Abstract
In weeks to months following implantation, neointimal hyperplasia (NIH) in vein grafts (VGs) transitions from a cellularized to a decellularized phenotype. The inhibition of early cellular proliferation failed to improve long-term VG patency. We have previously demonstrated that transforming growth factor-β1 (TGF-β1)/connective tissue growth factor (CTGF) pathways mediate a conversion of fibroblasts to myofibroblasts in the early VG (<2 wk). We hypothesize that these similar pathways drive fibrosis observed in the late VG lesion. Within rabbit VGs, real-time RT-PCR, Western blot analysis, ELISA, and immunohistochemistry were used to examine TGF-β/CTGF pathways in late (1–6 mo) NIH. All VGs exhibited a steady NIH growth (P = 0.006) with significant reduction in cellularity (P = 0.01) over time. Substantial TGF-β profibrotic activities, as evidenced by enhanced TGF-β1 activation, TGF-β receptor types I (activin receptor-like kinase 5)-to-II receptor ratio, SMAD2/3 phosphorylation, and CTGF production, persisted throughout the observation period. An increased matrix synthesis was accompanied by a temporal reduction of matrix metalloproteinase-2 (P = 0.001) and -9 (P < 0.001) activity. VG NIH is characterized by a conversion from a proproliferative to a profibrotic morphology. An enhanced signaling via TGF-β/CTGF coupled with reduced matrix metalloproteinase activities promotes progressive fibrotic NIH expansion. The modulation of late TGF-β/CTGF signaling may offer a novel therapeutic strategy to improve the long-term VG durability.
Keywords: transforming growth factor-β, stenosis, connective tissue growth factor
vein grafts occlude largely as a direct or an indirect consequence of neointimal hyperplasia. Despite the advances in vascular biology over the past decades, strategies effective for controlling neointimal hyperplasia growth remain to be emerged. A headline highlighted our current situation as “intimal hyperplasia—still here” (22). It is widely accepted that the critical cellular event taking place during neointimal development is the dedifferentiation of mature medial smooth muscle cells (SMCs) from a contractile to proliferative/synthetic phenotype (32). Developed on this theory, hundreds of therapeutic strategies aiming to inhibit cell proliferation have been experimentally tested, and a few promising ones have moved to clinical trials (22). However, none of them survived due to the marginal benefits in prolonging the long-term graft durability (7, 22). This frustration necessitates deeper insights into the biology of neointimal hyperplasia.
Vein grafts express dynamic phenotypes over the course of the adaptation. Within weeks, the highly cellularized proproliferative early neointima starts a transition to a phenotype with hypocellularity, where matrix components dominate the neointimal mass. Although neointimal cells become relatively quiescent at this stage, the neointimal volume continues to expand due to the progressive accumulation of the matrix (48). It appears that fibrosis becomes the prevailing event in late neointimal growth, and the interruption of this fibrotic process holds the potential to inhibit luminal narrowing as a result of progressive neointimal expansion. Unfortunately, most effort in the field of vascular biology has focused on the proproliferative properties of early neointima, with little attention being paid to the late fibrotic neointimal expansion.
Tissue fibrosis is characterized by excessive matrix accumulation, the balance of synthesis, and the degradation of matrix proteins. Profibrotic growth factors such as transforming growth factor (TGF)-β and connective tissue growth factor (CTGF) are potent factors that stimulate matrix production and deposition, with CTGF serving as a downstream effector in TGF-β1-induced fibrosis (3). Counterbalancing matrix deposition are proteolytic pathways, which in normal homeostasis maintain the system in equilibrium. In the vascular wall, matrix metalloproteinases (MMPs) serve as the predominate effector for matrix degradation. However, while it appears that the MMPs act in opposition to TGF-β/CTGF, tuning the balance of matrix synthesis and degradation and thus regulating matrix accumulation during early neointimal growth (1, 2, 31), their role in late neointimal expansion remains unclear. In contrast to early intimal growth, we hypothesize that enhanced TGF-β/CTGF activity, coupled with reduced MMP-2 and -9 expression, promotes progressive matrix accumulation and neointimal fibrosis during late neointimal expansion. Using a novel microdissection technique to examine exclusively the neointima, we evaluated the temporal profibrotic signaling pathway of TGF-β and the counterbalancing MMP activities within established vein graft neointima.
METHODS
Animal model.
This study was performed after securing approval from the Institutional Animal Care and Use Committee and conforms to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, Revised 1996). Male New Zealand White rabbits (3.0–3.5 kg; n = 18) were anesthetized with ketamine hydrochloride (30 mg/kg) and inhaled isoflurane (∼2.5–3.0%). Heparin (1,000 units) was given intravenously and a unilateral jugular vein graft placed into the common carotid, as previously described (18). Intimal hyperplasia formation within the graft was augmented by a complete ligation of the distal internal and partial ligation external carotid at the time of implantation.
Samples were harvested at 1 (n = 4), 3 (n = 6), and 6 mo (n = 4) following implantation, with bromodeoxyuridine (BrdU, 50 mg/kg) administered 24 h before harvest. At the time of harvest, the perianastomotic regions were discarded and the graft was divided into three segments, which were immersed in 10% buffered formalin or liquid nitrogen for histological, mRNA, and protein analyses. Normal jugular veins (n = 4) were harvested for baseline controls.
Morphometric and immunohistochemical analyses.
Following paraffin embedding, morphological analyses (Axiovision version 4.4, Zeiss) were completed using cross-sectional measurements on Masson's stained specimens. The lumen area and the area within the external elastic lamina were measured, and the neointimal area was calculated as previously described (18). The content of the proteoglycans was quantified on Movat's stained cross sections in vein grafts using the AxioVision interactive measurement. In Movat's staining, the proteoglycan appears in blue, and the density of the blue area correlates with proteoglycan production. A program that measures the density of the blue color was set up and kept identical for all measurements. The data presented are expressed as density per neointimal area.
Proliferating cells were identified using an antibody to BrdU (Zymed) on formalin-fixed sections. Neointimal cells positive for BrdU were counted in six high-power fields, evenly divided along the circumference of the lumen. Hematoxylin staining was used to obtain the total nuclear density, and the data were expressed as the ratio of BrdU positive to total nuclei. Monoclonal antibodies to the recombinant human TGF-β1 (R&D), CTGF, and phosphorylated Smad1 (pSmad1, Santa Cruz) were applied to detect the production of these growth factors, with isotype IgG or nonimmunized serum serving as a negative control. The antiserum to CTGF and pSmad1 used in this experiment requires antigen retrieval, and this was achieved via autoclaving sections in citra buffer (Bio-Nex). ABC and DAB kits (Vector) were used to visualize specific staining. The immunogenicity of the pSmad1 within the neointimal layer was measured with the AxioVision interactive measurement program. The density of the nuclear stain was averaged to the cross-sectional nuclear area and further calibrated to 1-mo vein grafts.
Neointimal separation.
To focus analysis exclusively on the neointima and exclude the confounding factors contained with the fibrotic adventitia, a novel microdissection technique was developed. Frozen (−80°C) samples were transferred to RNAlater-ICE (Ambion) and incubated overnight at −20°C. A 5-mm segment of graft was incised longitudinally, and the neointima was stripped from the underlying media using standard microdissection instrumentation. The extracted neointima was preserved for mRNA or protein analysis, whereas the remaining wall was histologically examined to ensure an appropriate cleavage plane.
Protein analyses.
Extracted neointimal specimens were pulverized with a mortar and pestle, and the protein was extracted with a buffer containing 0.05 M Tris (pH 7.5), 0.2% Triton X-100, 0.1% SDS, 1.0 mM PMSF, 1.0 μM leupeptin, and a cocktail of phosphatase inhibitors (Calbiochem). Pierce BCA assay (Thermo Scientific) was used to quantify sample protein concentrations.
Heat-denatured protein (10 μg per lane) was separated with 7.5% SDS-PAGE gel (Bio-Rad) electrophoresis. Rabbit antiserum to human activin receptor-like kinase 5 (Alk5, Santa Cruz), TGF-β receptor type II (TβRII, Santa Cruz), and pSmad2/3 (R&D) were applied to identify the specific bands. Proxidase-conjugated mouse antiserum to native rabbit IgG (Jackson Immuno Research) served as a secondary antibody. Following the development of the blots with chemiluminescence detection (ECL advanced kit), the density of each specific band was measured using Kodak Imager and normalized to the β-actin band density. Freshly harvested jugular vein served as the time zero control.
A TGF-β1 immunoassay ELISA kit (R&D) was used to determine the TGF-β1 protein content and the latent TGF-β1 activated, following the manufacturer's instructions. The total protein (20 μg) with and without activation treatment was loaded in the assay for the total and active TGF-β1 concentration.
Gelatin zymography was performed on protein samples extracted from neointimal tissue. A mixture of latent and active MMP-2 and -9 standards with series dilution and samples (10 μg total protein per lane) were loaded and separated with SDS-PAGE gel containing 0.1% gelatin (Invitrogen). Following the development of bands with colloidal blue staining (Invitrogen), the intensity of each band was quantified with Kodak Imager System.
Real-time PCR and ELISA assay.
Total RNA was isolated from neointimal samples, and TaqMan RT-PCR was performed as previously described (19), using the following primers (TGF-β1: forward, AAGGGCTACCACGCCAACT; reverse, CCGGGTTGTGCTGGTTGT; and 6-FAM-labeled probe, AGTACAGCAAGGTCCTGGCCCTG; and CTGF: forward, TCCAAGCCGGTCAAGTTTG; reverse, TGCATACTCCGCAGAACTTAGC; and 6-FAM labeled probe, TGGCTGCACCAGCGTGAAGACG). RT-PCR was simultaneously run for 18S RNA on all samples to normalize sample loading. The comparative threshold cycle method was used for analysis, and the results were expressed as relative fold change compared with those of the freshly harvested jugular vein.
Statistical analysis.
All data are expressed as means ± SE. Comparisons were done using ANOVA and Tukey's post hoc analysis, with P < 0.05 considered significant.
RESULTS
Morphology.
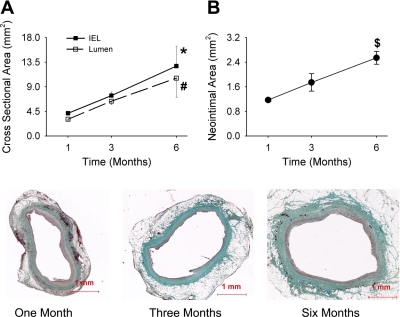
From 1 to 6 mo following implantation, the vein grafts displayed a progressive outward remodeling, as reflected by an increase in the lumen area and cross-sectional area within the external elastic lamina over time (Fig. 1A). Associated with this increase in area was a marked increase in neointimal mass, with 6-mo grafts demonstrating a neointimal area more than twice that of 1-mo vein grafts (Fig. 1B). Representative images of Masson's trichrome stain for vein grafts from each time point are shown in Fig. 1, bottom.
Fig. 1.
Morphological analyses of vein grafts 1 to 6 mo following implantation. With one-way ANOVA, significant differences were detected among time points for lumen cross-sectional area (A), area within the internal elastic lamina (IEL; A), and neointimal area (B). *P = 0.02; #P = 0.01; $P = 0.006 (one-way ANOVA). Bottom: representative images of Masson's trichrome stain for vein grafts harvested at 1, 3, and 6 mo following implantation.
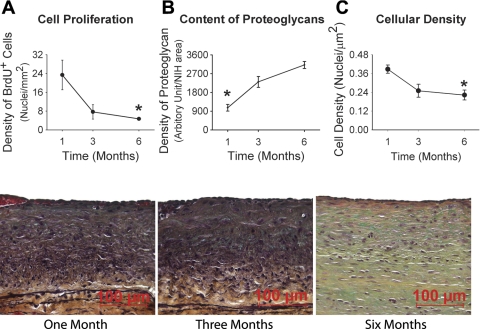
Unlike the early phases of vein graft adaptation that we have described in this model (18), cell proliferation during this late phase of neointimal expansion was relatively modest (Fig. 2A). While there was a sparse scattering of BrdU-positive neointimal SMCs at 1 mo, the neointima was devoid of proliferating SMCs at 3 and 6 mo with the few identified BrdU-positive cells lining on the luminal surface, consistent with the endothelium. Corresponding to the substantial increase in neointimal mass with limited SMC proliferation, the neointima exhibited a dramatic increase in the content of proteoglycans (Fig. 2B) and a 50% reduction in cell density (Fig. 2C), consistent with a substantial deposition and remodeling of the extracellular matrix and a transition from a cellularized to a hypocellularized phenotype during late-phase neointimal growth. A marked reduction in neointimal elastin content was also detected with Movat's stain (Fig. 2, bottom).
Fig. 2.
Temporal changes in cell proliferation (A), content of proteoglycans (B), and cellular density (C) during late vein graft neointimal hyperplasia (NIH). Significant reductions in cell proliferation (A) and nuclear density (C) along with dramatic increases in the content of proteoglycans within the neointima are observed in the 1- to 6- mo time frame. *P = 0.01 (one-way ANOVA). Brdu, bromodeoxyuridine. Bottom: representative images of Movat's stain for vein grafts harvested at 1, 3, and 6 mo following implantation.
Activation of the TGF-β1 and CTGF profibrotic signals in the late-stage neointima.
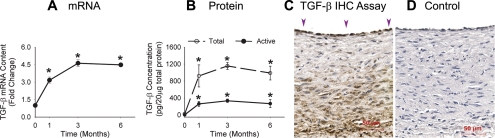
The transcription of TGF-β1 was upregulated more than threefold from the baseline level, and this elevation persisted throughout the 1- to 6-mo time frame (Fig. 3A). The temporal pattern of TGF-β1 protein production generally mirrors that of the mRNA levels, with marked increases in both latent and active forms of TGF-β1 (Fig. 3B). Immunohistochemistry (IHC) assays demonstrate the majority of the TGF-β1 to be localized to the endothelium and periluminal portion of the neointima (Fig. 3C). Figure 3D demonstrates negative staining where the primary antiserum was substituted with isotype antibody.
Fig. 3.
Transforming growth factor-β1 (TGF-β1) production during late vein graft remodeling. TGF-β1 mRNA (A) and total and activated TGF-β1 protein content (B) are significantly elevated from baseline. *P < 0.001 for each data point vs. baseline level. Cells with the most intensive TGF-β1 immunogenicity (arrowheads) are located at luminal surface of the graft wall (C, 3-mo vein graft). IHC, immunohistochemistry. D: negative control using isotype antibody.
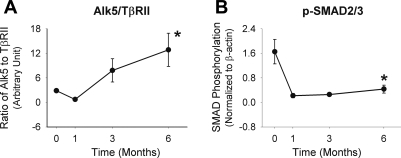
TβRII content was notably upregulated at 1 mo but demonstrated marked reductions by 3 and 6 mo following graft implantation (data not shown). Counterbalancing this decrease in the type II receptor was a slight increase in the TGF-β type I/Alk5 after 1 mo, and it is this balance between the Alk5 and TβRII that determines SMC responses to TGB-β1. An examination of this balance demonstrates a progressive increase in the Alk5-to-TβRII ratio from 1 to 6 mo, leading to a fivefold increase in this ratio at the 6-mo time point (Fig. 4A). Consistent with this relative decrease in type II receptor levels is the reduced phophorylation of the downstream signaling mediators Smad2/3 (Fig. 4B).
Fig. 4.
Western blot analyses for the production of TGF-β receptors types I [activin receptor-like kinase 5 (Alk5)] and TβRII (A) and the phosphorylation of Smad2/3 (B). Late vein graft neointimal expansion is associated with a progressive shift in the stoichiometry of Alk5 and TβRII (P < 0.001) and marked reduction of Smad2/3 phosphorylation (P = 0.001). *P = 0.001 (one-way ANOVA). pSmad2/3, phosphorylated Smad2/3.
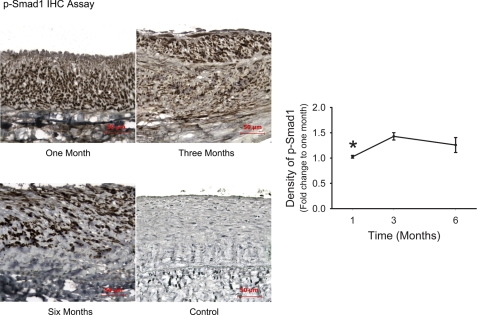
In addition to the Alk5/TβRII/Smad2/3 pathway, TGF-β can also signal through Alk1/TβRII/Smad1/5 to regulate the expression of target genes. Using IHC assay, we evaluated the activation of this pathway by measuring the phosphorylation of Smad1 in neointimal cells. As shown in Fig. 5, the majority of neointimal cells were positive for the immunogenicity of pSmad1. Whereas the absolute number of positive cells decreased over time, the density of the nuclear stain increased significantly 4 wk after graft implantation (P = 0.02), indicating an enhanced Smad1 phosphorylation during fibrotic neointimal expansion.
Fig. 5.
IHC assay for Smad1 phosphorylation. pSmad1 protein presented in the majority of the neointimal nuclei during late neointimal expansion. Whereas the absolute number of pSmad1-positive cells decreases, the averaged density of nuclear stain exhibits a significant increase a month after graft implantation. *P = 0.02 (one-way ANOVA).
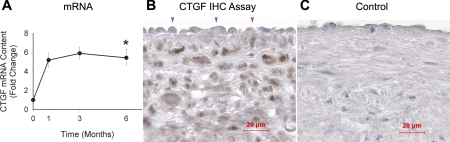
The expression of CTGF, a well-defined TGF-β1 responsive gene, was significantly upregulated throughout the 1- to 6-mo time frame (Fig. 6A). The IHC assay (Fig. 6B) demonstrates CTGF to be localized to a majority of neointimal cells. In contrast to TGF-β1, the cells lining the luminal surface appeared to be negative for CTGF, suggesting a marginal contribution of the endothelium to the increased CTGF production.
Fig. 6.
Connective tissue growth factor (CTGF) production during late vein graft remodeling. CTGF mRNA levels (A) are significantly elevated from the baseline. *P = 0.01 (one-way ANOVA). Cells lining the luminal surface (arrowheads) are negative for CTGF, whereas the majority of neointimal cells display intensive CTGF immunogenicity (B). Negative control using nonimmunized serum yielded completely negative staining (C).
Production and activation of gelatinases.
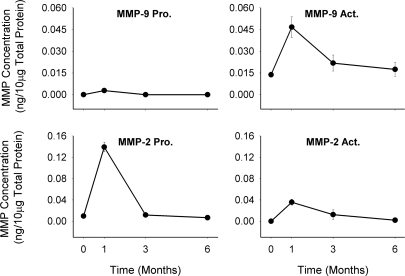
MMP-2 and -9 were noted to be significantly upregulated in the 1-mo neointima, with MMP-2 predominately found in proenzyme and MMP-9 in an active-enzyme form (Fig. 7). Both gelatinases exhibited a modest reduction at 3 and 6 mo from the peak levels observed at 1 mo.
Fig. 7.
Zymographic analysis of gelatinolytic activity of matrix metalloproteinase (MMP)-2 and -9. Significant reduction in pro-MMP-2 (P < 0.001), active (Act) MMP-2 (P < 0.001), and active MMP-9 (P = 0.036) activities were detected when growth of the matrix-dominant neointima is most pronounced.
DISCUSSION
Critical vein graft stenoses occur when a rapid growth of the neointima, composed of SMCs and deposited extracellular matrix, encroaches on the lumen. Previous studies from our group have demonstrated a highly cellularized, proproliferative phenotype of early (1 day to 1 mo) neointima (18, 19). While the majority of studies have focused on this early time frame as a key opportunity to modulate these events, it is in fact the progressive growth of the established neointima that leads to vein graft failure. An understanding of these critical events is lacking. In the current study, we determined that 1) the established neointima continued to expand via profibrotic processes with a limited contribution by SMC proliferation, 2) the late neointimal expansion was associated with the enhanced TGF-β1 production and activation and the reduced MMP activity, and 3) the stoichiometry of TGF-β receptors Alk5 and TβRII shifted toward a profile characteristic of profibrotic TGF-β function. Our earlier studies together with the present work demonstrate that the early neointima is established by a proproliferative, cellularized expansion but that the established neointima continues to expand through enhanced profibrotic pathways. The microenvironment in the neointimal tissue stimulates the expression, production, and activation of TGF-β1 and specifies a profibrotic function for the TGF-β1 signaling pathway. The enhanced matrix synthesis coupled with diminished MMP-mediated matrix degradation causes progressive matrix accumulation, driving late profibrotic neointimal expansion during vein graft adaptation.
When compared with cellularized neointimal growth, the fibrotic neointimal expansion has attracted much less investigative efforts in the field of vascular biology despite the fact that matrix constitutes up to 80% of neointimal volume in an advanced lesion of human vein grafts (12). In both coronary artery and lower extremity bypass vein grafts, lesions with hemodynamically significant stenosis demonstrate a very low level of neointimal cell proliferation (16, 44). In addition to the number of neointimal cells, the accumulation of neointimal matrix is another major determinant of the total volume of neointimal mass, and more importantly, the synthetic behavior of neointimal cells is not always coupled with the proliferative state (33). It is not surprising then that therapies aimed at inhibiting neointimal cell proliferation failed to attenuate vein graft stenosis or arterial restenosis (7). A few limited studies evaluated the accumulation of matrix in experimental (48) and occluded human (12, 13) vein grafts. Whereas these authors clearly demonstrated fibrotic neointimal expansion and proposed antifibrotic therapy to inhibit late neointimal expansion, the responsible signaling pathways and molecular targets were not explored. TGF-β and CTGF are potent profibrotic growth factors that promote matrix synthesis during pathological fibrosis of the vasculature (38) and other organs (3) and therefore stand as reasonable targets for the modulation of vein graft failure. We and others have demonstrated the upregulation of TGF-β during vein graft maturation (17, 18). The inhibition of TGF-β attenuated an early neointimal hyperplasia induced in bypass vein grafts as well as injured arteries (4, 45). Further mechanistic studies suggest that this is ascribed to its function in regulating fibroblast recruitment, macrophage survival, and neointimal cell proliferation (15, 19, 23). TGF-β is a pleiotropic cytokine with multiple bioactivities functioning in a context-dependent manner (8, 28). While the loss and gain of function studies (27, 30, 40, 45) demonstrate a critical role for TGF-β in early neointimal hyperplasia, the distinct pathological features of early and late neointima tissue point toward the disparity of TGF-β functions between early and late phases. In support of this notion are the findings from the present study, demonstrating that an enhanced profibrotic TGF-β function correlates with progressive matrix accumulation during late neointimal expansion.
TGF-β signals through a receptor complex consisted of Alk5 and TβRII. The activation of these receptors phosphorylates Smad2/3 or Smad1/5, regulating various physiological and pathophysiological processes (28). While mechanisms for the specification of TGF-β function remain to be fully elucidated, one of them is a shift in the stoichiometry of type I and type II receptors (9, 28). Studies for chronic organ fibrosis have defined that the increased ratio of Alk5 to TβRII levels specifies a profibrotic function for TGF-β (34, 35, 37). A similar relationship between the receptor profile and TGF-β function has also been observed in SMCs cultured from atherosclerotic lesions (29). While these studies indicate that a similar mechanism may underlie the fibrotic expansion of late vein graft neointimal hyperplasia, this hypothesis has to be verified due to the cellular context-dependent property of TGF-β function. In the present study, we assayed the protein levels of these receptors selectively collected from the neointima of vein grafts and identified an enhanced ratio of Alk5 to type II receptors. This finding provides the first piece of in vivo evidence demonstrating the association between the shift of TGF-β receptor profile and neointimal fibrosis within vein grafts.
The profibrotic signaling of TGF-β is mediated by Smad2/3 in many cell types, and thus Smad2/3 are considered as the major mediators for TGF-β-induced fibrotic pathogenesis. Our data show modest levels of Smad2/3 phosphorylation, indicating that neointimal fibrosis in the vein graft is not processed via Smad2/3 signaling. A recent study demonstrates that the shift of TGF-β receptor profile promotes the synthesis of matrix proteins via Smad1/ERK rather than Smad2/3 signaling pathways (36). Our data demonstrate a correlation of a progressive accumulation of matrix with an enhanced phosphorylation of Smad1, suggesting a role for Smad1 signaling in mediating neointimal fibrosis.
As a TGF-β responsive gene, CTGF is expressed in tissues with fibrotic diseases. However, its induction by TGF-β is cell-type dependent. In SMCs, for instance, CTGF is induced by TGF-β in a dose-dependent fashion (11). In contrast, it may or may not be induced in the endothelium, depending on the size of the vessel from which the cells are cultured (5, 41). Our previous study has shown that the transcription of CTGF mRNA correlates with the production of TGF-β protein in the graft wall (19). Using IHC assay, we demonstrate in the present study an incomplete spatial colocalization between TGF-β1 and CTGF production, suggesting a cell type-dependent CTGF expression in response to TGF-β during late neointimal expansion in vein grafts.
Matrix accumulation is the balance of synthesis and degradation of matrix proteins. MMPs are able to catalyze matrix proteins and thus act in concert with TGF-β, determining the eventual amount of matrix in the neointimal tissue. Our data show a diminished MMP-2 and -9 activity, suggesting the contribution of reduced matrix degradation to late fibrotic neointimal expansion. This temporal reduction appears to be consistent across species (42), indicating a potential role for reduced MMP activity in the development of fibrosis in human vein graft lesions. We and others have demonstrated that highly cellularized neointimal development correlates with a sequential upregulation and activation of MMP-9 and -2 during early vein graft adaptation (1). The pharmaceutical inhibition of MMP activities (25) or the overexpression of tissue inhibitors of metalloproteinases (14) attenuates cellular neointimal growth, suggesting MMP as a driving factor in this process. These studies, together with the current study, suggest distinct roles for MMP-2 and -9 regulating early cellular and late fibrotic neointimal expansion. Matrix proteins not only provide structural support to neointimal cells but also initiate “outside-in” and “inside-out” signals in neointimal cells through binding to their integrin receptors (43). Although not fully understood, MMPs actively reorganize the deposited matrix proteins and regulate cell-matrix interaction (20), which may serve as a potential mechanism to sustain the synthetic phenotype of neointimal cells. MMP-3, for instance, inhibits the migration of SMCs (21), whereas membrane type 1 MMP promotes SMC migration (10). More than 20 members have been identified in the MMP family (26). Although considerable functional redundancy exists among the MMP members, gene interruption experiments have shed light on the unique function of these members in vascular disorders (6). The modest levels of MMP-2 and -9 observed in the present study warrant further studies for the role of other MMP members in modulating the phenotype of neointimal cells during late neointimal expansion.
Whereas the total volume (area) of neointimal tissue continued to increase, the neointimal thickness remained unchanged due to a significant outward remodeling during the half-year observation period. The fibrotic expansion does not seem to have an immediate impact on the preservation of the luminal size of the vein grafts. However, it is increasingly recognized that the histological phenotype of the neointimal tissue, in addition to the neointimal mass, is another significant factor for the determination of long-term graft performance. The neointima is an inflamed tissue that provides fertile soil for the development of atherosclerosis (39). A recent study demonstrates that the inhibition of TGF-β1 induces apoptosis of macrophages, therefore leading to a healthier graft wall (15). Focusing on the graft wall remodeling, Wong et al. (46) demonstrated an association of persistent graft wall fibrosis with significant lumen dilation that predisposes the graft to the eventual formation of atherosclerosis. During past decades, hundreds of reagents have been developed in experimental studies and demonstrated a significant inhibition to neointimal thickening. Unfortunately, all failed to attenuate restenosis in the costly clinical trials (7, 22). While the failure can be ascribed to several factors, a critical factor is that the potential therapeutic effects of these reagents were evaluated against early neointimal thickening only, with the impacts on the modulation of late neointimal phenotype undefined. The present study demonstrates a sustained TGF-β activity coinciding with fibrotic neointimal expansion in late vein graft adaptation. Further studies are required to define the vulnerability of this phenotype to the development of lesions such as calcification and atherosclerosis.
There are limitations in this study. First, the statement of increased extracellular matrix accumulation is based on a semiquantification of proteoglycan content and the augmented neointimal mass with reduced cellularity in the neointimal layer. A reduction in cellularity can be a result of an increase in matrix accumulation and a decrease in the total number of cells caused by cell apoptosis. However, our unpublished data show that extensive cell apoptosis occurs within the first week after graft implantation, with rare apoptotic cells identified in the established 4-wk neointima. Next, the extracellular matrix is composed of proteins encoded by a variety of genes, and some of them may not be responsive to TGF-β signaling. While our data demonstrate the coincidence of enhanced TGF-β signaling with the increased content of proteoglycans and fibrotic neointimal expansion, the molecular targets were not defined. The identification of TGF-β responsive components would certainly bring deeper insight into the mechanisms underlying TGF-β-regulated late neointimal progression in vein grafts. Finally, TGF-β/Alk5/Smad2/3 signaling pathway generally exerts opposing effects on TGF-β/Alk1/Smad1/5 function (23, 24, 47). We observed the activation of both pathways during late neointimal expansion, as reflected by the increased production of active TGF-β1 and the phosphorylation of Smad2/3 and Smad1. The bioactivities of these pathways in modulating the phenotype of neointimal cells remain to be defined.
In conclusion, the present study, together with our previous work, demonstrates that the neointima expands via early cellularized expansion and late extracellular matrix-dependent growth following vein graft implantation. The shift of the stoichiometry of TGF-β type I and type II receptors specifies a profibrotic function for TGF-β1 signaling pathway. Coupled with the reduced MMP-2 and -9 activity, the TGF-β/CTGF pathway promotes late fibrotic neointimal expansion in vein grafts. While interventions inhibiting TGF-β activities successfully attenuate early neointimal hyperplasia, further studies to evaluate the effects of TGF-β inhibition on late neointimal expansion are warranted so that the long-term benefits of anti-TGF-β therapy to improve the long-term performance of vein grafts can be clarified.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants 1R01-HL-079135-01 and 1K08-HL-04070-01A1; the Lifeline Foundation; an American Heart Association Scientist Development Grant; and the James and Esther King Biomedical Research Program.
REFERENCES
- 1.Berceli SA, Jiang Z, Klingman NV, Pfahnl CL, Abouhamze ZS, Frase CD, Schultz GS, Ozaki CK. Differential expression and activity of matrix metalloproteinases during flow-modulated vein graft remodeling. J Vasc Surg 39: 1084–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Berceli SA, Jiang Z, Klingman NV, Schultz GS, Ozaki CK. Early differential MMP-2 and -9 dynamics during flow-induced arterial and vein graft adaptations. J Surg Res 134: 327–334, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol 21: 473–482, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol 26: 1712–1720, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Boes M, Dake BL, Booth BA, Erondu NE, Oh Y, Hwa V, Rosenfeld R, Bar RS. Connective tissue growth factor (IGFBP-rP2) expression and regulation in cultured bovine endothelial cells. Endocrinology 140: 1575–1580, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Chase AJ, Newby AC. Regulation of matrix metalloproteinase (matrixin) genes in blood vessels: a multi-step recruitment model for pathological remodelling. J Vasc Res 40: 329–343, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 43: 742–751, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, Roberts JD, Fay WP, Birkedal-Hansen H, Holmbeck K, Sabeh F, Allen ED, Weiss SJ. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med 202: 663–671, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu M, Zhang J, Zhu X, Myles DE, Willson TM, Liu X, Chen YE. Peroxisome proliferator-activated receptor gamma inhibits transforming growth factor beta-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J Biol Chem 276: 45888–45894, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Garratt KN, Edwards WD, Kaufmann UP, Vlietstra RE, Holmes DR., Jr Differential histopathology of primary atherosclerotic and restenotic lesions in coronary arteries and saphenous vein bypass grafts: analysis of tissue obtained from 73 patients by directional atherectomy. J Am Coll Cardiol 17: 442–448, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Gentile AT, Mills JL, Westerband A, Gooden MA, Berman SS, Boswell CA, Williams SK. Characterization of cellular density and determination of neointimal extracellular matrix constituents in human lower extremity vein graft stenoses. Cardiovasc Surg 7: 464–469, 1999 [DOI] [PubMed] [Google Scholar]
- 14.George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH. Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation 101: 296–304, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Heaton NS, Wolff RA, Malinowski RL, Hullett DA, Hoch JR. Antisense to transforming growth factor-beta(1) facilitates the apoptosis of macrophages in rat vein grafts. J Vasc Res 45: 365–374, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hilker M, Buerke M, Lehr HA, Oelert H, Hake U. Bypass graft disease: analysis of proliferative activity in human aorto-coronary bypass grafts. Heart Surg Forum 5, