Abstract
Heart valve disease and pulmonary hypertension, in patients with carcinoid tumors and people who used the fenfluramine-phentermine combination for weight control, have been associated with high levels of serotonin in blood. The mechanism by which serotonin induces valvular changes is not well understood. We recently reported that increased oxidative stress is associated with valvular changes in aortic valve stenosis in humans and mice. In this study, we tested the hypothesis that serotonin induces oxidative stress in human heart valves, and examined mechanisms by which serotonin may increase reactive oxygen species. Superoxide (O2·−) was measured in heart valves from explanted human hearts that were not used for transplantation. O2·− levels (lucigenin-enhanced chemoluminescence) were increased in homogenates of cardiac valves and blood vessels after incubation with serotonin. A nonspecific inhibitor of flavin-oxidases (diphenyliodonium), or inhibitors of monoamine oxidase [MAO (tranylcypromine and clorgyline)], prevented the serotonin-induced increase in O2·−. Dopamine, another MAO substrate that is increased in patients with carcinoid syndrome, also increased O2·− levels in heart valves, and this effect was attenuated by clorgyline. Apocynin [an inhibitor of NAD(P)H oxidase] did not prevent increases in O2·− during serotonin treatment. Addition of serotonin to recombinant human MAO-A generated O2·−, and this effect was prevented by an MAO inhibitor. In conclusion, we have identified a novel mechanism whereby MAO-A can contribute to increased oxidative stress in human heart valves and pulmonary artery exposed to serotonin and dopamine.
Keywords: serotonin, reactive oxygen species, carcinoid syndrome, superoxide, valvulopathy
carcinoid valve disease is present in ∼50–60% of patients with the carcinoid syndrome (2, 39, 54). Several circulating hormones including serotonin (5-hydroxytryptamine) and dopamine are released by carcinoid tumors (2, 18, 23, 44). Serotonin is released by carcinoid tumors, and its metabolism is associated with development and progression of myxomatous changes in heart valves and pulmonary hypertension (12, 38, 47). Similar findings were described in patients taking fenfluramine-phentermine for weight control (10, 16, 48, 52), pergolide for Parkinson's disease (60, 61), and other drugs such as ergot derivatives (48). Interestingly, fenfluramine also increases circulating serotonin levels (49). The combination fenfluramine-phentermine was removed from the market in 1997, and pergolide was removed in 2007, after Food and Drug Administration advisories about increased risk of valvular disease. However, mechanisms whereby serotonin elicits myxomatous valvular diseases have remained poorly understood.
Several studies in animals suggest a role for serotonin in carcinoid heart disease and drug-induced valve disease. Tryptophan hydroxylase I, the limiting enzyme in serotonin synthesis, is increased in canine myxomatous valve disease (14). In rats exposed to long-term administration of serotonin, increased cell proliferation and thickening of the heart valves, that resembles the changes reported in patients with carcinoid heart disease, are observed (15, 20). In addition, mice with the serotonin transporter gene knocked out also manifest valvular dysfunction, hyperplasia, and fibrosis of the valve leaflets (34).
In several cell types, including valvular interstitial cells (21, 43) and vascular smooth muscle cells (SMC) (9, 28–30), serotonin increases cell proliferation. There is evidence for activation and nuclear translocation of mitogen-activated protein kinases, transforming growth factor-β1, and other proliferative pathways by serotonin (28–30).
Reactive oxygen species (ROS), especially superoxide (O2·−), appear to participate in serotonin-induced mitogenesis (19, 26, 28–30). Antioxidants can prevent the mitogenic effects of serotonin (19, 26, 28–30). Nicotine adenine dinucleotide phosphate oxidase [NAD(P)H oxidase] may be a significant source of O2·− after stimulation of SMC with serotonin, because inhibitors of NAD(P)H oxidase such as diphenyliodonium (DPI) inhibit proliferative signals in response to serotonin in pulmonary artery SMC and rat mesangial cells (19, 29, 30). Furthermore, recent evidence suggests that ROS may contribute to development of structural changes in stenotic aortic valves in humans and mice (36, 37). However, it is not known if serotonin increases oxidative stress in heart valves. Thus it is possible that increased oxidative stress in heart valves exposed to high concentrations of serotonin might increase proliferation of valve interstitial cells and contribute to the development of myxomatous valve disease.
In this study, we tested the hypothesis that serotonin increases ROS in human heart valves and explored mechanisms for production of ROS by serotonin in heart valves. We found that high concentrations of serotonin increase O2·− in heart valves and blood vessels. Because amines, including serotonin and dopamine, are metabolized by monoamine oxidase (MAO) (4, 42, 55), we incubated the valves with an MAO inhibitor to prolong and augment the effects of serotonin. Surprisingly, the MAO inhibitor greatly reduced the serotonin-induced increase in O2·− in cardiac valves and blood vessels. We also found that MAO-dependent metabolism of dopamine increased O2·− levels. We used pharmacological interventions and recombinant human protein to demonstrate that MAO-A-mediated degradation of serotonin can be a critical contributor to O2·− production in heart.
