.
Lung colonization assay.
To examine the metastatic potential of melanoma cells, lung colonization assay was performed as described previously (11, 43). In brief, Cloudman S91 melanoma cells (clone M3, European Collection of Cell Cultures) were maintained in Ham's F-10 (Sigma) with 2.5% FCS and 15% normal horse serum. The cells were infected with adenovirus expressing Epac1 or green fluorescent protein (GFP) and incubated for 36 h. The expression of Epac1 was examined by Western blot analysis. The cells were harvested and injected (2 × 106 cells/0.2 ml) into tail veins of BALB/c nude mice (Charles River, male, 6 wk old). Two weeks after the injection, the number of metastatic colonies on the surface of the lungs was counted under a dissection microscope. This study was approved by the Animal Care and Use Committee at Yokohama City University.
Statistical analysis.
All results are expressed as means ± SE. Differences in all parameters between experimental groups were analyzed using Student's t-test or analysis of variances (ANOVA), followed by post hoc analysis Fischer test for multiple observations. Differences were considered significant when P values were <0.05.
RESULTS
Epac increases migration in melanoma.
We examined the effect of target proteins of cAMP, i.e, Epac and PKA, on cell migration in melanocyte (HEMA-LP) and melanoma cell lines (SK-Mel-2 and SK-Mel-24) (Fig. 1A). 8-pMeOPT, an Epac-specific agonist, increased cell migration in melanoma cells lines, but not in a melanocyte cell line. By contrast, 6-MB-cAMP, a PKA agonist, did not increase cell migration in any of the cell lines, whereas it increases phosphorylation of CREB (Fig. 1I). Adenoviral overexpression of Epac1 and Epac2, but not PKA (Fig. 1J), increased melanoma cell migration (Fig. 1A). Overexpression of Epacs also increased melanocyte cell migration, suggesting that Epac provides migratory ability.
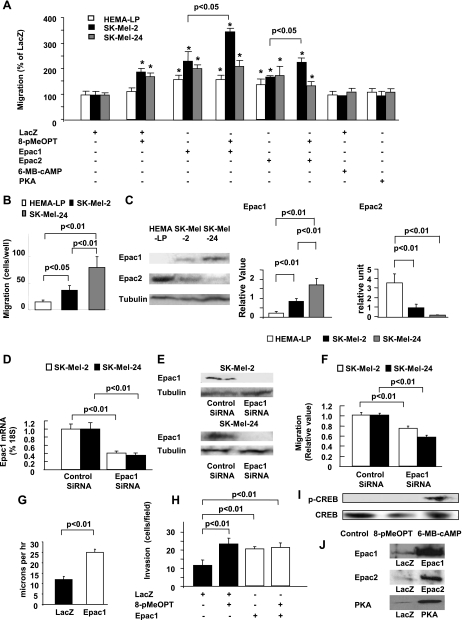
Fig. 1.
Epac increases melanoma cell migration. A: Epac increases melanoma cell migration. Cells were infected with adenovirus harboring LacZ, Epac1, Epac2, and protein kinase A (PKA) followed by the migration assay in the presence or absence of 50 μM 8-(4-methoxyphenylthio)-2'-O-methyladenosine-3',5'-cAMP (8-pMeOPT) or 50 μM N6-monobutyryl-cAMP (6-MB-cAMP). Both 8-pMeOPT and Epac overexpression, but neither 6-MB-cAMP nor PKA overexpression, increased melanoma cell migration. *P < 0.01 vs. LacZ. n = 4. B: basal migration assay in melanocytes and melanoma cell lines is shown. Basal migration was higher in melanoma cell lines than in melanocytes and in SK-Mel-24 cells than in SK-Mel-2 cells. n = 4. C: immunoblots for Epac1 and Epac2. Epac1 protein expression was more abundant in melanoma cell lines than in melanocytes and in SK-Mel-24 cells than in SK-Mel-2 cells. Epac2 protein expression was more abundant in melanocytes than in melanoma cell lines. Bar graphs show densitometric analyses of the blots. n = 4. D: quantitative real-time PCR (qPCR) showed that Epac1 small interfering RNA (siRNA) decreased mRNA expression in SK-Mel-2 cells or SK-Mel-24 cells are shown. n = 4. E: immunoblots showed that Epac1 siRNA decreased Epac1 protein expression in both SK-Mel-2 and SK-Mel-24 cells. F: Epac1 siRNA decreased basal cell migration in SK-Mel-2 cells or SK-Mel-24 cells. *P < 0.01 vs. control siRNA. n = 4. G: video-recorded analysis showed that SK-Mel-2 cells overexpressing Epac1 significantly increased cell motility. n = 10. Supplementary video files; video-recorded cell motility is shown. 1 second in video approximately corresponds to 1 h recording. H: invasion assay showed that both 8-pMeOPT and Epac1 overexpression increased invasion of SK-Mel-2 cells. n = 4. I: immunoblot showed that 6-MB-cAMP, but not 8-pMeOPT, increased phosphorylation of cAMP-response element-binding protein (CREB). J: immunoblot showed overexpression of Epac1, Epac2, and PKA with adenoviral infection in SK-Mel-2.
