Abstract
Carbon monoxide (CO) has anti-inflammatory properties. We previously reported that acute treatments with inhaled CO inhibit vascular inflammation and hypoxia-induced vasoocclusion in sickle cell disease mouse models. Therefore, we hypothesized that chronic CO inhalation would decrease vascular inflammation and organ pathology in a sickle cell disease mouse model. The treatment of sickle cell disease mice with 25 or 250 parts/million inhaled CO for 1 h/day, 3 days/wk for 8–10 wk significantly decreased the total mean white blood cell, neutrophil, and lymphocyte counts in peripheral blood. Eight weeks of 250 parts/million CO treatments reduced staining for myeloid and lymphoid markers in the bone marrow of sickle mice. Bone marrow from treated sickle mice exhibited a significant decrease in colony-forming unit granulocyte-macrophage during colony-forming cell assays. Anti-inflammatory signaling pathways phospho-Akt and phospho-p38 MAPK were markedly increased in CO-treated sickle livers. Importantly, CO-treated sickle mice had a significant reduction in liver parenchymal necrosis, reflecting the anti-inflammatory benefits of CO. We conclude that inhaled CO may be a beneficial anti-inflammatory therapy for sickle cell disease.
Keywords: inflammation, heme oxygenase
the hallmarks of sickle cell disease (SCD) are hemolysis and painful vasoocclusive crises that cause organ infarctions, leading to increased morbidity and mortality. In recent years the potential role of leukocytes in SCD vasoocclusion and pathogenesis has been appreciated (17, 29, 47). An elevated white blood cell (WBC) count in the absence of sepsis is a strong indicator of poor patient outcomes, including vasoocclusive pain, acute chest syndrome, stroke, and mortality (35, 41, 45). In murine models of SCD, leukocytes participate in vasoocclusion by adhering to the vessel wall and to sickled red blood cells; knockout or blockade of leukocyte adhesion molecules inhibits vasoocclusion and improves blood flow (6, 29, 47).
The mechanism behind the elevated WBC counts in SCD patients and mice is unclear but is likely related to hemolysis and the resulting oxidative stress characterizing SCD. Hemolysis induces proinflammatory adhesion molecules and anti-inflammatory heme oxygenase-1 (HO-1), the rate-limiting enzyme in the catabolism of heme. Not surprisingly, human patients and mice with SCD have elevated HO-1 (5, 36, 48). A further induction of HO-1 in SCD mice with hemin or adenovirus HO-1 inhibits vascular inflammation and vasoocclusion (5).
HO-1 has three enzymatic by-products: carbon monoxide (CO), biliverdin/bilirubin, and iron, which stimulates ferritin synthesis. These by-products have established antioxidant and anti-inflammatory properties. CO gas mimics many of the protective effects of HO-1. Short periods of inhaled exogenous CO, at 250 parts/million (ppm), and in some studies as low as 10 ppm (44), reduce inflammatory responses in models of oxidant injury in similar ways to HO-1 overexpression (39). The anti-inflammatory properties of CO are in part due to its ability to activate multiple signal transduction pathways such as p38 MAPK and Akt (37, 39, 43, 49, 53). CO interactions with hemoglobin (Hb) may also modulate hemolysis in SCD. CO binding to Hb S (HbS) can shift the oxygen dissociation curve to the left and inhibit HbS deoxygenation, HbS polymerization, and red blood cell hemolysis (9).
We hypothesize that inhaled CO in SCD mice will inhibit hemolysis, modulate leukocytosis and vasoocclusion, activate anti-inflammatory cell-cell signaling pathways, and diminish hepatic necrosis.
MATERIALS AND METHODS
Mice.
All animal experiments were approved by the University of Minnesota's Institutional Animal Care and Use Committee. We used male and female S+S-Antilles (16) transgenic sickle mice as models for human SCD. S+S-Antilles mice are on a C57BL/6J genetic background and are homozygous for the deletion of mouse β-major globin locus and express human α-, βS-, and βS-Antilles-globin transgenes. βS-Antilles-globins contain, in addition to the βS-mutation at β6, a second mutation at β23 (Val→Ile) (16). βS+S-Antilles mice have lower oxygen affinity than βS and decreased solubility under deoxygenated conditions, resulting in a more severe form of SCD. S+S-Antilles mice have severe congestion and pathology in the liver, lungs, and spleens (16). Normal male and female C57BL/6 mice from Jackson Laboratories were used as controls. All of the mice used in these studies were age matched at the initiation of CO treatments. The mice were housed in specific pathogen-free housing to prevent common murine infections that could cause an inflammatory response. All the mice were maintained on a standard chow diet and weighed between 20–40 g at the conclusion of the study period.
Four cohorts of S+S-Antilles and C57BL/6 mice were used for these studies. The first cohort was used to measure CO-Hb levels in whole blood after CO treatments (Table 1). The second cohort was treated with CO for 10 wk before euthanasia and was used in Table 2 and Figs. 1 and 8. The third cohort was treated with CO for 8 wk, followed by a 5-wk washout and was used to measure the kinetics of the effects of CO on white counts (Fig. 2). The fourth cohort was treated with CO for 8 wk before euthanasia and was used in all the remaining figures (Figs. 3–7). All four cohorts were housed as described above.
Table 1.
CO-Hb levels in mice treated with CO for 1 h
| Treatment Group | Hours after Treatment | C57BL/6J | S+S-Antilles |
|---|---|---|---|
| n | 4 | 5–9 | |
| No treatment | 0 | 1±0.0 | 1±0.0 |
| 1-h 25 ppm CO | 0 | 1±0.0 | 2±0.0 |
| 1-h 25 ppm CO | 24 | <1 | 1±0.0 |
| 1-h 250 ppm CO | 0 | 14±0.0 | 21.8±0.8 |
| 1-h 250 ppm CO | 24 | <1 | 1±0.0 |
Values are mean percentages ± SE; n, number of mice/group. CO, carbon monoxide; ppm, parts/million.
Fig. 1.
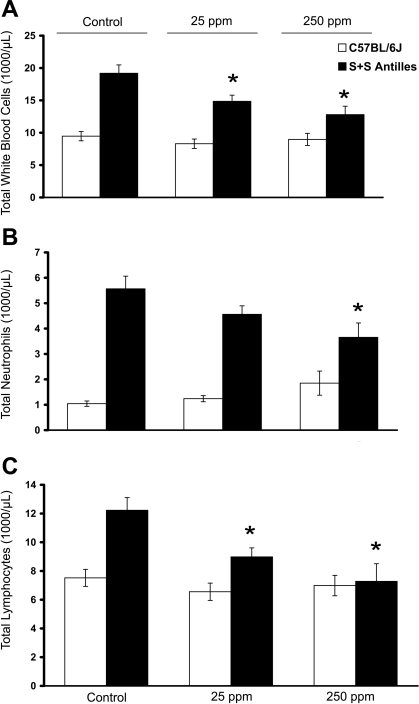
Inhaled carbon monoxide (CO) reduces white blood cell (WBC) counts in sickle cell disease mice. C57BL/6J (n = 10–12 mice/treatment group) and S+S-Antilles (n = 8 mice/treatment group) mice were treated with either 25 or 250 parts/million (ppm) inhaled CO for 1 h/day, 3 days/wk for 10 wk. Control animals were kept in ambient air. Blood was drawn at the end of 10 wk via cardiac puncture 24 h after the final CO treatment, and complete blood counts were measured. Total mean WBC (A), neutrophil (B), and lymphocyte (C) counts are shown by treatment group. Values are means ± SE. *P < 0.05 compared with untreated controls using ANOVA.
