Abstract
Hypochlorous acid (HOCl) is a unique oxidant generated by the enzyme myeloperoxidase that contributes to endothelial cell dysfunction and death in atherosclerosis. Since myeloperoxidase localizes with heme oxygenase-1 (HO-1) in and around endothelial cells of atherosclerotic lesions, the present study investigated whether there was an interaction between these two enzymes in vascular endothelium. Treatment of human endothelial cells with the myeloperoxidase product HOCl stimulated a concentration- and time-dependent increase in HO-1 protein that resulted in a significant rise in carbon monoxide (CO) production. The induction of HO-1 protein was preceded by a prominent increase in HO-1 mRNA and total and nuclear factor-erythroid 2-related factor 2 (Nrf2). In addition, HOCl induced a significant rise in HO-1 promoter activity that was blocked by mutating the antioxidant response element (ARE) in the promoter or by overexpressing a dominant-negative mutant of Nrf2. The HOCl-mediated induction of Nrf2 or HO-1 was blocked by the glutathione donor N-acetyl-l-cysteine but was unaffected by ascorbic or uric acid. Finally, treatment of endothelial cells with HOCl stimulated mitochondrial dysfunction, caspase-3 activation, and cell death that was potentiated by the HO inhibitor, tin protoporphyrin-IX, or by the knockdown of HO-1, and reversed by the exogenous administration of biliverdin, bilirubin, or CO. These results demonstrate that HOCl induces HO-1 gene transcription via the activation of the Nrf2/ARE pathway to counteract HOCl-mediated mitochondrial dysfunction and cell death. The ability of HOCl to activate HO-1 gene expression may represent a critical adaptive response to maintain endothelial cell viability at sites of vascular inflammation and atherosclerosis.
Keywords: vascular endothelium, oxidative stress, toxicity, bile pigments, atherosclerosis
myeloperoxidase (MPO) is a secreted protein found in phagocytes that participates in the innate immune response through the formation of microbicidal reactive oxidants (31). Hypochlorous acid (HOCl) is a unique oxidant formed by MPO that reacts with several biological targets to produce a variety of HOCl-modified compounds. There is increasing evidence that HOCl contributes to tissue injury in a number of inflammatory diseases, including ischemia-reperfusion injury, acute vasculitis, and atherosclerosis (34). Multiple lines of evidence suggest that MPO plays a role in atherogenesis. Immunohistochemical and biochemical analyzes localize the enzyme and HOCl-modified proteins within and around endothelial cells of human atherosclerotic lesions (8, 16). Interestingly, individuals with MPO deficiency are less likely to develop cardiovascular disease, whereas elevated systemic levels of MPO are associated with the presence of coronary artery disease and predict risk in patients with acute coronary syndromes (4, 57).
Several potential mechanisms contribute to the atherogenic property of MPO. In particular, MPO-derived HOCl oxidizes low-density lipoprotein converting it to a highly atherogenic form that is readily taken up by macrophages via scavenger receptors (15). In addition, HOCl oxidizes high-density lipoprotein and impairs its ability to promote cholesterol efflux (5). Furthermore, HOCl induces endothelial dysfunction, which is one of the earliest changes associated with the development of atherosclerosis. HOCl inhibits endothelium-dependent relaxation and suppresses nitric oxide (NO) synthesis from endothelial cells (22, 43). Moreover, low concentrations of HOCl stimulate tissue factor expression, whereas higher, but pathophysiologically relevant, concentrations, provoke endothelial cell death and desquamation (45). These latter findings suggest that HOCl may also participate in the pathogenesis of acute coronary syndromes by promoting superficial plaque erosion and increasing thrombogenicity.
Heme oxygenase-1 (HO-1) is a highly inducible enzyme that catalyzes the degradation of heme into equimolar amounts of carbon monoxide (CO), biliverdin, and free iron (see Ref. 10). Biliverdin is subsequently metabolized to bilirubin by biliverdin reductase. HO-1 is strongly induced by oxidative and nitrosative stress, and its induction in endothelial cells provides an important cellular defense mechanism against tissue injury (6, 33, 54). The cytoprotection afforded by HO-1 is mediated by several different mechanisms, including the catabolism of pro-oxidant heme to the antioxidant bile pigments biliverdin and bilirubin; the coordinated induction of ferritin, which chelates free iron; and the liberation of CO, which exerts significant anti-inflammatory and antiapoptotic effects. Considerable evidence suggests that HO-1 also protects against the development of atherosclerosis. HO-1 is highly expressed in endothelial and foam cells of atherosclerotic lesions in both humans and animals (49). In addition, pharmacological induction or adenovirus-mediated transfer of HO-1 inhibits lesion formation in various rodent models of atherosclerosis, whereas inhibition or gene deletion of HO-1 promotes lesion development (19, 24, 55). Consistent with these animal studies, the first human case of HO-1 deficiency exhibited early atherosclerotic disease as reflected by the presence of fatty streaks and fibrous plaque (26).
Since MPO and HO-1 are highly expressed and localized to endothelial cells in atherosclerotic lesions, the current study investigated whether there was an interaction between these two enzymes. In particular, we examined whether the MPO product HOCl influences HO-1 gene expression and CO production in human endothelial cells. In addition, we identified the signaling pathway responsible for regulating HO-1 expression and determined the physiological significance of the induction of HO-1 by HOCl in vascular endothelium.
MATERIALS AND METHODS
Materials.