Basal migration was higher in melanoma cell lines than in melanocytes. Between the two melanoma cell lines, SK-Mel-24 cells showed higher migration than SK-Mel-2 cells (Fig. 1B). When we examined the expression of Epacs, protein expression of Epac1 was higher in melanoma cell lines than in melanocytes, and higher in SK-Mel-24 than in SK-Mel-2 cells (Fig. 1C, left). By contrast, the protein expression of Epac2 was the highest in melanocytes among the cell lines (Fig. 1C, right). These data suggested the major role of endogenous Epac1 in melanoma cells, but the minor role of Epac2 in melanocytes, in cell migration.
We next examined whether deletion of Epac1 reduces melanoma cell migration. When Epac1 expression was decreased by siRNA (Fig. 1, D and E), cell migration was decreased (Fig. 1D). Video-recorded cell motility was also increased by Epac1 overexpression (Fig. 1G and supplemental video). Furthermore, Epac increased melanoma cell invasion (Fig. 1H), suggesting that Epac enhances not only cell migration but also invasion.
Epac-induced cell migration is mediated by the translocation of syndecan-2.
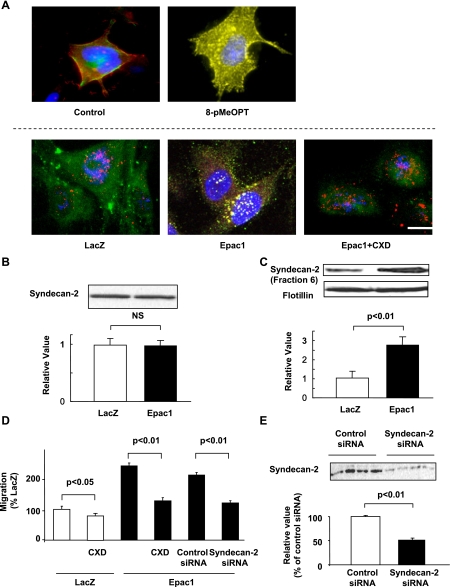
We investigated changes in cell surface molecules which regulate cell migration. Since HSPGs, such as syndecan-2 and glypicans, play a role in cell migration (19, 45), we examined the changes in localization and the expression of glypicans and syndecan-1, -2, and -4. Using immunocytochemistry, we found that Epac1 overexpression increased a particle size of the syndecan-2 immunofluorescent signal, but not of the other HSPGs (data not shown). We also examined expression changes of HSPGs including syndecan-2, however, Epac1 overexpression did not change the expression of syndecan-2 (Fig. 2B) or the expression of the other HSPGs (data not shown). Since syndecan is known to translocate to lipid rafts (40), which serve as platforms for molecules involved in cell migration (26, 30, 32), we hypothesized that such large particle size indicates accumulation of syndecan-2 in lipid rafts. Indeed, immunocytochemistry showed that both Epac agonist and Epac1 overexpression increased colocalization of syndecan-2 with lipid rafts. Cyclodextrin (CXD), a lipid rafts-disrupting agent, decreased such colocalization (Fig. 2A). Additionally, in lipid rafts-rich fraction purified from sucrose density gradient centrifugation, syndecan-2 expression was increased by Epac1 overexpression (Fig. 2C). These data suggested that Epac increases translocation of syndecan-2 to lipid rafts.
Fig. 2.
Epac activates syndecan-2 translocation into rafts in SK-Mel-2. A: immunocytochemistry for syndecan-2 (red) and FLAER (green) is shown. Top, cells were incubated in the presence or absence of 50 μM 8-pMeOPT for 15 min. 8-pMeOPT induced colocalization of syndecan-2 with lipid rafts (yellow). Bottom, cells overexpressing LacZ or Epac1 were incubated in the presence or absence of 10 μg/ml CXD. Epac1 overexpression increased colocalization of syndecan-2 with lipid rafts (yellow). CXD decreased such colocalization. B: immunoblot showed that syndecan-2 expression was not changed by Epac1 overexpression. n = 4. C: immunoblot showed that Epac1 overexpression increased syndecan-2 expression in rafts-rich fraction. Flotillin was used for a marker of lipid rafts. n = 4. D: migration assay showed that CXD (10 μg/ml) decreased basal and Epac1 overexpression-induced cell migration. Syndecan-2 siRNA also inhibited Epac1 overexpression-induced cell migration. n = 4. E: immunoblot showed decreased syndecan-2 expression with siRNA. n = 4.
We next examined whether translocation of syndecan-2 to rafts is involved in Epac-induced cell migration. When lipid rafts was disrupted by CXD, basal and Epac-induced cell migration was inhibited (Fig. 2D), suggesting that lipid rafts are necessary for Epac-induced cell migration. We also examined the effect of deletion of syndecan-2 on Epac-induced cell migration. When syndecan-2 expression was decreased with siRNA (Fig. 2E), Epac-induced cell migration was inhibited (Fig. 2D). These data suggested that the translocation of syndecan-2 to lipid rafts is involved in Epac-induced cell migration.
Epac translocates syndecan-2 to lipid rafts by tubulin polymerization.
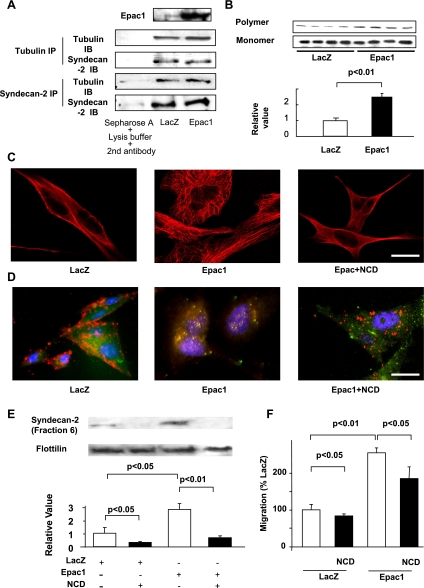
We investigated the mechanism by which Epac regulates syndecan-2 translocation. Since tubulin polymerization is known to mediate intracellular molecule transport (15), and syndecan-2 has a tubulin-binding motif in its intracellular domain (8), we hypothesized that tubulin polymerization mediates Epac-induced translocation of syndecan-2. In melanoma cells, indeed, syndecan-2 is physically bound to tubulin (Fig. 3A). Such binding was not augmented by Epac1 overexpression, suggesting that Epac mediates syndecan-2 translocation not by enhancing the physical interaction between syndecan-2 and tubulin. We thus examined whether Epac increases tubulin polymerization, and this polymerization leads to syndecan-2 translocation. Epac1 overexpression increased polymer form of tubulin (Fig. 3B). In support, immunocytochemistry showed increased tubulin fine structure (Fig. 3C), which is known to reflect tubulin polymerization (17). These data suggested that Epac increases tubulin polymerization.
Fig. 3.