Fig. 8.
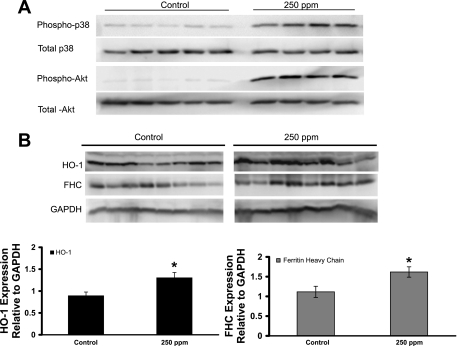
CO treatments (250 ppm) increases phospho-p38 MAPK, phospho-Akt, heme oxygenase-1 (HO-1), and ferritin heavy chain (FHC) protein expression in the livers of S+S-Antilles mice. Organ homogenates and nuclear extracts were isolated from the livers of control and 250-ppm CO-treated S+S-Antilles mice. A: Western blot showing phospho-p38 MAPK and phospho-Akt expression in nuclear extracts from the livers of S+S-Antilles mice treated for 8 wk with 250 ppm CO compared with untreated controls. Total p38 MAPK and Akt protein expression in the nuclear extracts did not change. B: Western blots of HO-1, FHC, and GAPDH protein expression with corresponding densitometry bar graph of the HO-1-to-GAPDH and FHC-to-GAPDH ratios in liver protein samples from S+S-Antilles mice treated for 10 wk with 250 ppm CO compared with untreated controls. Values are mean relative expression ± SE. *P < 0.05 compared with controls using a two-tailed t-test.
Fig. 2.
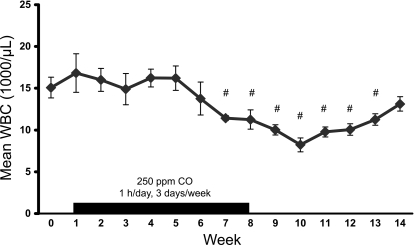
Kinetics of WBC decrement during CO treatment. Weekly WBC counts were performed on 7 age-matched S+S-Antilles mice for 2 consecutive wk to determine mean baseline WBC (week 0). The mice then received 250 ppm inhaled CO 1 h/day, 3 days/wk, 1 h/day, with a blood sample drawn weekly to assess total WBCs. After 7 wk of CO, there was a significant decrease in mean WBC counts. CO treatments were continued for an additional week (week 8) and then discontinued. The mean WBC counts of the mice remained significantly lower for 5 wk (weeks 9–13) after CO withdrawal. Values are means ± SE. #P < 0.05 compared with baseline using a one-way repeated-measure ANOVA.
Fig. 3.
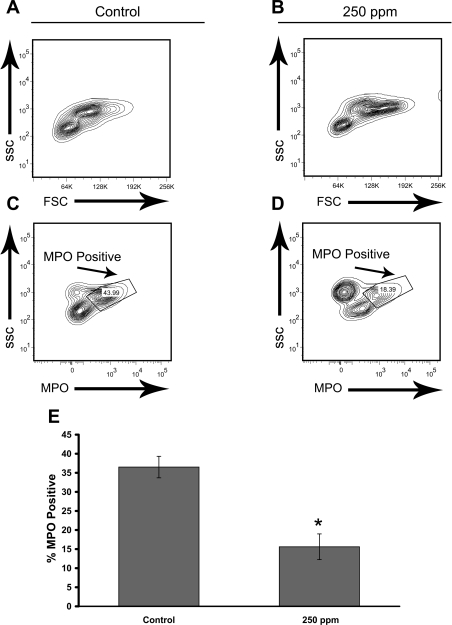
CO treatments (250 ppm) decrease mature myeloid myeloperoxidase (MPO positive) cells in the bone marrow of S+S-Antilles mice. S+S-Antilles mice (n = 4) were treated with 250 ppm CO, 3 days/wk, 1 h/day for 8 wk. After 8 wk, bone marrow from age-matched CO-treated and untreated mice (n = 5) was collected, stained for MPO, and analyzed by flow cytometry. A and B: representative fluorescence-activated cell sorting (FACS) plots of the forward scatter (FSC) vs. side scatter (SSC) profiles of control (A) and 250-ppm CO-treated (B) S+S-Antilles mice. C and D: representative FACS plots of the MPO vs. SSC gating of control (C) and 250-ppm CO-treated (D) S+S-Antilles mice. E: bar graph demonstrating a significant decrease in the percentage of MPO-positive cells in mouse bone marrow from 250-ppm CO-treated S+S-Antilles mice (15.6 ± 3.4%) compared with control S+S-Antilles mice (36.5 ± 2.8%). Mean %MPO ± SE. *P < 0.01 compared with controls using t-test.
Fig. 4.
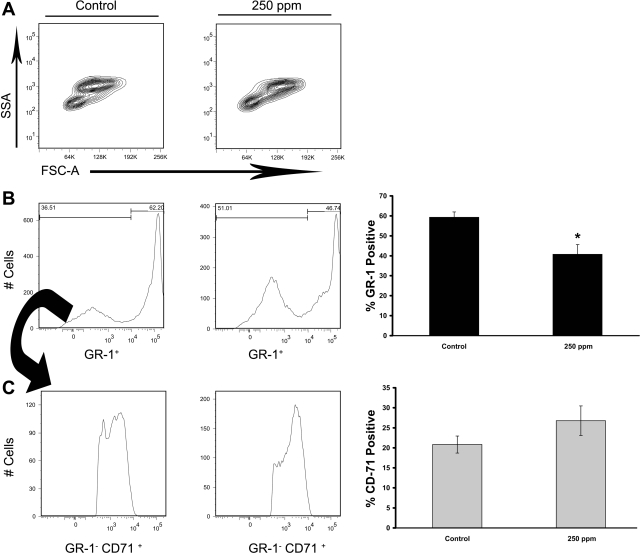
CO treatments (250 ppm) decrease myeloid precursors in S+S-Antilles bone marrow. S+S-Antilles mice (n = 4) were treated with 250-ppm CO 3 days/wk, 1 h/day for 8 wk. SSA, side-scatter area; FSC-A, forward-scatter area. After 8 wk, bone marrow from the age-matched CO-treated and untreated controls (n = 5) was collected and stained for GR-1 (Ly-6) and CD71 (transferrin receptor). A: representative FSC vs. SSA FACS plots demonstrating the difference between untreated control and 250-ppm CO-treated S+S-Antilles mice. B: representative histographs for GR-1+ population in control and 250-ppm CO-treated S+S-Antilles mice. Bar graph showing the significant decrease in the percentage of GR-1+ cells in mouse bone marrow in 250-ppm CO-treated S+S-Antilles mouse bone marrow compared with control S+S-Antilles mice. C: representative histographs for GR-1−CD71+ (erythroid) cells in mouse bone marrow from 250-ppm CO-treated S+S-Antilles mice compared with control S+S-Antilles mice. Bar graph demonstrates a trend (P = 0.14) toward increased GR-1−CD71+ population in 250-ppm CO-treated S+S-Antilles mice compared with controls. The CD71+ population was gated from the GR-1− populations as indicated by arrows. Values in bar graphs are mean %positive cells ± SE. *P < 0.05 compared with controls using two-tailed t-test.
Fig. 5.
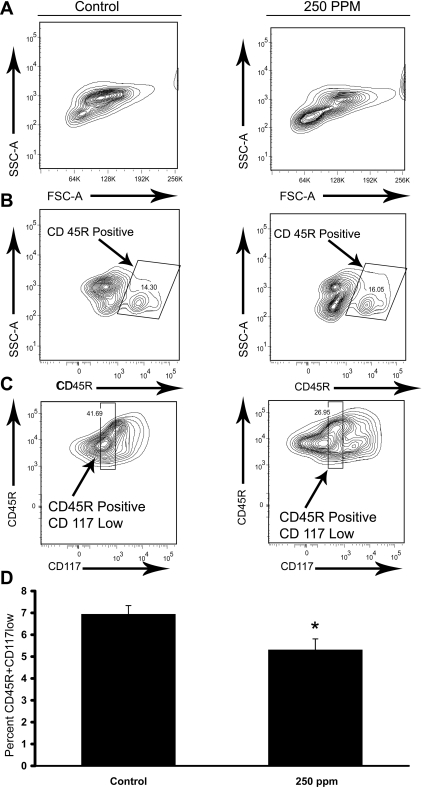
CO treatments (250 ppm) decrease the common lymphoid progenitor population in S+S-Antilles bone marrow. S+S-Antilles mice (n = 4) were treated with 250 ppm CO 3 days/wk, 1 h/day for 8 wk. After 8 wk, bone marrow from the treated mice and age-matched untreated controls (n = 5) was collected and stained for CD45R and CD117 (c-kit). A: representative FSC vs. SSC FACS plots for control and 250-ppm CO-treated S+S-Antilles mice. B: representative CD45R vs. SSC FACS plots of control and 250-ppm CO-treated S+S-Antilles mice featuring the gating for CD45R+ cells. C: representative CD117 vs. CD45R micrograph for the gating performed on the CD45R+ population for CD117low cells, with the gate set for expression levels between 100–1,000 fluorescence intensity. D: bar graph demonstrating the significant decrease in the percentage of CD45R+CD117low cells in mouse bone marrow in 250-ppm CO-treated S+S-Antilles mice compared with untreated control S+S-Antilles mice. Values are mean %positive cells ± SE, *P < 0.05 compared with controls using t-test.
Fig. 6.
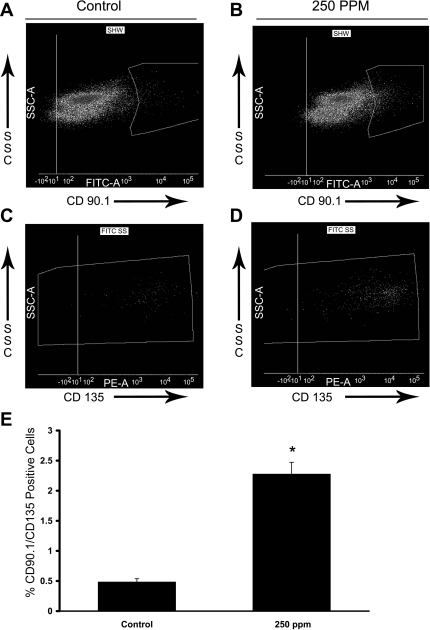
CO treatments (250 ppm) significantly increased immature hematopoietic cells in S+S-Antilles bone marrow. S+S-Antilles mice (n = 4) were treated with 250 ppm CO 3 days/wk, 1 h/day for 8 wk. After 8 wk, bone marrow from the CO-treated mice and untreated age-matched controls (n = 5) was collected and stained for CD135 (flk2) and CD90.1 (Thy1.1). A and B: representative SSC vs. CD90.1 FACS plots featuring the gating of CD90.1+ populations in control (A) and 250-ppm CO-treated (B) S+S-Antilles mice. C and D: representative SSC vs. CD135 FACS plots for the CD90.1+ populations in control (A) and 250-ppm CO-treated (B) S+S-Antilles mice. E: bar graph demonstrating the significant increase in the percentage of CD90.1+CD135+ cells in mouse bone marrow in 250-ppm CO-treated S+S-Antilles mice compared with control S+S-Antilles mice. Values are mean %positive cells ± SE. *P < 0.001 compared with controls using two-tailed t-test.
Fig. 7.
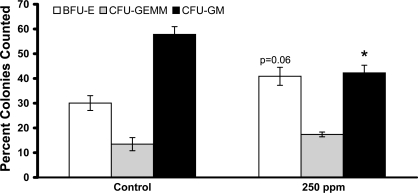
CO treatments (250 ppm) decrease the colony-forming unit (CFU) granulocyte-macrophage (GM) precursor population in S+S-Antilles bone marrow. S+S-Antilles mice (n = 4) were treated with 250 ppm CO 3 days/wk, 1 h/day for 8 wk. After 8 wk, bone marrow from the CO-treated mice and age-matched untreated controls (n = 5) was collected, and methycellulose CFU assays were performed with 3 assay dishes/animal. After 10 days in culture, blood-forming units-erythroid (BFU-E), CFU-granulocyte-erythroid-macrophage-megakaryocyte (GEMM), and CFU-GM were counted and the percentage of total colonies counted from each of the 3 dishes was calculated. Values are mean %colonies counted ± SE. *P < 0.01 using two-tailed t-test.
Treatments of mice.
Mice were exposed to inhaled CO 3 times/wk for a 1-h duration in the specific pathogen-free facility at the University of Minnesota for 8 to 10 wk. No treatment-related morbidity or mortality was seen in any mice during the course of the study. Briefly, mice were sorted by sex and placed in a universal rat cage with lid (VetEquip). The cage was placed within a certified class II biosafety fume hood (NuAire). Certified tanks of either 25 or 250 ppm CO (Minneapolis Oxygen) were connected to the cage by tubing, and the mice were provided with a continuous flow of CO at the desired concentration for 1 h. CO levels in the cage reached the levels of the tank within 5 min of treatment and remained at that level for the duration of the treatment. Controls were kept in the same housing room, which had CO concentrations < 1 ppm CO. CO concentrations were certified by the manufacturer and verified with the use of a three-gas photoionization detector set for CO (RAE Systems).
Blood and serum analysis.
Blood was collected via cardiac puncture at the time of euthanasia from mice into sodium EDTA or serum separator tubes 24 h after the last CO treatments were completed (1 h/day, 3 days/wk, 8–10 wk). Complete blood counts with differential, hematocrit levels, and reticulocytes were measured in EDTA blood as previously described (4, 5). For serum cytokine analysis, the blood was collected 24 h after the last CO treatment was completed (1 h/day. 3 days/wk, 8 wk) in a Capiject red top tube and allowed to clot at room temperature for a minimum of 20 min and centrifuged at 1,200 g for 10 min, and the serum was then separated off and stored at −84°C. The samples were transported on dry ice for cytokine immunoassay (Millipore) at the University of Minnesota Cytokine Analysis Laboratory.
A separate cohort of S+S-Antilles mice was used to follow the kinetics of the effects of CO on WBC counts. Nonterminal blood draws were conducted weekly 24 h post-CO treatment via saphenous venipuncture into sodium EDTA tubes. WBC counts were obtained by counting cells (n = 2–4 counts/sample) on a hemocytometer.
Mouse tissue collection.
After terminal blood draws, the mice were euthanized and tissues were harvested as previously described (6). Mice were asphyxiated in a CO2 chamber, and their organs were removed and weighed. Organ sections for homogenate preparation were wrapped in aluminum foil and immediately frozen in liquid nitrogen and stored at −84°C. Organ sections were collected for histopathology and placed in 10% buffered formalin before embedding in paraffin blocks and sectioning. For bone marrow, both femurs and a humerus were disarticulated and placed immediately in ice-cold saline. The muscle was removed from the bone, and the femoral head was removed with a sterile scalpel. A 19-gauge syringe was then used to flush the marrow out of the bone with ice-cold saline. The marrow was centrifuged at 500 g for 10 min and washed three times in Dulbecco's phosphate-buffered solution (DPBS). Red blood cells were lysed with distilled water, and the remaining cells were centrifuged at 500 g and resuspended in cold DPBS. The cells were counted and aliquoted for flow cytometry analysis and colony-forming unit (CFU) assays.
Flow cytometry.
Flow cytometry was performed using a BD FACSCanto benchtop cytometer (BD Biosciences). All antibodies were purchased from BD Bioscience with the exception of mouse monoclonal myeloperoxidase (MPO; Abcam). For MPO, the cells were fixed with 2% paraformaldehyde and permeabilized with 0.2% (vol/vol) Tween-20-PBS solution. Alexa Fluor 488-conjugated goat anti-mouse IgG1 secondary antibody (BD Biosciences) was used to label. For cell surface markers, the bone marrow was stained with phycoerythrin-conjugated rat anti-mouse antibodies to CD45R, CD117 (c-kit), CD135 (Flk2), or GR-1 (Ly-6G and Ly-6C) plus fluorescein isothiocyanate-conjugated rat anti-mouse CD71 or CD90.1 (Thy 1.1) (BD Biosciences). Data were analyzed using FlowJo software (Tree Star) or FCS Express software (DeNovo Software).
Methylcellulose colony assay.
Bone marrow cells were washed with DPBS and plated in 35-mm tissue culture dishes in 1.1 ml methylcellulose containing growth factors (StemCell Technologies) according to the manufacturer's instructions. The cells were seeded at 2.0 × 104 cells/dish and cultured at 37°C in a humidified atmosphere with 5% CO2-95% room air for 10 days before scoring colonies. The colonies were distinguished as blood-forming units-erythroid (BFU-E), CFU granulocyte-macrophage (CFU-GM), or CFU granulocyte-erythroid-macrophage-megakaryocyte based on their morphological appearance (manufacturer's atlas).
Western blot analysis.
For Western blots, the organ homogenates were prepared as previously described using frozen tissue (4–6). Homogenate DNA concentrations were measured using the PicoGreen DNA quantitation kit (Invitrogen) per the manufacturer's directions using a fluorimeter (Bio-Tek). An equal amount of homogenate DNA per lane was loaded and subjected to electrophoresis on a 15% Tris·HCl gel (Bio-Rad). Afterward, the samples were transferred electrophoretically to polyvinylidene difluoride membranes (Millipore), and an immunoblotting of the organ homogenates was performed with rabbit polyclonal anti-HO-1 antiserum (Assay Designs).
For nuclear signaling molecules, a nuclear extract was made from the organ homogenate using a nuclear extraction kit (Panomics). Organ homogenate (100 μl) was added to nuclear extraction buffer B (900 μl) prepared with 1 M dithiothreitol, 5 mM vanadate, 1 M β-glycerophosphate, 1 mg/ml leupeptin, 100 mM phenylmethane-sulfonylfluoride in ethanol, and 1 μg/ml protease inhibitor cocktail (Sigma-Aldrich). The samples were sonicated for 10 s, followed by a gentle shaking on ice. After 2 h, the samples were centrifuged for 10 min at 14,000 g at 4°C. The supernatant nuclear extract was removed, and the protein content was determined using a Bradford-based protein assay (Bio-Rad). An equal amount of nuclear extract protein was loaded per lane and subjected to electrophoresis on a 15% Tris·HCl gel (Bio-Rad). All signaling antibodies for p38 MAPK, Akt, phosphorylated (phospho) p38 MAPK (residue Thr180/Tyr182), and phospho-Akt (residue Ser473) were purchased from Cell Signaling Technology.
Sites of primary antibody binding were visualized with alkaline phosphatase-conjugated goat anti-rabbit or rabbit anti-goat IgG (Santa Cruz Biotechnology). The final detection of immunoreactive bands was performed using an ECF substrate (GE Healthcare) and visualized on a Storm Reader (GE Healthcare). All membranes were stripped using Restore stripping buffer (Thermo Scientific) and reprobed with rabbit anti-GAPDH (Sigma-Aldrich). The bands were quantitated using ImageJ software (National Institutes of Health).
Pathology.
Formalin-fixed tissues were processed routinely, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Two pathologists surveyed the organs in a blinded manner using the semiquantitative criteria designated by Manci and associates (33). Upon unblinding, contingency tables were made for statistical analysis. Bone marrow and blood smears were stained using Wright-Giemsa solution or anti-MPO antibody (Sigma-Aldrich). Differential counts were performed in a standard manner.
Statistics.
Data were entered into Excel spreadsheets for preliminary analysis, with final statistical analysis performed using SigmaStat software version 3.5 (SYSTAT). Comparisons between control, 25-ppm-, and 250-ppm-treated C57BL/6J and S+S-Antilles animals were conducted using one-way analysis of variances (ANOVA). A one-way repeated-measure ANOVA was performed for serial blood draws. For analysis of pathology scoring, χ2-tests were performed using assembled contingency tables. All other comparisons between control and 250-ppm-treated animals used a two-tailed Student's t-test. See figure legends for specific statistical tests used.
RESULTS
Inhaled CO treatments briefly elevated CO-Hb levels in normal and sickle mice.
After treatment with 250 ppm CO for 1 h, the CO-Hb levels in C57BL/6J mice increased from 1% at baseline to 14.0% in C57BL/6J mice and 20.0% in S+S-Antilles mice (Table 1). The elevation in CO-Hb levels after 1 h of 25 ppm CO treatment was minimal. The CO-Hb levels in all mice returned to baseline after 24 h, as predicted by the half-life of CO-Hb (46). No signs of acute physiological stress or mortality were seen in any mice during these studies or in any subsequent studies presented.
CO decreased mean total WBCs in sickle mice.
Ten weeks of inhaled CO treatments for 1 h/day, 3 days/wk with 25 and 250 ppm CO significantly (P < 0.05) decreased the mean total WBC count in S+S-Antilles mice (Fig. 1A). The mean total WBC count in normal C57BL/6J mice did not change with either the 25- or 250-ppm CO treatment. The drop in mean total WBC count in S+S-Antilles mice was primarily due to significant (P < 0.05) decreases in mean total neutrophil (Fig. 1B) and lymphocyte (Fig. 1C) counts (see supplemental Table 1; note: all supplemental materials can be found posted with the online version of this article). CO treatments did not significantly change mean neutrophil or lymphocyte counts in C57BL/6J mice. There were no significant changes in either the mean hematocrit or reticulocyte counts in any CO-treated mice (Table 2).
Table 2.
Reticulocyte count and hematocrit in mice treated for 10 wk (1 h/day, 3 days/wk) with inhaled CO
| Treatment |
Control |
25 ppm |
250 ppm |
|||
|---|---|---|---|---|---|---|
| Strain | C57BL/6J | S+S-Antilles | C57BL/6J | S+S-Antilles | C57BL/6J | S+S-Antilles |
| n | 11 | 8 | 12 | 8 | 12 | 8 |
| Hematocrit | 49.6±0.6 | 46.5±1.0 | 49.8±1.4 | 49.5±1.5 | 49.0±0.4 | 48.5±1.4 |
| Reticulocyte | 2.4±0.3 | 4.7±0.4 | 2.2±0.2 | 4.2±0.1 | 1.9±0.2 | 4.6±0.4 |
Values are mean percentages ± SE; n, number of mice/group.
The weekly mean WBC values were measured for 16 wk in a separate cohort of seven age-matched S+S-Antilles mice treated with 250 ppm CO for 1 h/day, 3 days/wk inhaled CO to determine the kinetics of the CO-induced changes. Before the treatment initiation, the mean baseline WBC value for the S+S-Antilles mice was 15.1 ± 1.2 × 103 μl−1 (Fig. 2, week 0, supplementary Table 2). There was a significant decrease in the mean total WBC count after 7 and 8 wk of CO treatment compared with that of baseline. The weekly mean WBC count was monitored after the withdrawal of the CO treatments (week 8). The mean WBC count remained significantly lower than baseline for 5 additional wk (weeks 9–13) after treatment withdrawal, returning within the range of the baseline value after 6 wk of no treatments (week 14).
CO decreased myeloid and lymphoid cells in sickle mouse bone marrow.
Bone marrow smears, which were collected after 8 wk of 250-ppm CO treatments and stained with Wright-Giemsa, showed a decrease in polymorphonuclear cells in CO-treated S+S-Antilles mice compared with untreated S+S-Antilles control mice (data not shown). To determine whether a change in myelopoiesis was occurring in CO-treated mice, the bone marrow from 250-ppm CO-treated and untreated S+S-Antilles mice was analyzed for markers of myelopoiesis. Bone marrow was collected from S+S-Antilles mice after 8 wk of CO treatment (250 ppm CO for 1 h/day, 3 days/wk), stained for MPO, and analyzed by flow cytometry (Fig. 3). The number of MPO-positive cells in CO-treated mice was decreased (P < 0.01) compared with that in the untreated controls (Fig. 3E).
Maturation of myeloid cells was examined by staining bone marrow cells for GR-1 (a myeloid differentiation antigen also known as Ly-6G and Ly-6C) and CD71 (the transferring receptor). The mean percent CD71+ cells in S+S-Antilles bone marrow was not significantly (P = 0.14) different in untreated controls versus 250-ppm CO-treated S+S-Antilles mice (Fig. 4C). However, the mean population percentage of CD71− GR-1+ cells was significantly (P < 0.05) decreased in bone marrow from 250-ppm CO-treated mice versus controls (Fig. 4B).
S+S-Antilles bone marrow was also examined for evidence of decreased lymphopoiesis. Bone marrow from CO-treated and untreated S+S-Antilles mice was stained for lymphoid antigen markers CD117 (c-kit) and CD45R (13, 52) (Fig. 5). Cells positive for CD45R+ were gated (Fig. 5B), followed by subsequent gating for cells with decreased expression of CD117 (CD117low) (Fig. 5C). In S+S-Antilles mice, the mean population percentage of CD45R+CD117low (lymphoid) cells was significantly (P < 0.05) decreased in CO-treated mice versus controls (Fig. 5D).
CO increased immature hematopoietic cells in sickle mouse bone marrow.
A less mature hematopoietic progenitor subset was examined by staining bone marrow cells with CD135 (flk-2) and CD90.1 (Thy1.1) (13). When gating on the cells expressing CD90.1 (Fig. 6, A and B), there was a pronounced shift in the CO-treated cell population toward increased CD90.1 expression. This increase in cell population was carried over into the CD135-positive cells (Fig. 6, C and D), leading to a highly significant (P < 0.001) increase in the mean population percentage of CD90.1+CD135+ cells in S+S-Antilles mice treated with 250 ppm CO compared with untreated mice.
CO decreased the percentage of GM-CFU colonies in sickle mouse bone marrow but did not significantly change BFU-E colonies.
CFU assays were performed on bone marrow from S+S-Antilles mice treated 1 h/day, 3 days/week for 8 wk with 250 ppm CO to determine the fates of the progenitor cells from the bone marrow. After 10 days in culture, there was no significant difference in the total number of CFU colonies counted between control and 250-ppm CO-treated mice. However, when broken down into colony types, there was a significant (P < 0.05) decrease in the percent CFU-GM colonies in CO-treated mice compared with controls (Fig. 7). There was also a modest, but not significant (P = 0.06), increase in the percent blood forming unit-erythroid (BFU-E) colonies in the mice treated with CO compared with controls.
CO treatments did not change the cytokine profile of sickle mice.
Patients with SCD have elevated IL-6 cytokine levels (14, 15). To explore whether a change in cytokine levels was occurring in CO-treated mice, serum samples were collected from age-matched untreated control S+S-Antilles mice and 250-ppm (1 h/day, 3 days/wk for 8 wk) CO-treated S+S-Antilles mice. There were no significant differences between the treatment groups for any of the cytokines analyzed (Table 3).
Table 3.
Serum cytokine levels in S+S-Antilles Mice Treated for 8 wk (1 h/day, 3 days/wk) with inhaled CO
| Treatment | Control | 250 ppm |
|---|---|---|
| n | 5 | 4 |
| IL-6 | 53.8±38.1 | 61.2±56.7 |
| G-CSF | 418.6±138.3 | 368.0±75.6 |
| IP-10 | 56.9±14.2 | 50.1±7.7 |
| MIP-1α | 58.0±24.0 | 62.4±36.3 |
| RANTES | 28.9±6.9 | 26.6±7.4 |
| VEGF | 4.5±1.5 | 3.4±0.4 |
Values are means pg/ml ± SE; n, number of mice/group. G-CSF, granulocyte colony-stimulating factor; IP-10, interferon-inducible protein-10; MIP-1α, macrophage inflammatory protein-1α; RANTES, regulated upon activation, normal T-cell expressed, and secreted factor.
CO treatments increased expression of activated p38 MAPK and Akt, HO-1, and ferritin heavy chain in the livers of sickle mice.
We examined liver samples from untreated and 250-ppm CO-treated S+S-Antilles mice to investigate anti-inflammatory signaling molecules known to interact with CO (18, 55). Phospho-p38 MAPK and Akt were markedly increased in nuclear extracts from the livers of S+S-Antilles mice treated with 250 ppm CO for 8 wk (1 h/day, 3 days/wk) compared with untreated control S+S-Antilles mice (Fig. 8A). There was no change in the nuclear expression of either total p38 MAPK or total Akt between treated and control livers. We also measured the expression of HO-1 and another cytoprotective protein, ferritin heavy chain (FHC), in liver homogenates by Western blot analysis (Fig. 8B). There was an increase (P < 0.01) in liver HO-1 and FHC expression in CO-treated mice compared with controls.
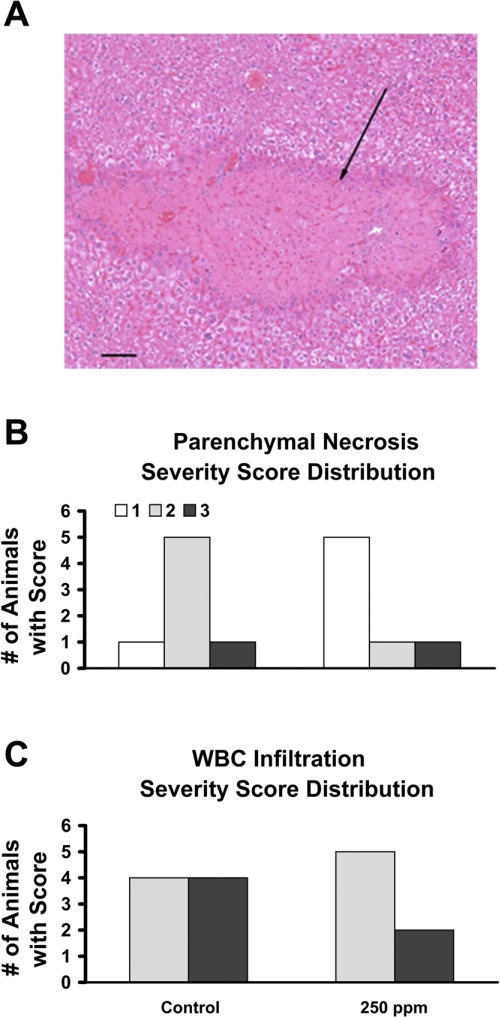
CO treatments decreased liver parenchymal necrosis severity in sickle mice.
We systematically evaluated the histopathology sections from the lungs, livers, and spleens of CO-treated and untreated S+S-Antilles mice (33). No specific lesions were observed in the spleen and lungs except for severe red blood cell congestion. Numerous histopathology lesions were observed in the livers of the S+S Antilles mice. The hepatic lesions comprised irregularly shaped and variably sized areas of coagulation necrosis within the liver that involved single or small groups of hepatic lobules (see Fig. 9A). Some of these necrotic lesions had an associated cellular response consisting of variable numbers of neutrophils, macrophages, and fibroblasts or a stromal response within or at the margins of the lesion. The robust nature of the pathology in the livers of the S+S-Antilles allowed for a semiquantitative analysis of the sections, which revealed a significant (P < 0.001) decrease in the extent of parenchymal necrosis severity score in 250-ppm CO-treated S+S-Antilles mice (1 h/day, 3 days/wk, 10 wk total) compared with untreated control animals (Fig. 9B). In addition, there was a trend (P = 0.06) toward a decreased severity score for WBC infiltration (Fig. 9C) in CO-treated sickle mice.
Fig. 9.
CO (250 ppm) reduces parenchymal necrosis severity in the livers of S+S-Antilles mice. Histopathology slides of the livers from S+S-Antilles mice treated with 250 ppm CO for 10 wk (1 h/day, 3 days/wk), and untreated control mice were analyzed semiquantitatively by 2 pathologists (n = 7 to 8 animals/group). Results were analyzed using χ2-statistics. A: representative micrograph of liver from an S+S-Antilles mouse demonstrating a clearly delineated, irregular area of hepatic coagulative necrosis (arrow). Bar = 100 μm. Parenchymal necrosis severity (B) and WBC infiltration severity (C) bar graphs compare the number of animals assigned the severity scores from 1 (less severe) to 3 (most severe) in untreated control and 250-ppm CO-treated S+S-Antilles mice.
DISCUSSION
We report here that the treatment of S+S-Antilles SCD mice with 25 or 250 ppm inhaled CO for 1 h/day, 3 days/wk for 8–10 wk decreased the total mean WBC, neutrophil, and lymphocyte counts in peripheral blood. Decreased WBC counts were accompanied by a reduced staining for myeloid and lymphoid markers in bone marrow and a decrease in CFU-GM in colony-forming cell assays. Anti-inflammatory signaling pathways phospho-p38 MAPK and phospho-Akt were markedly increased in CO-treated livers, and the cytoprotective proteins HO-1 and FHC were also increased. Most importantly, CO-treated SCD mice had reduced hepatic parenchymal necrosis after only 10 wk of treatment.
In SCD, the insufficient amounts of HO-1 activity and CO in response to hemolysis can result in both increased inflammation and deficient cytoprotection (5). In previous studies, we demonstrated that SCD mouse models have inadequate levels of HO-1 to compensate for hemolysis and that they benefit from additional upregulation of HO-1 (5). For example, in a dorsal skinfold chamber model, inhaled CO (250 ppm, 1 h/day, 3 days in a row) significantly reduces vasoocclusion in S+S-Antilles mice (4, 5, 28). In the current study, we present experiments that address the hypothesis that the chronic inflammation in SCD can be reduced by prolonged therapy with CO. Our results demonstrate that CO treatments are effective in decreasing leukocytosis, increasing anti-inflammatory protection, and reducing organ pathology in SCD.
CO therapy for SCD has been previously explored by other groups. In the 1970s, Beutler (9) gave 1,000–2,000 ppm inhaled CO to two patients with SCD for brief intervals and achieved CO-Hb levels of up to 23.6%. The study concluded that moderate elevations of the CO-Hb percentage may be beneficial for treating patients with SCD who are admitted to the hospital for pain crisis (9). This study was limited in its duration of CO exposure and in its final therapeutic end points. Additionally, Yallop and associates (54) determined that environmental levels of CO (in parts per billion) are inversely correlated with hospital admission rates for SCD, with low levels of environmental CO resulting in a higher incidence of hospitalization (54).
Our study is novel in that it provided exposure to low doses of CO to SCD mouse models over several weeks with the hope of inhibiting inflammation and preventing the pathophysiology of SCD, rather than alleviating acute symptoms. Levels of CO-Hb in patients exposed to CO do not correlate with the presence of symptoms or with outcomes when patients are admitted for CO poisoning (51). Both the CO dose and duration of exposure are elements that determine toxicity in both humans and animals. In our studies, the dose of 250 ppm CO 1 h/day, 3 days/wk, was both nontoxic and therapeutic for sickle mice as the WBC counts fell, liver necrosis was reduced, and the mice appeared normal. Additionally, none of the sickle or normal mice died during the treatments, despite levels of CO-Hb reaching upward of 20% (Table 1).
The most striking finding in our study was the significant reduction in leukocytes that occurred after 8–10 wk of 250-ppm therapy. Patient outcome studies have identified the elevation in the steady-state absolute neutrophil count as a parameter that correlates highly with clinical disease severity (38). Therefore, a reduction of circulating leukocytes may prove highly beneficial to patients with SCD.
Several mechanisms were explored to explain why CO treatments significantly decreased leukocytosis in SCD mice. The sequestration of leukocytes was an unlikely mechanism, since normal C57BL/6J mice treated with CO had no change in WBC, CO-treated S+S-Antilles mice displayed no increase in organ weights (data not shown), and the organs of CO-treated mice displayed no increase in leukocytes upon histopathological examination.
The data presented provides evidence that CO treatments were affecting stress hematopoiesis in SCD mice. Under normal physiological conditions, a balance exists in the bone marrow between multiple cell-cycle regulators that control the ability of hematopoietic stem cells (HSCs) to remain quiescence or to proliferate and differentiate into hematopoietic multipotent progenitor cells (MPPs) (11). If the HSCs differentiate and proliferate into MPPs, they are further committed to myeloid or lymphoid progenitor cells via the interaction and controlled expression of multiple transcription factors, cytokines, and cell-cycle regulators (2, 11, 23, 25, 42, 52). Under stress conditions, the restrictions on HSCs are reduced, pushing them toward proliferation into MPPs, which are then further pushed via cytokines and transcription factors toward an increased mature cell production (11, 19, 20, 32). The steady-state elevation of leukocytes in the SCD mice is similar to models of hematopoietic stress (11, 20). Therefore, the data presented in this study provide strong evidence for a decreased rate of stress hematopoiesis as the primary mechanism for the decreased leukocytosis in CO-treated mice.
A recent publication that explores the importance of HO-1 in the response of HSCs to stress in mice heterozygous for HO-1 supports our observations (11). This study demonstrated that the HO-1 heterozygous mice have an accelerated response to chemotherapy and radiation-induced stress than normal animals, with HO-1+/− mice displaying leukocytosis and reticulocytosis 2 to 3 wk after treatment. The authors hypothesized that the insufficiencies of HO-1 in HSCs lead to inadequate amounts of endogenous CO needed to maintain HSC homeostasis. Although S+S-Antilles mice have an increased expression of HO-1 relative to C57BL/6J controls, S+S-Antilles mice may be HO-1 deficient relative to the amount of heme overload created by the HbS mutation, making them similar to the heterozygous HO-1 mice. The addition of CO to stressed sickle mice may affect the signaling pathways that inhibit the hematopoietic stress response which occurs in sickle mice.
CO is an “anti-inflammatory” molecule because it activates several anti-inflammatory signaling pathways through the generation of low levels of oxidants via the blockade of the mitochondrial respiratory chain at complex IV (10). Frank inhibition of the respiratory chain by CO also mimics hypoxia by slowing ATP production and cell growth (40) and stimulating the hypoxia-inducible factor-1α pathway. Additionally, hypoxia leads to a differentiation arrest in HSCs and is important in the maintenance of quiescence (21, 24, 26, 31, 50). CO-treated mice also had a significant decrease in bone marrow CFU-GM (Fig. 7), a finding that mirrors what occurs when human CD34+ HSCs are cultured in hypoxic conditions (21). Therefore, CO treatments in SCD may increase the hypoxic environment and hypoxia-inducible factor-1α levels in the hematopoietic compartment needed to decrease HSC differentiation. Further investigation into this potential mechanism is warranted.
In our CO-treated SCD mice, we saw a reduced WBC infiltration in the livers, with a significantly decreased severity in parenchymal necrosis (Fig. 9B), suggesting that CO treatment is able to protect SCD livers from damage. CO has many multifaceted interactions with heme proteins that allow for the activation of an integrated adaptive response to oxidants which include p38 MAPK, Akt, and other signaling pathways (10, 12, 27, 40). Therefore, we surveyed liver samples for changes in these pathways. CO-treated SCD mice had a significant increase in activated phospho-p38 MAPK and Akt (Fig. 8A). The activation of both of these pathways leads to the downstream expression of numerous cytoprotective genes, including a further upregulation HO-1 and FHC expression (Fig. 8B) (8, 10, 12, 43). This activation of anti-inflammatory signaling pathways and increased expression of cytoprotective proteins may allow for a better adaptation to hemolysis and the decrease in liver pathology observed in our SCD mice.
In conclusion, this study demonstrates that a prolonged exposure to CO significantly reduces leukocytosis and liver pathology in SCD mice without toxicity. Overall, these findings suggest that a prolonged treatment with CO initiates a homeostatic-conditioning program in the inflamed SCD mice that involves multiple overlapping signaling pathways. The activation of these pathways, in turn, reduces the inflammation in SCD that creates stress hematopoiesis and organ pathology.
GRANTS
This work was funded by National Institutes of Health Grants F30-AG-030909 (to J. D. Beckman) and R01-HL-67367 and P01-HL-055552 (to G. M. Vercellotti and R. P. Hebbel). Support for M. G. O'Sullivan was through the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30-CA-77598.
DISCLOSURES
Drs. Vercellotti, Hebbel, and Belcher receive funding from Lundbeck, Inc., for research on Panhematin.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the following individuals for their support and assistance during the completion of this project: Dr. Fuad Abdullah, Dr. Timothy Singelton, Colleen Forester, Thomas E. Schmidt, Casey Yang, and Paul Marker.
REFERENCES
- 1.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol 132: 108–113, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, Graf T, Mayfield R, Chan S, Kastner P, Akashi K. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell 1: 416–427, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Aslan M, Canatan D. Modulation of redox pathways in neutrophils from sickle cell disease patients. Exp Hematol 36: 1535–1544, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood 101: 3953–3959, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest 116: 808–816, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, Bowlin PR, Bischof JC, Hebbel RP, Vercellotti GM. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol 288: H2715–H2725, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood 96: 2451–2459, 2000 [PubMed] [Google Scholar]
- 8.Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K, Csizmadia E, Tyagi S, Otterbein LE, Brouard S, Tobiasch E, Bach FH, Kupiec-Weglinski JW, Soares MP. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J 17: 1724–1726, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Beutler E. The effect of carbon monoxide on red cell life span in sickle cell disease. Blood 46: 253–259, 1975 [PubMed] [Google Scholar]
- 10.Bilban M, Haschemi A, Wegiel B, Chin B, Wagner O, Otterbein L. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med 86: 267–279, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Cao YA, Wagers AJ, Karsunky H, Zhao H, Reeves R, Wong RJ, Stevenson DK, Weissman IL, Contag CH. Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood 112: 4362–4363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin BY, Jiang G, Wegiel B, Wang HJ, MacDonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA 104: 5109–5114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA 98: 14541–14546, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conran N, Saad S, Costa F, Ikuta T. Leukocyte numbers correlate with plasma levels of granulocyte-macrophage colony-stimulating factor in sickle cell disease. Ann Hematol 86: 255–261, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Croizat H. Circulating cytokines in sickle cell patients during steady state. Br J Haematol 87: 592–597, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Fabry ME, Sengupta A, Suzuka SM, Costantini F, Rubin EM, Hofrichter J, Christoph G, Manci E, Culberson D, Factor SM, Nagel RL. A second generation transgenic mouse model expressing both hemoglobin S (HbS) and HbS-Antilles results in increased phenotypic severity. Blood 86: 2419–2428, 1995 [PubMed] [Google Scholar]
- 17.Fadlon E, Vordermeier S, Pearson TC, Mire-Sluis AR, Dumonde DC, Phillips J, Fishlock K, Brown KA. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood 91: 266–274, 1998 [PubMed] [Google Scholar]
- 18.Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, Yoshida K, Nagai R. Carbon monoxide protects against cardiac ischemia—reperfusion injury in vivo via MAPK and Akt—eNOS pathways. Arterioscler Thromb Vasc Biol 24: 1848–1853, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 10: 1923–1940, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta SK, Gupta M, Hoffman B, Liebermann DA. Hematopoietic cells from gadd45a-deficient and gadd45b-deficient mice exhibit impaired stress responses to acute stimulation with cytokines, myeloablation and inflammation. Oncogene 25: 5537–5546, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hermitte F, Brunet de la Grange P, Belloc F, Praloran V, Ivanovic Z. Very low O2 concentration (0.1%) favors G0 return of dividing CD34+. Cell Stem Cell 24: 65–73, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hofstra TC, Kalra VK, Meiselman HJ, Coates TD. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood 87: 4440–4447, 1996 [PubMed] [Google Scholar]
- 23.Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell Signal 18: 174–182, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Ivanovic Z, Belloc F, Faucher JL, Cipolleschi MG, Praloran V, Sbarba PD. Hypoxia maintains and interleukin-3 reduces the pre-colony-forming cell potential of dividing CD34+ murine bone marrow cells. Exp Hematol 30: 67–73, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity 26: 726–740, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaczorowski DJ, Zuckerbraun BS. Carbon monoxide: medicinal chemistry and biological effects. Curr Med Chem 14: 2720–2725, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kalambur VS, Mahaseth H, Bischof JC, Kielbik MC, Welch TE, Vilback A, Swanlund DJ, Hebbel RP, Belcher JD, Vercellotti GM. Microvascular blood flow and stasis in transgenic sickle mice: utility of a dorsal skin fold chamber for intravital microscopy. Am J Hematol 77: 117–125, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kaul DK, Liu XD, Choong S, Belcher JD, Vercellotti GM, Hebbel RP. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am J Physiol Heart Circ Physiol 287: H293–H301, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lard LR, Mul FP, de Haas M, Roos D, Duits AJ. Neutrophil activation in sickle cell disease. J Leukoc Biol 66: 411–415, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Lin Q, Lee YJ, Yun Z. Differentiation Arrest by Hypoxia. J Biol Chem 281: 30678–30683, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Macleod KF. The role of the RB tumour suppressor pathway in oxidative stress responses in the haematopoietic system. Nat Rev Cancer 8: 769–781, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood 107: 1651–1658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFaul SJ, Bowman PD, Villa VM. Hemoglobin stimulates the release of proinflammatory cytokines from leukocytes in whole blood. J Lab Clin Med 135: 263–269, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, Wethers DL, Smith J, Kinney TR. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med 342: 83–89, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 158: 893–903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohta K, Yachie A. Development of vascular biology over the past 10 years: heme oxygenase-1 in cardiovascular homeostasis. J Endovasc Ther 11, Suppl 2: II140–II150, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Okpala I. The intriguing contribution of white blood cells to sickle cell disease—a red cell disorder. Blood Rev 18: 65–73, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24: 449–455, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Piantadosi CA. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med 45: 562–569, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn CT, Lee NJ, Shull EP, Ahmad N, Rogers ZR, Buchanan GR. Prediction of adverse outcomes in children with sickle cell anemia: a study of the Dallas Newborn Cohort. Blood 111: 544–548, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbauer F, Tenen DG. Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol 7: 105–117, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234–235: 249–263, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J 18: 854–856, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Sebastiani P, Nolan VG, Baldwin CT, Abad-Grau MM, Wang L, Adewoye AH, McMahon LC, Farrer LA, Taylor JG, 4th, Kato GJ, Gladwin MT, Steinberg MH. A network model to predict the risk of death in sickle cell disease. Blood 110: 2727–2735, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart RD. The effect of carbon monoxide on humans. Annu Rev Pharmacol 15: 409–423, 1975 [DOI] [PubMed] [Google Scholar]
- 47.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci USA 99: 3047–3051, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagener FA, Abraham NG, van Kooyk Y, de Witte T, Figdor CG. Heme-induced cell adhesion in the pathogenesis of sickle-cell disease and inflammation. Trends Pharmacol Sci 22: 52–54, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev 55: 551–571, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Walmsley SR, McGovern NN, Whyte MK, Chilvers ER. The HIF/VHL pathway: from oxygen sensing to innate immunity. Am J Respir Cell Mol Biol 38: 251–255, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Weaver LK. Carbon monoxide poisoning. N Engl J Med 360: 1217–1225, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112: 3543–3553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57: 585–630, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Yallop D, Duncan ER, Norris E, Fuller GW, Thomas N, Walters J, Dick MC, Height SE, Thein SL, Rees DC. The associations between air quality and the number of hospital admissions for acute pain and sickle-cell disease in an urban environment. Br J Haematol 136: 844–848, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Zuckerbraun BS, Chin BY, Bilban M, de Costa d'Avila J, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J 21: 1099–1106, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.