M199 medium, streptomycin, penicillin, gelatin, ascorbic acid, uric acid, trypan blue, sodium dodecyl sulfate (SDS), sodium fluoride, EDTA, HEPES, N-tris-(hydroxymethyl) methyl-2-aminoethanesulfonic acid (TES), iron, tricarbonyldichlororuthenium (II) dimer (CORM2), N-acetyl-l-cysteine (NAC), and HOCl were purchased from Sigma-Aldrich Chemical (St. Louis, MO); tin protoporphyrin-IX (SnPP), bilirubin, and biliverdin were from Frontier Scientific (Logan, UT); a polyclonal antibody against HO-1 was from Assay Designs (Ann Arbor, MI); antibodies against nuclear factor-erythroid 2-related factor 2 (Nrf2), and β-actin were from Santa Cruz Biotechnologies (Santa Cruz, CA); Alexa Fluor 488 was from Invitrogen (Carlsbad, CA); γ-[32P]ATP (3,000 Ci/mmol) was from NEN-DuPont (Boston, MA); p-nitroanilide-conjugated Asp-Glu-Val-Asp (DEVD) was from Clontech (Palo Alto, CA); the HO-1 small interference RNA (siRNA; sense: 5′-AUGCUGAGUUCAUGAGGA AUU-3′, antisense: 5′-PUUCCUCAUGAACUCAGCAUUU-3′) and a nontargeting siRNA (5′-AAUGGAAGACCACUCCCACUC-3′) were purchased from Dharmacon (Lafayette, CO).
Cell culture.
Human umbilical vein endothelial cells (HUVEC) or human aortic endothelial cells (HAEC) were purchased from Lonza (Allendale, NJ) and serially cultured on gelatin-coated dishes, as we previously described (28). Cells were propagated in M199 medium supplemented with 20% bovine calf serum, 2 mM l-glutamine, 50 μg/ml endothelial cell growth factor, 90 μg/ml heparin, and 100 U/ml of penicillin and streptomycin in an atmosphere of 95% air-5% CO2.
Western blotting.
Cells were lysed in sample buffer (125 mM Tris, pH 6.8, 12.5% glycerol, 2% SDS, 50 mM sodium fluoride, and trace bromophenol blue) and proteins separated by SDS-PAGE. After transfer to nitrocellulose membrane, blots were blocked with PBS and nonfat milk (5%) and then incubated with antibodies directed against HO-1 (1:1,500), Nrf2 (1:200), or β-actin (1:200). Membranes were then washed in PBS, incubated with horseradish peroxidase-conjugated goat anti-rabbit, rabbit anti-mouse, or donkey anti-goat antibody, and developed with commercial chemoluminescence reagents (Amersham, Arlington Heights, IL). Protein expression was quantified by scanning densitometry and normalized with respect to β-actin.
Northern blotting.
Total RNA was loaded onto 1.2% agarose gels, fractionated by electrophoresis, and blot transferred to Gene Screen Plus membranes (Perkin Elmer Life Sciences, Waltham, MA). Membranes were prehybridized for 4 h at 68°C in rapid hybridization buffer (Amersham, Arlington Heights, IL) and then incubated overnight at 68°C in hybridization buffer containing [32P]DNA probes (1 × 108 cpm) for HO-1, Nrf2, or 18S mRNA. DNA probes were generated by RT-PCR and labeled with [32P]dCTP using a random priming kit (Amersham, Arlington Heights, IL) (28). After hybridization, membranes were washed and exposed to X-ray film at −70°C, and HO-1 expression was quantified by scanning densitometry and normalized with respect to 18S rRNA.
HO-1 promoter analysis.
HO-1 promoter activity was determined using HO-1 promoter/firefly luciferase constructs that were generously provided by Dr. Jawed Alam at the Ochsner Clinic Foundation, New Orleans, LA. These constructs consisted of the wild-type enhancer (E1) coupled to a minimum HO-1 promoter (E1) as well as the mutant enhancer (M739) that had its three antioxidant responsive element (ARE) core sequences mutated. In some experiments, a plasmid expressing a dominant-negative Nrf2 mutant (dnNrf2) that had its transactivation domain deleted was used. A plasmid encoding Renilla luciferase ([hRluc/TK]-Renilla luciferase) was included in all samples to control for transfection efficiency. Cells were transfected with plasmids (1 μg/ml) using lipofectamine, incubated for 48 h, and then exposed to HOCl. After an additional 6 h incubation, cells were collected and lysed, and luciferase activity was measured by using the Promega Dual-Light assay system and a Glomax luminometer (Promega, Madison, WI). Firefly luciferase activity was normalized with respect to Renilla luciferase activity, and this ratio was expressed as fold induction over control cells.
CO production.
Carbon monoxide (CO) formation was determined by solid-phase gas chromatography (23). In brief, cells were washed with KH2PO4 (100 mM) buffer, scraped into microcentrifuge tubes, and sonicated. Cell debris was sedimented at 1,000 g for 5 min, and supernatants were collected and stored at −80°C. For CO analysis, cell sonicates (60 μl) were placed in amber glass vials sealed with silicon septum caps. The headspace was purged with CO-scrubbed air and incubated for 1 h at 37°C. Matched samples maintained at 0°C, to inhibit HO activity, served as background controls. CO levels in the headspace gas were quantified using solid phase gas chromotography (model: Customized Peak Performer 1, Peak Analytical, Mountain View, CA) The analyzer was calibrated with a mixture of CO in air (Scott Specialty Gases, Plumsteadville, PA) and corrected immediately before measurements.
Immunofluorescence microscopy.
Cellular localization of Nrf2 was determined by immunofluorescence. Cells were grown on glass coverslips, and following treatment, cells were fixed with cold acetone, washed with PBS, and incubated with blocking buffer (3% BSA, 5% goat serum, 0.1% Triton X-100 in PBS) for 30 min at room temperature. Cells were then washed with PBS and incubated with an antibody against Nrf2 (1:100 dilution) in blocking buffer for 1 h at room temperature. Coverslips were washed and incubated with Alexa Fluor 488 goat anti-rabbit IgG (1:1,000) in blocking buffer for 45 min at room temperature. Coverslips were then washed and mounted on glass slides, and images were obtained with a Bio-Rad Radiance 2000 Confocal system coupled to an inverted IX70 microscope and digital camera.
Antioxidant activity.
Antioxidant activity was determined by measuring superoxide anion scavenging activity using a modification of the procedure described by Marklund and Marklund (32). The spontaneous oxidation of a pyrogallol solution (0.6 mM in 0.2 mM Tris·HCl, pH 7.6) to purpurogallin was followed by absorbance spectroscopy at 235 nm. Rates of pyrogallol oxidation in the presence of various antioxidants were determined by measuring the slope of the absorbance curve during the first 5 min of the reaction.
Cell viability.
Cell viability was determined by measuring the uptake of the membrane impermeable stain trypan blue. Cells were treated with trypsin (0.25%), collected, and diluted (1: 4) with trypan blue. Viable cells that exclude trypan blue were counted with a hemocytometer, as we previously described (29).
Mitochondrial membrane potential.
Mitochondrial membrane potential was determined using the cationic dye, MitoCapture (BioVision Research Products, Mountain View, CA). In healthy cells, the dye accumulates and aggregates in the mitochondria as a function of inner mitochondrial membrane potential resulting in a red fluorescence. However, in apoptotic cells where mitochondrial membrane potential is disrupted, the reagent cannot aggregate in the mitochondria and remains in its monomer form generating a green fluorescence. Endothelial cells were grown on coverslips, and after treatment, they were incubated with the dye in incubation buffer for 20 min at 30°C. Cells were then washed three times in PBS and images obtained using a Zeiss LSM 510 NLO scanning confocal microscope for red (excitation/emission = 488 nm/590 nm): green (excitation/emission = 488 nm/530 nm) fluorescence ratio detection.
Caspase-3 activity.
Caspase-3 activity was measured by monitoring the cleavage of the p-nitroanilide-conjugated caspase-3 substrate DEVD (29). Briefly, cells were trypsinized, pooled with detached cells, washed in ice-cold PBS, and suspended in lysis buffer (50 mM HEPES, pH 7.5, 10% sucrose, 0.1% Triton X-100) on ice for 10 min. After centrifugation at 13,000 g for 5 min at 4°C, supernatants were incubated with 50 μM of DEVD and absorbance was measured at 405 nm with a μQuant spectrophotemeter (Bio-Tek Instruments, Winooski, VT).
Statistics.
Results are expressed as the means ± SE. Statistical differences between groups were evaluated with a Student's two-tailed t-test or by ANOVA with Bonferroni's t-test when multiple groups were compared. P values <0.05 were considered statistically significant.
RESULTS
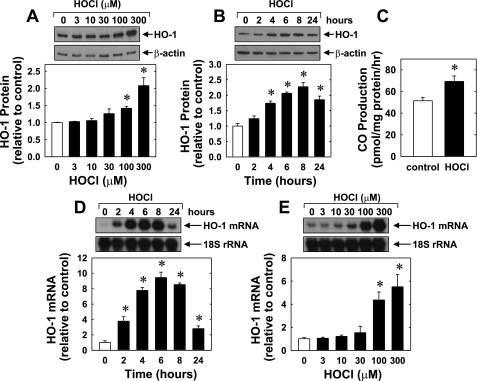
Treatment of HUVEC with HOCl stimulated a concentration- and time-dependent increase in HO-1 protein. An increase in HO-1 protein was detected after 24 h with 100 μM of HOCl, and a higher concentration of HOCl (300 μM) showed a further increase in HO-1 protein (Fig. 1A). The induction of HO-1 protein by HOCl was delayed, with a significant increase in HO-1 protein appearing 4 h after HOCl administration, and levels remained elevated following 24 h of treatment (Fig. 1B). The induction of HO-1 protein in HUVEC was associated with a significant 40% increase in CO production (Fig. 1C). HOCl also stimulated the expression of HO-1 mRNA in HUVEC in a manner that preceded the increase in HO-1 protein (Fig. 1, D and E). An increase in HO-1 mRNA was detected 2 h after HOCl exposure, and transcript levels peaked between 4 and 8 h and remained elevated after 24 h of HOCl treatment. In addition, HOCl induced a concentration- and time-dependent rise in HO-1 protein in HAEC that was associated with an increase in HO-1 mRNA (Fig. 2).
Fig. 1.
Hypochlorous acid (HOCl) stimulates heme oxygenase-1 (HO-1) expression and carbon monoxide (CO) production in human umbilical vein endothelial cells (HUVEC). A: concentration-dependent effect of HOCl (3–300 μM for 24 h) on HO-1 protein expression. B: time course of HO-1 protein expression following the administration of HOCl (300 μM). C: HOCl (300 μM for 24 h) stimulates endothelial cell CO formation. D: time-dependent increase in HO-1 mRNA expression following the administration of HOCl (300 μM). E: concentration-dependent increase in HO-1 mRNA following the administration of HOCl (3–300 μM for 4 h). HO-1 protein and mRNA expression was quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE (n = 3–4). *Statistically significant effect of HOCl.
Fig. 2.
HOCl stimulates HO-1 protein and mRNA expression in HAEC. A: concentration-dependent effect of HOCl (3–300 μM for 24 h) on HO-1 protein expression. B: time course of HO-1 protein expression following the administration of HOCl (300 μM). C: time-dependent increase in HO-1 mRNA expression following the administration of HOCl (300 μM). HO-1 protein and mRNA expression was quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE (n = 3). *Statistically significant effect of HOCl.
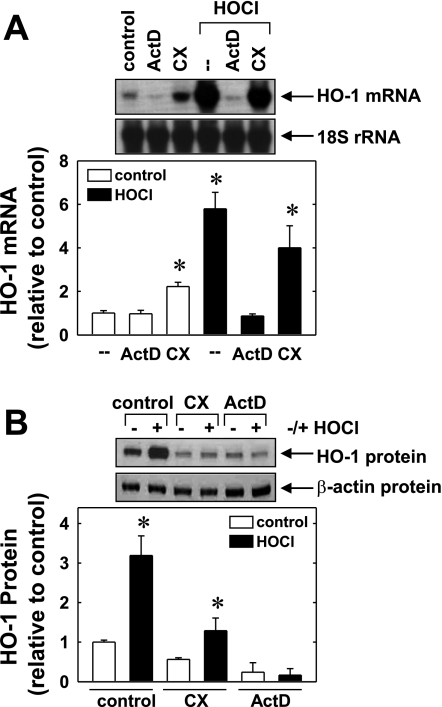
Incubation of HUVECs with the transcriptional inhibitor actinomycin D (2 μg/ml) completely blocked the induction of HO-1 mRNA and protein by HOCl (Fig. 3, A and B). In contrast, the protein synthesis inhibitor cycloheximide (5 μg/ml), minimally affected the increase in HO-1 mRNA while totally suppressing the rise in HO-1 protein induced by HOCl. In the absence of HOCl, cycloheximide and actinomycin D suppressed basal HO-1 protein expression (Fig. 3B). Actinomycin D had no significant effect on basal HO-1 mRNA levels, but cycloheximide resulted in an elevation in HO-1 message (Fig. 3A).
Fig. 3.
HOCl-mediated HO-1 gene expression requires de novo RNA synthesis. A: effect of actinomycin D (ActD, 2 μg/ml) or cycloheximide (CX, 5 μg/ml) on HOCl (300 μM for 4 h)-mediated increase in HO-1 mRNA. B: effect of ActD (2 μg/ml) or CX (5 μg/ml) on HOCl (300 μM for 24 h)-mediated increases in HO-1 protein. HO-1 protein or mRNA was quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE (n = 4). *Statistically significant increase in HO-1 expression.
To further examine the molecular mechanism by which HOCl induces HO-1 gene expression, HUVEC were transiently transfected with an HO-1 promoter construct, and promoter activity was monitored. Treatment of HUVEC with HOCl stimulated a concentration-dependent increase in HO-1 promoter activity (Fig. 4A). Interestingly, mutation of the ARE (M739) attenuated basal activity and abolished the response to HOCl, suggesting that HOCl activates HO-1 gene transcription via the ARE. Since the transcription factor Nrf2 plays a predominant role in ARE-mediated gene expression (1), we investigated whether Nrf2 was involved in the activation of HO-1 by HOCl. Transfection of HUVEC with a dominant-negative mutant of Nrf2 that had its activation domain deleted inhibited the HOCl-mediated increase in HO-1 promoter activity. Furthermore, incubation of HUVEC with HOCl stimulated a rapid rise in Nrf2 protein beginning 1 h after HOCl exposure (Fig. 4B). Immunofluorescence experiments demonstrate weak cytosolic staining of Nrf2 in control, untreated HUVEC; however, HOCl-treatment resulted in marked increase in both cytosolic and nuclear Nrf2 staining (Fig. 4C). In contrast, HOCl failed to stimulate Nrf2 mRNA expression (Fig. 4D).
Fig. 4.
HOCl stimulates HO-1 promoter activity and nuclear factor-erythroid 2-related factor 2 (Nrf2) protein expression in HUVEC. A: effect of HOCl on HO-1 promoter activity. Cells were cotransfected with a HO-promoter construct (E1) or a mutated HO-1 promoter construct (M739) and a Renilla luciferase construct and treated with HOCl (100 or 300 μM) for 6 h and then analyzed for luciferase activity. In some experiments, a dominant-negative mutant Nrf2 construct was cotransfected into cells. Results are means ± SE (n = 6). *Statistically significant increase in promoter activity. B: Western blot of Nrf2 protein following treatment of cells with HOCl (300 μM). Nrf2 protein was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SE (n = 4). *Statistically significant effect of HOCl. C: immunofluorescence microscopy (magnification, ×60) demonstrating Nrf2 expression (green) with propidium iodide (red) nuclear counterstaining after treatment of cells with HOCl (300 μM for 4 h). Cytosolic localization of Nrf2 is indicated in green, whereas nuclear localization is given in yellow. Similar findings were observed in 3 separate experiments. D: Northern blot of Nrf2 mRNA expression after treatment of endothelial cells with HOCl (300 μM). Nrf2 mRNA was quantified by scanning densitometry, normalized with respect to 18S rRNA and expressed relative to that of control, untreated cells. Results are means ± SE (n = 3).
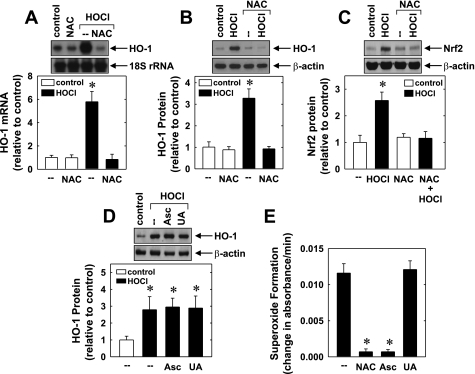
In subsequent experiments, we determined the upstream signaling pathway that stimulates Nrf2 and HO-1 expression. Since HOCl is an oxidant and because reactive oxygen species (ROS) have been implicated in the activation of Nrf2 (1, 21, 56), the involvement of oxidative stress was examined. Treatment of HUVEC with the glutathione donor and ROS scavenger NAC (10 mM) completely abrogated the induction of HO-1 mRNA and protein expression in response to HOCl (Fig. 5, A and B). In addition, NAC prevented the HOCl-mediated increase in Nrf2 protein (Fig. 5C). In contrast, other antioxidants including ascorbic (500 μM) and uric acid (300 μM) failed to block the HOCl-mediated induction of HO-1 (Fig. 5D). Interestingly, NAC and ascorbic acid were equally effective in scavenging superoxide anions, whereas uric acid was ineffective (Fig. 5E).