Epac increases syndecan-2 translocation by tubulin polymerization in SK-Mel-2. A: immunoprecipitation showed that syndecan-2 physically interacts with tubulin; however, Epac1 overexpression did not enhance the binding between syndecan-2 and tubulin. Epac1 was increased by Epac1 overexpression (top). B: immunoblots showed that Epac1 overexpression increases polymer form of tubulin. A bar graph shows the densitometric analysis of ratios of tubulin polymers to tubulin monomers. n = 4. C: immunocytochemistry for tubulin (red) is shown. Epac1 overexpression increased fine tubulin network, and such network formation was inhibited by nocodazol (NCD, 10 μM). Scale bar, 3 μm. D: immunocytochemistry for syndecan-2 (red) and lipid rafts (green) is shown. NCD (10 μM) decreased Epac1-induced syndecan-2 colocalization with lipid rafts (yellow). Scale bar, 3 μm. E: immunoblot showed that NCD (10 μM) decreased basal and Epac overexpression-induced syndecan-2 expression in lipid rafts-rich fraction. n = 4. F: migration assay showed that NCD (10 μM) decreased basal and Epac1 overexpression-induced cell migration. n = 4.
We next examined whether inhibition of tubulin polymerization prevents syndecan-2 translocation to lipid rafts. Immunocytochemistry showed that nocodazole (NCD), a tubulin polymerization inhibitor, decreased the colocalization of syndecan-2 with lipid rafts (Fig. 3D). NCD also decreased the expression of syndecan-2 in the lipid rafts-rich fraction (Fig. 3E). These data suggested that tubulin polymerization mediates Epac-induced syndecan-2 translocation to lipid rafts. Furthermore, NCD inhibited Epac-induced cell migration (Fig. 3F), supporting the concept that tubulin polymerization is involved in Epac-induced cell migration.
Epac mediates tubulin polymerization via PI3 kinase.
We next explored the mechanism by which Epac increases tubulin polymerization. Although a recent study demonstrated a direct binding of Epac to tubulin (29), this was not the case, at least, in melanoma cells; neither immunocytochemical studies nor immunoprecipitation assays showed association of Epac1 with tubulin in our study (data not shown). It is well known that the phosphoinositol-3 kinase (PI3K) regulates tubulin polymerization via the Akt/GSK3β pathway (48, 49). Also, a report demonstrated that Epac activates PI3K (7, 22, 31). Therefore, we hypothesized that Epac increases tubulin polymerization via PI3 kinase. Wortmannin, a PI3K inhibitor, inhibited Epac-induced tubulin polymerization (Fig. 4A). Wortmannin also inhibited Epac-induced phosphorylation of Akt (Fig. 4B) and GSK3β (Fig. 4C). These data suggested that the PI3K/Akt/GSK3β pathway is involved in Epac-induced tubulin polymerization. Wortmannin also decreased Epac-induced syndecan-2 localization in lipid rafts (Fig. 4, D and E) and migration (Fig. 4F), further supporting the involvement of PI3K in Epac-induced cell migration.
Fig. 4.
Epac regulates tubulin polymerization via phosphoinositol-3 kinase (PI3K) in SK-Mel-2. A–C: immunoblots for polymer and monomer form of tubulin (A), phosphorylated and total form of Akt (B), or GSK3β (C) are shown. Bar graphs show densitometric analysis of ratios of tubulin polymers to tubulin monomers (A) and ratios of phosphorylated form and total protein (B and C). Wortmannin (10 μM) decreased Epac1-induced tubulin polymerization, basal and Epac1 overexpression-induced phosphorylation of Akt and GSK3β. n = 4. D: immunoblot showed that wortmannin (10 μM) decreased syndecan-2 expression in lipid rafts-rich fraction in cells with either LacZ or Epac1 overexpression. n = 4. E: immunocytochemistry for syndecan-2 (red) and lipid rafts (green) is shown. Wortmannin (10 μM) decreased Epac1 overexpression-induced syndecan-2 colocalization with lipid rafts (yellow). Scale bar, 3 μm. F: migration assay was performed in the presence or absence of wortmannin (10 μM). Wortmannin inhibited basal and Epac1-induced migration n = 4.
Epac increases melanoma cell migration via HS production.
Since lipid rafts serve as a platform for the binding of syndecans to the ECMs (24), the translocation of syndecan-2 is likely to augment the binding between melanoma cells and ECMs via syndecan-2. Because HS is a major component among syndecan-2-bound ECMs (19), we examined whether translocation of syndecan-2 augments its binding to extracellular HS. We found that the glycanated form of syndecan-2, which reflects the HS-bound form of syndecan-2 (35), was increased by Epac1 overexpression (Fig. 5A). These data indicate the possibility that Epac increases the amount of extracellular HS itself. We thus examined whether Epac increases HS production in melanoma cells. Interestingly, Epac1 overexpression increased HS production as demonstrated by HS ELISA (Fig. 5B) and immunocytochemistry (Fig. 5C). These data suggested that, in addition to syndecan-2 translocation, HS production is also involved in Epac-induced cell migration. To investigate the involvement of HS production in Epac-induced cell migration, we examined whether HS degradation inhibits cell migration. When the amount of HS was decreased enzymatically with heparitinase (Fig. 5B), both basal and Epac-induced cell migration were decreased (Fig. 5D). These data were further confirmed by decreased Epac-induced cell migration with sodium chlorate, which chemically degrades HS (data not shown).
We next explored the mechanism by which Epac increases HS production. We examined changes in expressions of HS-biosynthetic enzymes and found that Epac1 overexpression markedly increased expression of NDST-1 (Fig. 5E). We also found that Epac1 overexpression neither changes NDST-1 mRNA expression nor protein degradation (data not shown), but, rather, at the level of proteins, as shown by increased protein translation in [ 35S] methionine pulse-labeling assay (Fig. 5F). We also examined whether deletion of NDST-1 decreases HS production and migration. When NDST-1 expression was reduced by siRNA (Fig. 5, G and H), both basal and Epac-induced HS production were decreased (Fig. 5B), paralleled with decreased basal- and Epac-induced cell migration (Fig. 5D). Put together, these data suggested that increased HS production with enhanced NDST-1 expression is involved in Epac-induced cell migration.
Epac increases melanoma metastasis to lung in mice.
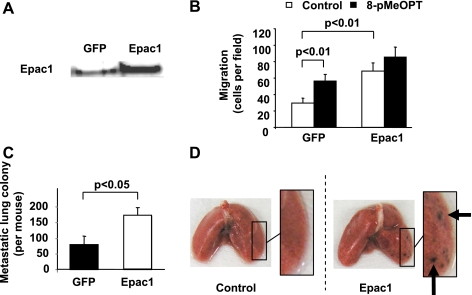
Migratory ability is essential for cancer metastasis not only in the detachment from cancer origin but also in transendothelial extravasation. To examine whether Epac-induced cell migration enhances melanoma metastasis, we performed lung colonization assay in mice, which can reflect the ability of extravasation (2, 46). In mouse Cloudman S91 melanoma cell line, Epac1 was endogenously expressed (Fig. 6A). Both 8-pMeOPT and Epac1 overexpression increased cell migration (Fig. 6B). We thus examined the effect of overexpression of Epac1 on melanoma metastasis. The number of metastatic colonies in the lung was significantly higher in the Epac1-overexpression group than in control group (GFP overexpression) (Fig. 6, C and D). These data suggested that Epac enhances melanoma metastasis presumably by increased cell migration.
Fig. 6.
Epac increases lung metastasis of Cloudman S91 melanoma in mice. A: immunoblot showed that Epac1 is endogenously expressed in S91 cells (left lane) and Epac1 adenovirus infection further increased Epac1 expression (right lane). B: migration assay showed that both 8-pMeOPT (50 μM) and Epac1 overexpression increased cell migration. n = 4. C: lung colonization assay demonsrated that the number of metastatic colonies was increased by Epac1 overexpression compared with control (GFP). n = 10. D: representative pictures of lungs from mice subjected to the melanoma cell injection are shown. Metastatic nodules on the lung surface are indicated by arrows.
DISCUSSION
The central finding of this study is that Epac increases melanoma cell migration/metastasis. The role of cAMP in melanoma metastasis is largely unknown. In the 1980s, a report demonstrated that intracellular cAMP positively correlates with the melanoma metastatic ability in mice (27). In contrast, treating melanoma cells with cholera toxin, which increases cAMP, reduced melanoma colony formation in mice (33). To obtain data of the role of cAMP, we examined the effect of target proteins, i.e., PKA and Epac, on melanoma cell migration/metastasis. We demonstrated that Epac increases melanoma cell migration, but PKA did not. Epac also enhanced melanoma metastasis in mice, indicating that Epac plays a major role in enhancing the metastatic ability of melanoma cells.
We demonstrated that Epac regulates localization of syndecan-2. Syndecan is known to regulate cell migration in various cancer cells through its binding to extracellular HS (19). Syndecan-translocation to lipid rafts plays a role in cell migration (39). Lipid rafts are known to serve as platforms for molecules (42), and translocation of molecules, such as ICAM-1 (30), calpain (32), and integrins (25), to lipid rafts is necessary to activate cell migration. We demonstrated that Epac increased translocation of syndecan-2 to lipid rafts. Deletion of syndecan-2 as well as disruption of lipid rafts decreased Epac-induced cell migration. These data suggested that translocation of syndecan-2 to lipid rafts plays a major role in Epac-induced cell migration. We have also demonstrated that Epac increased glycanation of syndecan-2, which plays a role in connecting the cell surface to extracellular HS (19, 45). On the other hand, studies have demonstrated that the clustering of syndecan in lipid rafts/caveolae is necessary for enhancing cell migration (13). To examine involvement of syndecan-2 clustering in Epac-induced cell migration, further experiments, such as those using overexpression of recombinant syndecan-2 lacking PDZ domain, which mediates syndecan clustering (39), is required. In addition, recent reports have demonstrated that syndecans increases cell migration via integrin α2 (10), laminin α3 (1), focal adhesion formation, and protein kinase C (10). Further investigation may be required to examine whether Epac also regulates these syndecan-related mechanisms in cell migration.
It is also well known that microtubule formation plays a role in the transportation of molecules (15, 18). A previous report demonstrated that Epac increased tubulin growth by direct binding to tubulin (29). Our data, however, did not show interaction between Epac1 and tubulin in immunoprecipitation assays. Instead, we demonstrated that Epac increased tubulin polymerization via activation of the PI3K/Akt/GSK3β pathway. Since GSK3β plays a central role in tubulin polymerization (48, 49), it is reasonable to speculate that Epac mediates tubulin polymerization via the PI3K/Akt/GSK3β pathway.
Even though HS plays a central role in cell migration (14), little is known about the mechanism of HS production. We demonstrated that Epac stimulates HS production in melanoma cells by NDST-1 expression. Such Epac-induced NDST-1 expression occurs at the level of protein translation but not at the transcription level. As far as we know, this is the first report showing that a particular molecule, i.e., Epac, can enhance NDST-1 expression. On the other hand, a report showed that cAMP upregulates 3-O-sulfotransferase-1, a HS-biosynthesis enzyme, in F9 embryonic carcinoma cells (47); however, this is not the case in our study. We did not find that 3-O-sulfotransferase-1 was increased by Epac (data not shown). A remaining question is how Epac regulates NDST-1 translation. Since Epac stimulates GSK3β, which plays a major role in protein translation, it is tempting to speculate that Epac regulates NDST-1 translation via GSK3β. Indeed, our data showed that wortmannin decreased Epac-induced NDST-1 translation (data not shown), supporting the idea that Epac regulates protein translation via GSK3β.
Migratory ability increases as the melanoma stage progress; when migration was compared in human melanoma cell lines, migration was greater in metastatic melanoma than in primary melanoma (9). We showed that SK-Mel-24 cells, derived from metastatic lymph node melanoma, showed higher Epac1 expression than in SK-Mel-2 cells, derived from regional metastasis. Also, melanoma cell lines showed increased Epac1 expression than melanocytes. Accordingly, it is likely that Epac1 expression increases as melanoma stage progress and provides metastatic ability. However, we examined only two melanoma cell lines. Thus, to obtain conclusive evidence, further study, i.e., investigation of the relation between Epac expression and metastatic ability by the use of different stages of melanoma in cell lines and/or human melanoma samples, is required to obtain conclusive evidence.
In conclusion, the robust finding of our study suggests that Epac may serve as a target molecule in melanoma cancer thereby inhibiting melanoma metastasis, even though we do not yet know whether Epac similarly regulates HS signaling in other cancer cell types.
GRANTS
This study was supported in part by grants from the Japan Space Forum, the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and the Takeda Science Foundation and National Institutes of Health Grants GM-067773 and HL-059139 (to Y. Ishikawa), and the American Heart Association (SDG 0835596D) and the Foundation of UMDNJ (K. Iwatsubo).
ACKNOWLEDGMENTS
We thank Lauren Danridge for critical reading of the manuscript.
REFERENCES
- 1.Araki E, Momota Y, Togo T, Tanioka M, Hozumi K, Nomizu M, Miyachi Y, Utani A. Clustering of Syndecan-4 and integrin β1 by iaminin α3 chain-derived peptide promotes keratinocyte migration. Mol Biol Cell 20: 3012–3024, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer 8: 212–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoist F and Grand-Perret T. Cotranslational degradation of apolipoprotein B100 by the proteasome is prevented by microsomal triglyceride transfer protein synchronized translation studies on HepG2 cells treated with an inhibitor of microsomal triglyceride transfer protein. J Biol Chem 272: 20435–20442, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Ann Rev Biochem 68: 729–777, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Berwick M, Wiggins C. The current epidemiology of cutaneous malignant melanoma. Front Biosci 11: 1244–1254, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Brennesvik EO, Ktori C, Ruzzin J, Jebens E, Shepherd PR, Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal 17: 1551–1559, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Brockstedt U, Dobra K, Nurminen M, Hjerpe A. Immunoreactivity to cell surface syndecans in cytoplasm and nucleus: tubulin-dependent rearrangements. Exp Cell Res 274: 235–245, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Byers HR, Etoh T, Doherty JR, Sober AJ, Mihm MC., Jr Cell migration and actin organization in cultured human primary, recurrent cutaneous and metastatic melanoma. Time-lapse and image analysis. Am J Pathol 139: 423–435, 1991 [PMC free article] [PubMed] [Google Scholar]
- 10.Choi S, Kim Y, Park H, Han IO, Chung E, Lee SY, Kim YB, Lee JW, Oh ES, Yi JY. Syndecan-2 overexpression regulates adhesion and migration through cooperation with integrin alpha2. Biochem Biophys Res Commun 384: 231–235, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res 56: 172–181, 1996 [PubMed] [Google Scholar]
- 12.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ethell IM, Yamaguchi Y. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J Cell Biol 144: 575–586, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fjeldstad K, Kolset SO. Decreasing the metastatic potential in cancers–targeting the heparan sulfate proteoglycans. Curr Drug Targets 6: 665–682, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Galbraith JA, Gallant PE. Axonal transport of tubulin and actin. J Neurocytol 29: 889–911, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Feng Y, Bowers R, Becker-Hapak M, Gardner J, Council L, Linette G, Zhao H, Cornelius LA. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res 66: 7880–7888, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Hadjidemetriou S, Toomre D, Duncan J. Motion tracking of the outer tips of microtubules. Med Image Anal 2008 [DOI] [PubMed] [Google Scholar]
- 18.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281: 26391–26399, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Iozzo RV. Series Introduction: Heparan sulfate proteoglycans: intricate molecules with intriguing functions. J Clin Invest 108: 165–167, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwatsubo K, Minamisawa S, Tsunematsu T, Nakagome M, Toya Y, Tomlinson JE, Umemura S, Scarborough RM, Levy DE, Ishikawa Y. Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J Biol Chem 279: 40938–40945, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Iwatsubo K, Suzuki S, Li C, Tsunematsu T, Nakamura F, Okumura S, Sato M, Minamisawa S, Toya Y, Umemura S, Ishikawa Y. Dopamine induces apoptosis in young, but not in neonatal, neurons via Ca2+-dependent signal. Am J Physiol Cell Physiol 293: C1498–C1508, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jing H, Yen JH, Ganea D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J Biol Chem 279: 55176–55186, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 286: H1845–H1852, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kopatz I, Remy JS, Behr JP. A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J Gene Med 6: 769–776, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Krauss K, Altevogt P. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J Biol Chem 274: 36921–36927, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci 115: 963–972, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lester BR, Greig RG, Buscarino C, Sheppard JR, Corwin SP, Poste G. cAMP metabolism in B16 melanoma clones during the formation of experimental and spontaneous metastases. Int J Cancer 38: 405–411, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, and Fernandez-Borja M. Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol 80: 1542–1552, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mei FC, Cheng X. Interplay between exchange protein directly activated by cAMP (Epac) and microtubule cytoskeleton. Mol BioSys 1: 325–331, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol 8: 113–123, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Misra UK, Pizzo SV. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J Biol Chem 280: 38276–38289, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Nuzzi PA, Senetar MA, Huttenlocher A. Asymmetric localization of calpain 2 during neutrophil chemotaxis. Mol Biol Cell 18: 795–805, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ormerod EJ, Hart IR. Different growth responses to agents which elevate cAMP in human melanoma cell lines of high and low experimental metastatic capacity. Clin Exp Metast 7: 85–95, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Oshikawa J, Otsu K, Toya Y, Tsunematsu T, Hankins R, Kawabe J, Minamisawa S, Umemura S, Hagiwara Y, Ishikawa Y. Insulin resistance in skeletal muscles of caveolin-3-null mice. Proc Natl Acad Sci USA 101: 12670–12675, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao D, Meyer K, Mundhenke C, Drew SA, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem 278: 16045–16053, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Raats CJI, Bakker MAH, van den Born J, Berden JHM. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem 272: 26734–26741, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Rickard A, Portell C, Siegal J, Goeckeler Z, Lagunoff D. Measurement of the motility of endothelial cells in confluent monolayers. Microcirculation 10: 193–203, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Thomas LA, Yamada KM. Contact stimulation of cell migration. J Cell Sci 103: 1211–1214, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Tkachenko E, Elfenbein A, Tirziu D, Simons M. Syndecan-4 clustering induces cell migration in a PDZ-dependent manner. Circ Res 98: 1398–1404, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tkachenko E, Simons M. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains. J Biol Chem 277: 19946–19951, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, Hirotani S, Ishikawa Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol 293: H1662–H1672, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wolter KG, Verhaegen M, Fernandez Y, Nikolovska-Coleska Z, Riblett M, de la Vega CM, Wang S, Soengas MS. Therapeutic window for melanoma treatment provided by selective effects of the proteasome on Bcl-2 proteins. Cell Death Differ 14: 1605–1616, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, Iwasaki S, Iwamoto M, Misra S, Tamura K, Hori H, Yokota S, Toole BP, Sugimoto Y, Ishikawa Y. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest 116: 3026–3034, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoneda A, Couchman JR. Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol 22: 25–33, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Yu HR, Schultz RM. Relationship between secreted urokinase plasminogen activator activity and metastatic potential in murine B16 cells transfected with human urokinase sense and antisense genes. Cancer Res 50: 7623–7633, 1990 [PubMed] [Google Scholar]
- 47.Zhang L, Schwartz JJ, Miller J, Liu J, Fritze LMS, Shworak NW, Rosenberg RD. The retinoic acid and cAMP-dependent up-regulation of 3-O-sulfotransferase-1 leads to a dramatic augmentation of anticoagulantly active heparan sulfate biosynthesis in F9 embryonal carcinoma Cells. J Biol Chem 273: 27998–28003, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Zhou FQ, Snider Cell biology WD. GSK-3beta and microtubule assembly in axons. Science 308: 211–214, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Zumbrunn J, Kinoshita K, Hyman AA, Nathke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol 11: 44–49, 2001 [DOI] [PubMed] [Google Scholar]