METHODS
Normal human cardiac valves (pulmonary, tricuspid, and mitral) and proximal segments of pulmonary artery and aorta were obtained from donor hearts that were not suitable for transplantation. Hearts were obtained through the Iowa Donor Network and the National Disease Research Interchange (Philadelphia, PA) <12 h after organ harvesting and maintained in cold University of Wisconsin solution as described previously (36). Because clinical information was not obtained from the donor patients (except for age and sex), the University of Iowa Institutional Review Board indicated that informed consent was not required from each patient. Tissue was homogenized in a cocktail of protease inhibitors in PBS and stored at −80°C until analysis.
O2·− measurement.
Homogenized heart valve and vascular tissue were used to examine the levels of O2·− using lucigenin-enhanced chemoluminescence. Tissue homogenates have been used in the past to study the activity of MAO in several animal tissues, including brain, heart, and liver (1, 5, 17, 22). Homogenates from pulmonary artery were used as a positive control because it is known that serotonin increases O2·− in this tissue (28–30). Protein was quantified in each sample. Tissue homogenate containing 250 μg of protein was placed in a cuvette containing 5 μM lucigenin in PBS to obtain a total volume of 500 μl. Samples were then incubated in the presence of serotonin at 37°C in a mixture of 95% O2 and 5% CO2 for 4 h. Oxygen concentration was calibrated with an oxygen analyzer (Beckman OM11). These conditions have been used for examination of responses to serotonin in rat aorta (40) and evaluation of NAD(P)H oxidase activity in mouse blood vessels (7, 50). Luminescence in each vial was read in an FB12 luminometer (Berthold, Germany).
Enzyme inhibitors were added to the samples 30 min before the addition of serotonin and maintained during the incubation period. The following drugs were used: 10 μM DPI (a nonspecific flavin-containing enzyme inhibitor), 10 μM NG-nitro-l-arginine methyl ester [l-NAME, an inhibitor of all three isoforms of the nitric oxide synthase (NOS)], 10 μM allopurinol (a xanthine oxidase inhibitor), 100 μM apocynin [an NAD(P)H oxidase inhibitor], 10 μM indomethacin (a cyclooxygenase inhibitor), 200 U/ml catalase (36), 1–10 μM tranylcypromine (an MAO-A/B inhibitor), and 1 μM clorgyline (an MAO-A inhibitor) (56). All reagents were obtained from Sigma.
In addition, some samples were incubated with 1 mM dopamine dissolved in PBS. Clorgyline (1 μM) was added 30 min before dopamine to inhibit MAO. Luminescence in each vial was read in a luminometer after 4 h of incubation.
To test the specificity of the MAO inhibitors, we examined effects of the inhibitors on NAD(P)H oxidase, which is another potential source of O2·− that has been associated with serotonin-induced oxidative stress, in pulmonary artery SMC (29, 30). For the evaluation of NAD(P)H oxidase-mediated O2·− production, homogenates were preincubated for 30 min in the presence of DPI, tranylcypromine, clorgyline, or vehicle (PBS). Samples were exposed to 100 μM NAD(P)H, and luminescence in each cuvette was measured.
O2·− generation by recombinant MAO-A.
Recombinant human MAO-A (Sigma) was incubated with serotonin for 4 h in 5 μM lucigenin in PBS at 37°C in a mixture of gases of 95% O2 and 5% CO2. MAO-A was preincubated in the presence of tranylcypromine (10 μM) or PBS (vehicle) for 30 min before the addition of 100 μM serotonin or PBS (control). O2·− generation was detected by lucigenin-enhanced chemoluminescence in a luminometer.
Electron paramagnetic resonance.
Recombinant human purified 100 μg/ml MAO-A (Sigma) was added to a solution containing 0.1 mM serotonin or vehicle (PBS), 100 mM phosphate buffer, 250 μM of the iron-chelating agent diethylenetriaminepentaacetic acid (DETAPAC), 1% albumin, and 50 mM 5,5-dimethylpyrroline-1-oxide (DMPO) as a spin trap. Electron paramagnetic resonance (EPR) was performed with a Bruker EMX spectrometer. Data were collected during 30 min at room temperature and ambient air. EPR parameters were 3510.3 G center field; 80 G scan width; 9.854 GHz microwave frequency; 20 mW power; 2 × 105 receiver gain; modulation frequency of 100 kHz; modulation amplitude of 1.0 G; with the conversion time and time constant both being 40.96 ms with five scans for each 1,024-point spectrum.
Quantitative real-time RT-PCR.
Heart valve and vascular tissues were homogenized in 0.5 ml of TRIzol (Invitrogen) and stored at −80°C until collection was complete. RNA extraction, quantification, and RT were performed as described previously (8). RT (1 μl) was used for TaqMan real-time PCR to obtain the cycle threshold (Ct) for MAO-A and β-actin. TaqMan Primers/probes were obtained from Applied Biosystems. Expression levels of MAO-A were determined relative to that of β-actin (which was constant among samples), using the ΔΔCt method as described previously (8, 36).
Statistics.
Results are expressed as means ± SE. Statistical significance was determined by one-way ANOVA and post hoc analysis with the Tukey test using the statistical program SAS (SAS Institute, Cary, NC) and VassarStats calculator (Vassar College, Poughkeepsie, NY). A significant difference was considered as P < 0.05.
RESULTS
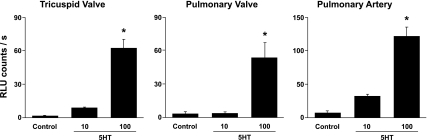
Incubation of homogenates of human heart valves (tricuspid and pulmonary) and pulmonary artery with 100 μM serotonin significantly increased levels of O2·− (Fig. 1). Serotonin also increased O2·− levels in the mitral valve and proximal aorta [Supplemental Fig. 1 (Supplemental material for this article can be found on the American Journal of Physiology: Heart and Circulatory Physiology website)].
Fig. 1.
Superoxide levels are increased in human tricuspid and pulmonary valves, and pulmonary artery, after incubation with serotonin (5-HT, 10 μM and 100 μM) compared with control tissue [incubated with vehicle (PBS)]. *P < 0.05 vs. control; n = 8–10 valves and pulmonary arteries.
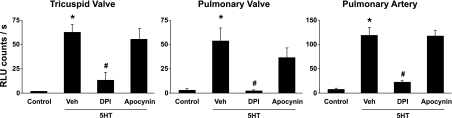
DPI (a nonspecific inhibitor of flavin oxidases) prevented the serotonin-induced increase in O2·− in heart valves and pulmonary artery. Apocynin [an inhibitor of NAD(P)H oxidase] did not attenuate the increase in O2·− in response to serotonin in any of the tissues (Fig. 2). A cyclooxygenase inhibitor (indomethacin) did not prevent the increase in O2·− after serotonin treatment (Supplemental Fig. 2). Similarly, inhibitors of NOS (l-NAME), xanthine oxidase (allopurinol), did not attenuate the serotonin-induced increase in O2·− in a subset of samples (data not shown).
Fig. 2.
5-HT (100 μM)-induced increase in superoxide in tricuspid and pulmonary valves, and pulmonary artery, is attenuated by diphenyliodonium (DPI, 10 μM), an inhibitor of several flavin-containing oxidases. The increase in superoxide was not attenuated significantly by apocynin (100 μM), an inhibitor of NAD(P)H oxidase. Vehicle (Veh) was PBS at pH 7.4. P < 0.05 vs. control (*) and vs. 5-HT only-Veh (#); n = 4 for tricuspid valve, n = 6 for pulmonary valve, and n = 7 for pulmonary artery. Analysis used controls shown in Fig. 1.
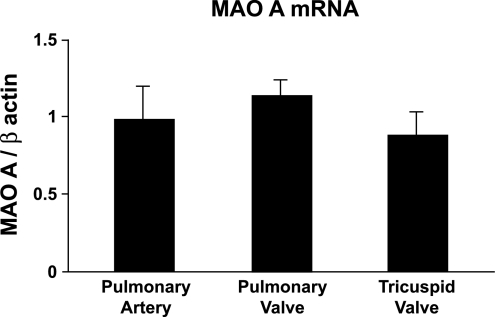
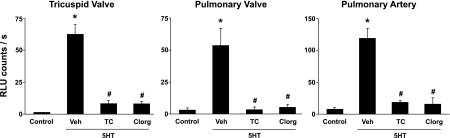
We found that MAO-A is expressed in human tricuspid and pulmonary valves, and in pulmonary artery (Fig. 3). Incubation of homogenates of tricuspid and pulmonary valves, or pulmonary artery, with tranylcypromine (a nonselective MAO-A/B inhibitor) or clorgyline (an MAO-A inhibitor) abolished the increase in O2·− in response to serotonin (Fig. 4). Tranylcypromine and clorgyline also prevented the increase in O2·− after treatment with serotonin in mitral valve and aorta (Supplemental Fig. 1).
Fig. 3.
Transcripts for monoamine oxidase (MAO)-A (real-time PCR) are expressed in pulmonary artery, and in pulmonary and tricuspid values. There were no significant differences between tissues (n = 11 valves and pulmonary arteries).
Fig. 4.
Inhibition of MAO with tranylcypromine (TC, 10 μM) significantly attenuated the increase in superoxide in tricuspid and pulmonary valves, and pulmonary artery homogenates incubated with 100 μM 5-HT. A specific inhibitor of MAO-A [1 μM clorgyline (Clorg)] also attenuated increases in superoxide. Vehicle was PBS at pH 7.4. P < 0.05 vs. control (*) and vs. 5-HT only-Veh (#); n = 4 for tricuspid valve, n = 6 for pulmonary valve, and n = 7 for pulmonary artery. Analysis used controls shown in Fig. 1.
Addition of exogenous NADPH to homogenates of tricuspid or pulmonary valves or pulmonary artery increased O2·− (Supplemental Fig. 3). Preincubation with DPI [NAD(P)H oxidase and flavin oxidases inhibitor] attenuated the increase in O2·−. MAO inhibitors (tranylcypromine and clorgyline) did not attenuate the increase in O2·− after addition of NADPH to homogenates of cardiac valves or pulmonary artery.
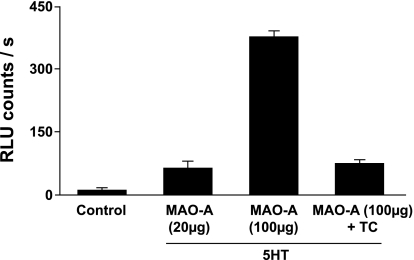
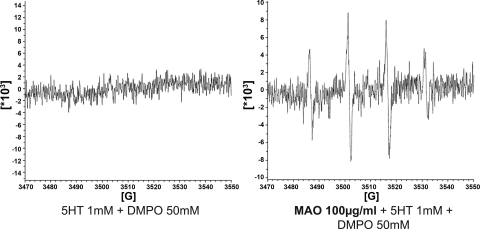
Incubation of recombinant human MAO-A with serotonin produced a significant increase in O2·− levels, which was attenuated by tranylcypromine (Fig. 5). Recombinant human MAO-A was also studied with EPR. A DMPO-OH signal, suggestive of the presence of O2·− in the sample, was obtained when serotonin and MAO-A were added together (Fig. 6).
Fig. 5.
MAO-A-derived superoxide. Superoxide is generated by human recombinant purified MAO-A incubated with 1 mM 5-HT or PBS as vehicle (control). The increase in superoxide was markedly attenuated by coincubation with an inhibitor of MAO (10 μM tranylcypromine). Results were obtained from 2 independent experiments, with 2 samples of MAO in each.
Fig. 6.
Superoxide generation by MAO-A. Left: 1 mM 5-HT dissolved in a buffer solution [1% albumin, 50 mM 5,5-dimethylpyrroline- 1-oxide (DMPO), 250 mM DETAPAC] was analyzed in the spectrometer. There is no change in the baseline recording during a 30-min period. Right: a signal indicative of DMPO-OH adducts (product of the DMPO + superoxide reaction) appears when human purified recombinant MAO-A is added to a buffer solution containing 1 mM 5-HT. Figure shows a representative trace.
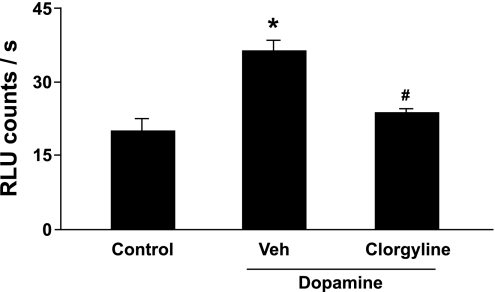
Incubation of pulmonary valve homogenates with another MAO-A substrate, dopamine, increased O2·− significantly (Fig. 7). The dopamine-induced increase in O2·− was significantly attenuated by clorgyline.
Fig. 7.
Dopamine-induced increase in superoxide in homogenates of human pulmonary valve (n = 3). Dopamine (1 mM) significantly increased superoxide levels in pulmonary valve compared with control tissue (incubated with PBS). The increase in superoxide produced by dopamine was attenuated by an MAO-A inhibitor (1 μM clorgyline). P < 0.05 vs. control (*) and vs. dopamine treatment (#).
DISCUSSION
Elevated levels of serotonin are associated with development of myxomatous valve disease and pulmonary artery hypertension by mechanisms that are currently not well understood (2, 11, 38, 47). Because recent findings from our laboratory suggest a role for oxidative stress in the pathogenesis of aortic valve disease (36, 37), we hypothesized that oxidative stress may be found in heart valves exposed to high concentrations of serotonin. In the present study, we report two major findings. First, high concentrations of serotonin or dopamine increase O2·− radicals in human heart valves and in vascular tissue. Second, MAO-A is a novel source of O2·− in human heart valves. The data support a model in which increased O2·−, derived from metabolism of amines by MAO-A, may contribute to the pathogenesis of valve disease.
About two-thirds of patients with carcinoid syndrome, especially those who tend to have the highest concentrations of serotonin in plasma (47), and high serotonin metabolism (38), develop carcinoid heart disease. Carcinoid heart disease in humans and in animal models is characterized by cellular proliferation, fibro-myxoid changes, and thickening of heart valves (2, 11, 15, 20, 34, 38, 47, 54). These changes are more frequent in the tricuspid and pulmonary valves than in the left side valves (2, 38, 39), presumably because lungs are a major site of metabolism for serotonin and other amines (24, 41), and the aortic and mitral valves therefore are exposed to lower concentrations of serotonin. In the present study, we examined human cardiac valves, and proximal segments of pulmonary artery and aorta. We focused especially on the tricuspid and pulmonary valves, and the pulmonary artery, because they are more commonly involved in carcinoid heart disease.
The data indicate that serotonin increases O2·− in heart valves and blood vessels. Data from cultured pulmonary artery SMC suggest that O2·− may participate in serotonin-induced mitogenesis in multiple pathways including: 1) activation and translocation of mitogen-activated protein kinases (28), and the phosphatidylinositol 3-kinase pathway (29); 2) activation of cell cycle proteins (53); and 3) transactivation of other mitogenic receptors such as the platelet-derived growth factor receptor (30). Antioxidants attenuate the mitogenic effects of serotonin in pulmonary artery SMC (28–30). Similarly, dexfenfluramine-induced proliferation of SMC requires ROS and is attenuated by antioxidants (27). Therefore, we speculate that serotonin or fenfluramine-induced oxidative stress may also play a critical role in proliferation of valve interstitial cells and valvular thickening in vivo.
A key finding in this study is that serotonin-mediated increases in O2·− are not primarily dependent on activation of NAD(P)H oxidase. Some studies in which DPI [an NAD(P)H oxidase inhibitor] was used concluded that NAD(P)H oxidase is the primary source of O2·− in serotonin or dexfenfluramine-induced oxidative stress in cultured pulmonary artery SMC (27, 29, 30). DPI, however, inhibits multiple flavin oxidases and is not specific for NAD(P)H oxidase (45). Apocynin [a somewhat more specific inhibitor of NAD(P)H oxidase] did not attenuate the increase in O2·− mediated by serotonin.
A major finding in this study is that the increase in O2·− induced by serotonin was attenuated by inhibition of the flavin-containing enzyme MAO (46). Serotonin is metabolized avidly by MAO, which is localized in the outer mitochondrial membrane (4, 42, 55). MAO is present in two isoforms, MAO-A (which is expressed in a variety of tissues, including brain, liver, heart, kidney, and blood vessels and catalyzes the degradation of serotonin, dopamine, and norepinephrine) and MAO-B (which is expressed predominantly in the central nervous system and degrades dopamine and phenethylamine) (4, 55). In this study, we found that MAO-A was expressed in tricuspid and pulmonary valves, and in pulmonary artery. Furthermore, incubation with tranylcypromine (an MAO-A/B inhibitor) or clorgyline (an MAO-A inhibitor) attenuated significantly the increase in O2·− in tissue homogenates exposed to serotonin. Moreover, two different assays (lucigenin-enhanced chemoluminescence and EPR) also indicated that MAO-A is a source of O2·− when recombinant human MAO-A is incubated with serotonin.
Although the chemistry of metabolism of serotonin and other amines by MAO is not clear (51), it appears that ROS are released as a byproduct during the reaction (58, 59). In another flavin oxidase, xanthine oxidase, the reaction with xanthine produces hydrogen peroxide or O2·−. Generation of different ROS varies with electron flux through the flavin group (6, 32). We speculate that a similar mechanism may exist for MAO.
MAO-dependent oxidative stress is associated with multiple pathological processes, including proliferation of SMCs (9) and renal epithelial cells (57), cardiomyocyte hypertrophy (3), and renal ischemia-reperfusion injury (25). Oxidative stress produced by MAO-dependent degradation of amines is also increased in aged hearts in rats (31). Therefore, ROS generated from MAO-dependent degradation of serotonin may be important for the understanding of proliferation of valve interstitial cells in carcinoid heart disease and drug-induced valvulopathies.
We found that, in homogenates of cardiac valves, MAO (and not other oxidases), appears to be the primary source of O2·− after stimulation with serotonin. Because we used two different MAO inhibitors (tranylcypromine and clorgyline) to study the role of MAO in serotonin-induced oxidative stress, it was important to test the selectivity of these compounds. This is critical, because it is known that tranylcypromine may inhibit eicosanoid metabolism (33). Therefore, we examined whether other common sources of O2·− (cyclooxygenase, NOS, xanthine oxidase) were important sources of O2·− in this preparation and assessed NAD(P)H oxidase activity in the presence of the MAO inhibitors. Inhibition of cyclooxygenase with indomethacin did not significantly attenuate the increase in O2·−, after incubation with serotonin, in the tricuspid valve or pulmonary artery. Similarly, inhibitors of the three isoforms of NOS (with l-NAME) and xanthine oxidase (allopurinol) did not attenuate the serotonin-induced increase in O2·− in a subset of samples of pulmonary artery. Importantly, we found that the MAO inhibitors, tranylcypromine and clorgyline, do not affect the NADPH-stimulated increase in O2·− levels in homogenates of heart valve or pulmonary artery. Thus it is unlikely that the findings were the result of a nonspecific effect of the MAO inhibitors on other enzymes. In addition, NAD(P)H oxidase, NOS, xanthine oxidase, and cyclooxygenase do not appear to contribute importantly, in this preparation, to increases in O2·− in heart valves or blood vessels exposed to high concentrations of serotonin.
Finally, incubation of pulmonary valve with dopamine (which, like serotonin, is a substrate for MAO) also increased O2·− levels, and the response was inhibited by clorgyline. This is of interest because about one-third of patients with carcinoid syndrome also have high concentrations of dopamine in blood and urine (18, 23). The role of dopamine in myxomatous valve disease is not clear. Pergolide, a dopamine agonist, however, has been associated with valvulopathies in humans (60, 61).
We acknowledge important limitations in the present study. First, we used high concentrations of serotonin. Second, it is not possible to study the role of membrane signaling in homogenates. Although the intracellular concentration of serotonin in vascular tissue is not known, it appears to be higher than circulating levels in other tissues (35). It seems reasonable to speculate that intracellular serotonin and/or dopamine concentrations in patients with carcinoid syndrome may be even higher than blood levels because of active uptake of the amines. Two mechanisms are responsible for serotonin uptake: a high-affinity low-capacity uptake through the serotonin transporter and a low-affinity high-capacity uptake by the norepinephrine transporter (13). There are no data about mechanisms of serotonin uptake, or expression and function of these transporters, in human heart valves. Some authors speculate that, in circumstances where reduced expression or knockout of the serotonin transporter in animals has been associated with valve disease, increased circulating serotonin activates serotonin 2B receptors, leading to increased valve cell proliferation and valve disease (15, 34). We did not address the potentially important role of activation of serotonin receptors or transporter in serotonin-induced oxidative stress. We speculate, however, that serotonin uptake through the serotonin or norepinephrine transporter may be increased in conditions with high serotonin concentrations, leading to increased availability of serotonin for MAO. Increased metabolism of amines is associated with progression of carcinoid heart disease (38), and may be an important source of ROS in heart valves.
In summary, these findings identify a novel pathway whereby serotonin increases oxidative stress in heart valves through an MAO-A-dependent mechanism. MAO-dependent generation of ROS may be important for the understanding of mitogenic actions of serotonin in carcinoid valve disease, drug-induced valvulopathies, and pulmonary artery hypertension. A deeper understanding of MAO-A-dependent oxidative stress may also facilitate the avoidance of unwanted side effects from pharmaceutical drugs under development, as well as serious cardiovascular side effects from recreational drugs such as 3,4- methylenedioxymethamphetamine (MDMA; ecstasy) that may alter the normal concentration of serotonin in human blood (49, 62).
GRANTS
This work was supported by National Institutes of Health Grants HL-62984 and NS-24621 (D. D. Heistad.) and HL-092235 (J. D. Miller), support from the faculty development program from the Fulbright Commission and the Universidad de los Andes (R. Peña-Silva), a fellowship from the American Heart Association (0815525G) to R. Peña-Silva, and funds from a Carver Trust Research Program of Excellence at the University of Iowa (D. D. Heistad).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Gary Buettner and the spectroscopy facility at the University of Iowa for assistance with the EPR experiments.
REFERENCES
- 1.Alper G, Girgin FK, Ozgonul M, Mentes G, Ersoz B. MAO inhibitors and oxidant stress in aging brain tissue. Eur Neuropsychopharmacol 9: 247–252, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya S, Davar J, Dreyfus G, Caplin ME. Carcinoid heart disease. Circulation 116: 2860–2865, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J 19: 641–643, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Billett EE. Monoamine oxidase (MAO) in human peripheral tissues. Neurotoxicology 25: 139–148, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Blatchford D, Holzbauer M. Ontogenesis of enzyme systems deaminating different monoamines. Br J Pharmacol 57: 279–293, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britigan BE, Pou S, Rosen GM, Lilleg DM, Buettner GR. Hydroxyl radical is not a product of the reaction of xanthine oxidase and xanthine. The confounding problem of adventitious iron bound to xanthine oxidase. J Biol Chem 265: 17533–17538, 1990 [PubMed] [Google Scholar]
- 7.Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension 51: 872–877, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol 22: 611–616, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Coatrieux C, Sanson M, Negre-Salvayre A, Parini A, Hannun Y, Itohara S, Salvayre R, Auge N. MAO-A-induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radic Biol Med 43: 80–89, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337: 581–588, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Connolly HM, Schaff HV, Mullany CJ, Rubin J, Abel MD, Pellikka PA. Surgical management of left-sided carcinoid heart disease. Circulation 104: I36–I40, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Cote F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med 10: 232–238, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Daws LC, Montanez S, Owens WA, Gould GG, Frazer A, Toney GM, Gerhardt GA. Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperometry. J Neurosci Methods 143: 49–62, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Disatian S, Orton EC. Autocrine serotonin and transforming growth factor beta 1 signaling mediates spontaneous myxomatous mitral valve disease. J Heart Valve Dis 18: 44–51, 2009 [PubMed] [Google Scholar]
- 15.Elangbam CS, Job LE, Zadrozny LM, Barton JC, Yoon LW, Gates LD, Slocum N. 5-hydroxytryptamine (5HT)-induced valvulopathy: compositional valvular alterations are associated with 5HT2B receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp Toxicol Pathol 60: 253–262, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Fishman AP. Aminorex to fen/phen: an epidemic foretold. Circulation 99: 156–161, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Gentili F, Pizzinat N, Ordener C, Marchal-Victorion S, Maurel A, Hofmann R, Renard P, Delagrange P, Pigini M, Parini A, Giannella M. 3-[5-(4,5-dihydro-1H-imidazol-2-yl)-furan-2-yl]phenylamine (Amifuraline), a promising reversible and selective peripheral MAO-A inhibitor. J Med Chem 49: 5578–5586, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Otten U, Suda K, Heitz PU, Stalder GA, Obrecht JP, Holzach P, Allgower M. Dopamine, norepinephrine and serotonin production by an intestinal carcinoid tumor. Cancer 45: 104–107, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Grewal JS, Mukhin YV, Garnovskaya MN, Raymond JR, Greene EL. Serotonin 5-HT2A receptor induces TGF-beta1 expression in mesangial cells via ERK: proliferative and fibrotic signals. Am J Physiol Renal Physiol 276: F922–F930, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson BI, Tommeras K, Nordrum I, Loennechen JP, Brunsvik A, Solligard E, Fossmark R, Bakke I, Syversen U, Waldum H. Long-term serotonin administration induces heart valve disease in rats. Circulation 111: 1517–1522, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, Levy RJ. Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-beta1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol 161: 2111–2121, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayama M, Kobayashi S, Oguchi K, Yasuhara H. Properties of monoamine oxidase in monkey heart. Jpn J Pharmacol 35: 425–431, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Kema IP, de Vries EG, Slooff MJ, Biesma B, Muskiet FA. Serotonin, catecholamines, histamine, and their metabolites in urine, platelets, and tumor tissue of patients with carcinoid tumors. Clin Chem 40: 86–95, 1994 [PubMed] [Google Scholar]
- 24.Kerstein MD, Cronau LH, Mandel SD, Gillis CN. Effect of hemorrhagic shock on 5-hydroxytryptamine removal by the lung. Am Surg 48: 644–646, 1982 [PubMed] [Google Scholar]
- 25.Kunduzova OR, Bianchi P, Pizzinat N, Escourrou G, Seguelas MH, Parini A, Cambon C. Regulation of JNK/ERK activation, cell apoptosis, and tissue regeneration by monoamine oxidases after renal ischemia-reperfusion. FASEB J 16: 1129–1131, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Lee SL, Simon AR, Wang WW, Fanburg BL. H2O2 signals 5-HT-induced ERK MAP kinase activation and mitogenesis of smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 281: L646–L652, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lee SL, Wang WW, Fanburg BL. Dexfenfluramine as a mitogen signal via the formation of superoxide anion. FASEB J 15: 1324–1325, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol Lung Cell Mol Physiol 277: L282–L291, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol 34: 182–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT transporter transactivates the PDGFbeta receptor in pulmonary artery smooth muscle cells. FASEB J 21: 2725–2734, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Frances B. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol 284: H1460–H1467, 2003 [DOI] [PubMed] [Google Scholar]
- 32.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 243: 5753–5760, 1968 [PubMed] [Google Scholar]
- 33.McCormick DL, Spicer AM, Hollister JL. Differential effects of tranylcypromine and imidazole on mammary carcinogenesis in rats fed low and high fat diets. Cancer Res 49: 3168–3172, 1989 [PubMed] [Google Scholar]
- 34.Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, Seif I, haiem-Sigaux N, Kirsch M, Hamon M, Adnot S, Eddahibi S. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation 113: 81–89, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Meulemans A, Poulain B, Baux G, Tauc L. Changes in serotonin concentration in a living neurone: a study by on-line intracellular voltammetry. Brain Res 414: 158–162, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 52: 843–850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JD, Weiss RM, Serrano KM, Brooks RM, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation 119: 2693–2701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med 348: 1005–1015, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Moller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation 112: 3320–3327, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ogden KK, Falck JR, Watts SW. The cytochrome p450 inhibitor ketoconazole potentiates 5-hydroxytryptamine-induced contraction in rat aorta. J Pharmacol Exp Ther 323: 606–613, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Olson EB, Jr, Ghias-Ud-Din M, Rankin J. Serotonin uptake and metabolism in isolated, perfused fetal, newborn and adult rabbit lungs. Lung 161: 173–179, 1983 [DOI] [PubMed] [Google Scholar]
- 42.Pizzinat N, Girolami JP, Parini A, Pecher C, Ordener C. Serotonin metabolism in rat mesangial cells: involvement of a serotonin transporter and monoamine oxidase A. Kidney Int 56: 1391–1399, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Rajamannan NM, Caplice N, Anthikad F, Sebo TJ, Orszulak TA, Edwards WD, Tajik J, Schwartz RS. Cell proliferation in carcinoid valve disease: a mechanism for serotonin effects. J Heart Valve Dis 10: 827–831, 2001 [PubMed] [Google Scholar]
- 44.Richter G, Stockmann F, Conlon JM, Creutzfeldt W. Serotonin release into blood after food and pentagastrin. Studies in healthy subjects and in patients with metastatic carcinoid tumors. Gastroenterology 91: 612–618, 1986 [DOI] [PubMed] [Google Scholar]
- 45.Riganti C, Gazzano E, Polimeni M, Costamagna C, Bosia A, Ghigo D. Diphenyleneiodonium inhibits the cell redox metabolism and induces oxidative stress. J Biol Chem 279: 47726–47731, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Rigby SE, Basran J, Combe JP, Mohsen AW, Toogood H, van TA, Sutcliffe MJ, Leys D, Munro AW, Scrutton NS. Flavoenzyme catalysed oxidation of amines: roles for flavin and protein-based radicals. Biochem Soc Trans 33: 754–757, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 92: 790–795, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Roth BL. Drugs and valvular heart disease. N Engl J Med 356: 6–9, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Rothman RB, Zolkowska D, Baumann MH. Serotonin (5-HT) transporter ligands affect plasma 5-HT in rats. Ann NY Acad Sci 1139: 268–284, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol 22: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Scrutton NS. Chemical aspects of amine oxidation by flavoprotein enzymes. Nat Prod Rep 21: 722–730, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Setola V, Roth BL. Screening the receptorome reveals molecular targets responsible for drug-induced side effects: focus on fen-phen. Expert Opin Drug Metab Toxicol 1: 377–387, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Simon AR, Severgnini M, Takahashi S, Rozo L, Andrahbi B, Agyeman A, Cochran BH, Day RM, Fanburg BL. 5-HT induction of c-fos gene expression requires reactive oxygen species and Rac1 and Ras GTPases. Cell Biochem Biophys 42: 263–276, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Simula DV, Edwards WD, Tazelaar HD, Connolly HM, Schaff HV. Surgical pathology of carcinoid heart disease: a study of 139 valves from 75 patients spanning 20 years. Mayo Clin Proc 77: 139–147, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Sivasubramaniam SD, Finch CC, Rodriguez MJ, Mahy N, Billett EE. A comparative study of the expression of monoamine oxidase-A and -B mRNA and protein in non-CNS human tissues. Cell Tissue Res 313: 291–300, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Ulus IH, Maher TJ, Wurtman RJ. Characterization of phentermine and related compounds as monoamine oxidase (MAO) inhibitors. Biochem Pharmacol 59: 1611–1621, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Vindis C, Seguelas MH, Lanier S, Parini A, Cambon C. Dopamine induces ERK activation in renal epithelial cells through H2O2 produced by monoamine oxidase. Kidney Int 59: 76–86, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Weyler W, Hsu YP, Breakefield XO. Biochemistry and genetics of monoamine oxidase. Pharmacol Ther 47: 391–417, 1990 [DOI] [PubMed] [Google Scholar]
- 59.Wouters J. Structural aspects of monoamine oxidase and its reversible inhibition. Curr Med Chem 5: 137–162, 1998 [PubMed] [Google Scholar]
- 60.Zadikoff C, Duong-Hua M, Sykora K, Marras C, Lang A, Rochon P. Pergolide associated cardiac valvulopathy based on Ontario administrative data. Can J Neurol Sci 35: 173–178, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Zadikoff C, Rochon P, Lang A. Cardiac valvulopathy associated with pergolide use. Can J Neurol Sci 33: 27–33, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary disease. J Pharmacol Exp Ther 318: 604–610, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.