Fig. 5.
N-acetyl-l-cysteine (NAC) inhibits the induction of HO-1 and Nrf2 expression by HOCl. A: effect of NAC (10 mm) on HOCl (300 μM for 4 h)-mediated HO-1 mRNA expression. B: effect of NAC (10 mM) on HOCl (300 μM for 6 h)-mediated HO-1 protein expression. C: effect of NAC (10 mM) on HOCl (300 μM for 4 h)-mediated Nrf2 protein expression. D: effect of ascorbic acid (Asc; 500 μM) or uric acid (UA; 500 μM) on HOCl (300 μM for 6 h)-mediated HO-1 protein expression. E: superoxide radical scavenging potential of NAC (10 mM), Asc (500 μM), or UA (300 μM) as measured by the rate of pyrogallol autooxidation. Protein or mRNA was quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE (n = 3–4). *Statistically significant effect of HOCl, NAC, or Asc.
In another series of experiments, the functional role of HO-1 induction in endothelial cells by HOCl was investigated. Treatment of HUVEC with a high concentration of HOCl (300 μM) for 24 h resulted in a significant decline in endothelial cell viability. Interestingly, the addition of the HO inhibitor tin protoporphyrin-IX (10 μM) enhanced HOCl-mediated cell death by nearly 50% (Fig. 6A). Similarly, treatment of HUVEC with a HO-1 siRNA (0.1 μM) potentiated HOCl-mediated cell toxicity, whereas the nontargeting siRNA had no effect (Fig. 6A). In the absence of HOCl, SnPP, HO-1 siRNA, or the nontargeting siRNA had no adverse effect on cell survival (data not shown). In addition, transfection of HUVEC with the HO-1 siRNA abolished the induction of HO-1 protein by HOCl (Fig. 6B). In contrast, the nontargeting siRNA had no effect on the HOCl-mediated increase in HO-1 protein expression, confirming the efficacy and selectivity of the HO-1 knockdown approach (Fig. 6B). These findings suggested that the induction of HO-1 by HOCl functions in an adaptive manner to limit cell death.
Fig. 6.
Induction of HO-1 by HOCl promotes the survival of HUVEC. A: cell viability following treatment of cells with HOCl (300 μM for 24 h) and transfection with HO small interfering RNA (siRNA, 1 μM) or nontargeting (NT) siRNA (0.1 μM) or incubation with the HO inhibitor, tin protoporphyrin-IX (SnPP, 10 μM). Results are means ± SE (n = 6). *Statistically significant effect of HOCl. †Statistically significant effect of SnPP or HO-1 siRNA. B: HO-1 protein expression in cells treated with HOCl (300 μM for 24 h) and transfected with HO siRNA (0.1 μM) or NT siRNA (0.1 μM). HO-1 protein was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SE (n = 4). *Statistically significant effect of HOCl.
Subsequently, we determined which of the HO-1 products mediates this cytoprotective effect. Interestingly, incubation of HUVEC with bilirubin (10 μM), biliverdin (10 μM), or the CO donor CORM2 (10 μM) reversed the cytotoxic effect of HOCl. In contrast, the addition of ferrous iron (10 μM) had no effect on cell toxicity (Fig. 7A). Since cell death often results from the loss of mitochondrial function, we explored the effect of HOCl on mitochondrial membrane potential. Incubation of HUVEC with HOCl (300 μM) resulted in a significant decline in mitochondrial membrane potential, as reflected by the decrease in the measured red-to-green fluorescence ratio of cells loaded with the voltage-sensitive Mitocapture dye (Fig. 7, B and C). In addition, the HOCl-mediated dissipation of mitochondrial membrane potential was associated with a twofold increase in caspase-3 activity (Fig. 7D). Interestingly, either bilirubin (10 μM) or CORM2 (10 μM) prevented the disruption of mitochondrial membrane potential as well as the activation of caspase-3 by HOCl (Fig. 7, B–D). In the absence of HOCl, bilirubin or CORM2 had no effect on mitochondrial membrane potential or caspase-3 activity.
Fig. 7.
CO and bile pigments prevent HOCl-mediated cytotoxicity in HUVEC. A: cell viability following treatment of HUVEC with HOCl (300 μM for 24 h) in the presence or absence of biliverdin (BV; 10 μM), bilirubin (BR; 10 μM), CORM2 (10 μM), or iron (10 μM). B: effect of HOCl on mitochondrial membrane potential. Cells were treated with HOCl (300 μM for 4 h) in the presence or absence of bilirubin (10 μM) or CORM2 (10 μM) and mitochondrial membrane potential determined in MitoCapture-loaded cells by confocal microscopy (magnification, ×20). C: quantification of the red-to-green fluorescence ratio in cells treated with HOCl (300 μM) in the presence or absence of BR (10 μM) or CORM2 (10 μM). D: caspase-3 activity following treatment of cells with HOCl (300 μM for 24 h) in the presence or absence of BR (10 μM) or CORM2 (10 μM). Results are means ± SE (n = 4–6). *Statistically significant effect of HOCl.
DISCUSSION
The present study demonstrates that physiologically relevant concentrations of HOCl stimulate HO-1 gene expression and CO production in human vascular endothelium. The induction of HO-1 is mediated via the Nrf2-ARE signaling pathway and is selectively blocked by the glutathione donor NAC. In addition, the induction of HO-1 functions to limit the cytotoxic effect of HOCl by generating CO, biliverdin, and bilirubin. Thus the ability of HOCl to induce HO-1 in endothelial cells may represent a fundamental adaptive response to preserve endothelial cell function and viability at sites of vascular inflammation.
Treatment of endothelial cells with HOCl results in a concentration- and time-dependent increase in HO-1 protein and mRNA production. Increases in both HO-1 mRNA and protein are observed with concentrations of HOCl (100–300 μM) that are within the physiological range. It has been estimated that during moderate inflammation the concentration of HOCl within the extracellular space can reach 340 μM (24). However, HOCl concentration may be substantially higher near activated leukocytes (50), although, precise tissue or cellular levels of HOCl are currently unknown. Significantly, HOCl increases HO-1 expression in both human umbilical vein and aortic endothelial cells, suggesting that HOCl upregulates HO-1 in both the venous and arterial vessel wall.
The induction of HO-1 mRNA expression by HOCl is dependent on de novo RNA synthesis, as the transcriptional inhibitor actinomycin D blocks the induction of HO-1 message. Interestingly, stimulation of HO-1 mRNA is largely preserved in the presence of cycloheximide indicating that HOCl-mediated HO-1 expression is predominantly independent of protein synthesis. Moreover, the induction of HO-1 gene transcription requires the presence of AREs since mutation of AREs abolishes the stimulation of promoter activity by HOCl. Whereas several transcription factors can bind to AREs, recent work indicates that Nrf2 plays the dominant role in ARE-dependent HO-1 gene expression (1). Consistent with this notion, we observed that HOCl increases total and nuclear Nrf2 in endothelial cells. This latter finding is in-line with a recent report showing that HOCl activates Nrf2 and HO-1 in a murine macrophage cell line (37). Interestingly, the induction of Nrf2 protein by HOCl is not associated with an increase in Nrf2 mRNA, suggesting that HOCl stimulates Nrf2 protein expression via a posttranscriptional mechanism that may involve protein stabilization (21, 54). Furthermore, we found that transfection of endothelial cells with a dominant-negative Nrf2 construct abolishes the activation of HO-1 promoter activity in response to HOCl. Thus the mobilization of Nrf2 plays an integral role in mediating HO-1 gene transcription by HOCl. Although the mechanism by which HOCl activates Nrf2 is not known, the oxidation of cysteine residues in Kelch-like erythroid cell-derived protein 1 (Keap1) is likely to be involved since several cysteine residues in Keap1 are capable of undergoing redox-dependent alterations that result in the liberation and/or inhibition of Keap1-dependent ubiquitination and degradation of Nrf2 (21, 54).
Since HOCl is a potent oxidant that rapidly reacts with thiols and results in the almost complete oxidation of intracellular glutathione in endothelial cells (38, 45), we investigated whether administration of the glutathione donor NAC influences the induction of HO-1. Indeed, we found that NAC blocks the induction of HO-1 mRNA and protein and the expression of Nrf2 protein by HOCl. In contrast, treatment of endothelial cells with ascorbic acid, which is equally effective as NAC in scavenging superoxide anions, fails to inhibit HOCl-stimulated HO-1 expression. Furthermore, uric acid, which is a poor scavenger of superoxide but an efficient scavenger of peroxynitrite, had no effect on the induction of HO-1 (39). Our finding that NAC selectively antagonizes the induction of HO-1 by HOCl is consistent with an earlier report showing that NAC restores endothelium-dependent relaxation in HOCl-treated vessels, whereas ascorbic and uric acid were ineffective (43). Failure of ascorbic and uric acid to block the induction of HO-1 may reflect their relative ineffectiveness in preventing glutathione oxidation in endothelial cells exposed to HOCl (48).
Our finding that HOCl induces HO-1 gene expression adds to a growing list of atherogenic molecules that are capable of stimulating HO-1 expression. Previous studies identified oxidized low-density lipoprotein, peroxynitrite, homocysteine, and inflammatory cytokines as potent inducers of HO-1 (3, 8, 11, 12, 30). Furthermore, growth factors such as platelet-derived growth factor, transforming growth factor-β, and angiotensin II, which are present in vascular lesions, are also able to upregulate HO-1 expression (17). Interestingly, the induction of HO-1 by HOCl and several atherogenic factors is mediated through the activation of Nrf2 (3, 12, 30). However, the upstream signaling pathways that lead to the activation of Nrf2 may differ between stimuli. Protein kinase C, mitogen-activated protein kinases, and phosphatidylinositol-3-kinase have all been implicated in the direct activation of Nrf2, whereas oxidants, such as HOCl, may indirectly mobilize Nrf2 by interfering with its Keap1-mediated sequestration and/or degradation (1, 21, 56). Alternatively, vascular mitogens and inflammatory mediators may stimulate HO-1 expression via the activation of other transcription factors, including the Smad and activator protein-1 family of transcription factors (17). Thus atherogenic factors are capable of activating multiple signaling pathways and transcription factors that converge to trigger HO-1 gene transcription.
In the present study, we are the first to show that HOCl stimulates the generation of CO by endothelial cells. This finding contrasts with previous work showing that HOCl inhibits endothelial NO synthesis, indicating that HOCl exerts a divergent regulatory effect on the production of gaseous monoxides by vascular endothelium (22, 43). In this respect, the HOCl-mediated increase in CO synthesis may function in a compensatory manner to offset the loss of NO and help maintain vascular homeostasis. Similar to NO, CO exerts many beneficial effects in the circulation, including inhibition of vascular tone, platelet aggregation, vascular smooth muscle cell proliferation, and inflammation (see Ref. 10). In agreement with this view, the exogenous administration of CO has been shown to restore endothelial function in an animal model of diabetes (9). However, CO can also inactive endothelial NO synthase by binding to the heme moiety of the enzyme (51), and this may provide an additional mechanism by which HOCl compromises endothelial NO formation.
The induction of HO-1 in endothelial cells likely represents an important adaptive response to counteract the harmful oxidative effects of HOCl. Consistent with previous reports demonstrating that HOCl induces endothelial cell apoptosis (38, 45), we found that HOCl stimulates mitochondrial membrane disruption, caspase-3 activation, and endothelial cell death. Interestingly, HOCl-mediated cell toxicity is potentiated following inhibition of HO activity or the selective knockdown of HO-1 protein, indicating that the induction of HO-1 by HOCl promotes cell survival. This is in agreement with earlier work showing that genetic deletion of HO-1 sensitizes endothelial cells to the deleterious effects of heme or oxidized fatty acids (7, 54). The cytoprotective action of HO-1 is likely mediated via the generation of CO, biliverdin, and bilirubin, since the exogenous administration of these three heme metabolites mimics the protection mediated by HO-1. In contrast, the other HO-1 product iron does not protect cells from HOCl. The cytoprotection elicited by bile pigments and CO may reflect their ability to counteract the detrimental effect of HOCl on mitochondrial function. We found that HOCl evokes a pronounced loss in endothelial cell mitochondrial membrane potential that is associated with a significant increase in the activity of caspase-3, the terminal effector caspase of the apoptotic cascade. This disruption in mitochondrial membrane potential likely reflects the ability of HOCl to activate the mitochondrial permeability transition pore (52). Significantly, both CO and bilirubin maintain endothelial mitochondrial membrane potential in the presence of HOCl and prevent the activation of caspase-3. The capacity of bilirubin to preserve cell viability and mitochondrial function may arise from its capability to efficiently scavenge HOCl as well as other secondary oxidants generated by HOCl that target mitochondria (44). Alternatively, CO may protect against oxidative mitochondrial injury by increasing the activity of mitochondrial superoxide dismutase and mitochondrial glutathione peroxidase (46).
The ability of HOCl to induce HO-1 gene expression may be of pathophysiological significance. Abundant MPO- and HOCl-modified proteins have been detected within and around endothelial cells of human atherosclerotic lesions where HO-1 is also highly expressed (8, 16, 49). Our results suggest that the induction of HO-1 in atherosclerotic lesions exert an important cytoprotective role to defend endothelial cells against the harmful ROS generated by MPO. Since endothelial cell damage and denudation promotes plaque erosion and thrombosis, the induction of HO-1 may serve to reduce thrombosis and stabilize atherosclerotic plaques. In this respect, HO-1 has recently been found to exert an antithrombotic effect following oxidative damage to the endothelium (47). Moreover, a recent clinical study found that HO-1 expression is more prevalent in carotid atherosclerotic plaques obtained from asymptomatic compared with symptomatic patients (2). Thus the induction of HO-1 by HOCl may serve to promote homeostasis in vascular lesions by limiting thrombosis and plaque rupture.
Finally, the ability of HOCl to induce HO-1 may also be important in infectious states. Endotoxemia is associated with prominent neutrophil activation and infiltration, HOCl formation, and HO-1 expression in multiple organs (13, 14, 36). In this pathological setting, the induction of HO-1 has been shown to ameliorate oxidative stress, end-organ dysfunction, and death (53). Aside from mitigating HOCl-mediated tissue damage, HO-1 may exert critical anti-inflammatory actions. In this respect, our finding that HOCl stimulates the production of CO may be highly relevant, since CO inhibits lipopolysacharide-mediated production of pro-inflammatory cytokines while increasing the synthesis of the anti-inflammatory cytokine, interleukin-10 (35). In addition, CO has an inhibitory action on the formation of granulocyte-macrophage colony stimulating factor, which is known to promote the secretion of pro-inflammatory mediators and the differentiation of hematopoetic progenitor cells into macrophages and neutrophils (42). Similarly, the HO-1 product biliverdin was recently demonstrated to reduce the severity of the inflammatory response to polymicrobial sepsis by inhibiting the expression of interleukin-6 and stimulating the production of interleukin-10 (40). Moreover, bile pigments interfere with the expression of adhesion molecules and leukocyte-endothelial interactions (41). Significantly, both CO and biliverdin have been demonstrated to reduce mortality in animal models of sepsis (27, 40).
In conclusion, the present study demonstrates that HOCl induces HO-1 gene transcription and CO production via the Nrf2/ARE pathway in human vascular endothelium. In addition, it found that the activation of Nrf2 is blocked by NAC, and that the induction of HO-1 counteracts HOCl-mediated mitochondrial dysfunction and cell death through the formation of CO, biliverdin, and bilirubin. The ability of MPO-derived HOCl to stimulate HO-1 gene expression may play a critical role in preserving endothelial cell function and survival in atherosclerosis and other inflammatory disorders.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-59976, HL-74966, HL-76187, and HL-62467, the American Heart Association South Central Affiliate Grant 0865241F, and a grant from the Semp Russ Foundation of the San Antonio Area Foundation.
REFERENCES
- 1.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response pathway. Curr Pharm Des 9: 2499–2511, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ameriso SF, Villamil AR, Zedda C, Parodi JC, Garrido S, Sarchi MI, Shultz M, Boczkowski J, Sevlever GE. Heme oxygenase-1 is expressed in carotid artherosclerotic plaque infected by Helicobacter pylori and is more prevalent in asymptomatic patients. Stroke 36: 1896–1890, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase-1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen activated protein kinases and Nrf2. Free Radic Biol Med 39: 227–236, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 108: 1440–1445, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bergt C, Pennathur S, Fu X, Byun J, O'Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA 101: 13032–13037, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J Exp Med 192: 1015–1026, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Sega M, Agarwal A. “Lumen digestion” technique for isolation of aortic endothelial cells from heme oxygenase-1 knockout mice. Biotechniques 37: 84–86, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94: 437–444, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Pascoli M, Rodella L, Sacerdoti D, Bolognesi M, Turkseven S, Abraham NG. Chronic CO levels have a beneficial effect on vascular relaxation seen in diabetes. Biochem Biophys Res Commun 340: 935–943, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Durante W, Johnson FK, Johnson RA. Role of carbon monxide in cardiovascular function. J Cell Mol Med 10: 672–686, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res 80: 557–564, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Foresti R, Sarathchandra P, Clark Green CJ JE, Motterlini R. Peroxynitrite induces heme oxygenase-1 in vascular endothelial cells: a link to apoptosis. Biochem J 339: 729–736, 1999 [PMC free article] [PubMed] [Google Scholar]
- 13.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richer GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci USA 98: 11961–11966, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol 287: G243–G252, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Hazell LJ, Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J 290: 165–172, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 99: 2075–2081, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill-Kapturczak N, Jarmi T, Agarwal A. Growth factors and heme oxygenase-1: perspectives in physiology and pathology. Antioxid Redox Signal 9: 2197–2207, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits monocyte transmigration induced by mildly oxidized LDL. J Clin Invest 100: 1209–1216, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa K, Sugawara D, Goto J, Watanabe K, Kawamura S, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation 104: 1831–1836, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Ishizaka N, de Leon H, Laursen JB, Fukui T, Wilcox JN, de Keulenaer G, Griendling KK, Alexander RW. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation 96: 1923–1929, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor Y, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuffling and degradation of Nrf2 in response to electrophiles. Genes Cells 8: 379–391, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Jaimes EA, Sweeney C, Raij L. Effects of the reactive oxygen species hydrogen peroxide and hypochlorite on endothelial nitric oxide production. Hypertension 38: 877–883, 2001 [PubMed] [Google Scholar]
- 23.Johnson RA, Johnson FK. Heme oxygenase-derived endogenous carbon monoxide impairs flow-induced dilation in resistance vessels. Shock 29: 526–30, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase- gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation 104: 1519–1525, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Katrantzis M, Baker MS, Handley CJ, Lowther DA. The oxidant hypochlorite (OCl-), a product of the myeloperoxidase system, degrades articular cartilage proteoglycan aggregate. Free Radic Biol Med 10: 101–109, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Kawashima A, Oda T, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol 33: 125–130, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther 329: 641–648, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Lin CC, Liu XM, Peyton KJ, Wang H, Yang WC, Lin SJ, Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol 28: 739–745, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu XM, Ensenat D, Wang H, Schafer AI, Durante W. Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett 541: 52–56, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle: role in cell survival. J Biol Chem 280: 872–877, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol 152: 838–854, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469–474, 1974 [DOI] [PubMed] [Google Scholar]
- 33.Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous protection against oxidative stress in the endothelium. Am J Physiol Heart Circ Physiol 270: H107–H114, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25: 1102–1111, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Otterbein LE, Bach FH, Alam J, Soares M, Lu HT, Wysk M, Davis RJ, Flavell RA, Choi AMK. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Pellacani A, Wiesel P, Sharma A, Foster LC, Huggins GS, Yet SF, Perrella MA. Induction of heme oxygenase-1 during endotoxemia is downregulated by transforming growth factor-beta1. Circ Res 83: 396–403, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Pi J, Zhang Q, Woods CG, Wong V, Collins S, Andersen ME. Activation of Nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol Appl Pharmacol 226: 236–243, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Pullar JM, Winterbourn CC, Vissers MC. Loss of GSH and thiol enzymes in endothelial cells exposed to sublethal concentrations of hypochlorous acid. Am J Physiol Heart Circ Physiol 277: H1505–H1512, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophs 372: 285–294, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 289: L1131–L1137, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Soares MP, Seldon MO, Gregoire IP, Vassilevskaia T, Berberato PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial activation. J Immunol 172: 3553–3563, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Song R, Ning W, Lin F, Ameredes BT, Calhoun WJ, Otterbein LE, Choi AM. Regulation of IL-1β-induced GM-CSF production in airway smooth muscle by carbon monoxide. Am J Physiol Lung Cell Mol Physiol 284: L50–L56, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Stocker R, Huang A, Jeranian E, Hou JY, Wu TT, Thomas SR, Keaney JF., Jr Hypochlorous acid impairs endothelium-derived nitric oxide bioactivity through a superoxide-dependent mechanism. Arterioscler Thromb Vasc Biol 24: 2028–2033, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Stocker R, Peterhans E. Antioxidant properties of conjugated bilirubin and biliverdin: biologically relevant scavenging of hypochlorous acid. Free Rad Res Comms 6: 57–66, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol 24: 1309–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Suliman HB, Carraway MS, Ali S, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The HO/CO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest 117: 3730–3741, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, Gladwin MT, Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res 101: 893–901, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Vissers MC, Lee WG, Hampton MB. Regulation of apoptosis by vitamin C. Specific protection of the apoptotic machinery against exposure to chlorinated oxidants. J Biol Chem 276: 46835–46840, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of HO-1 in atherosclerotic lesions. Am J Pathol 152: 711–720, 1988 [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 320: 365–376, 1989 [DOI] [PubMed] [Google Scholar]
- 51.White KA, Marletta MA. Nitric oxide synthase is a cytochrome P450 hemoprotein. Biochemistry 31: 6627–6631, 1992 [DOI] [PubMed] [Google Scholar]
- 52.Whiteman M, Rose P, Siau JL, Cheung NS, Tan GS, Halliwell B, Armstrong JS. Hypochlorous acid-mediated mitochondrial dysfunction and apoptosis in human hepatoma HepG2 and human fetal liver cells: role of mitochondrial permeability transition. Free Radic Biol Med 38: 1571–1584, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Wiesel P, Patel AP, DiFronzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation 102: 3015–3022, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Yachie A, Niida Y, Wada T, Igarishi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129–135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerosis lesion formation and vascular remodeling. FASEB J 17: 1759–1761, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Zhang DD, Hannink M. Distinct cysteine residues in keap1 are required for keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23: 8137–8151, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 286: 2136–2142, 2001 [DOI] [PubMed] [Google Scholar]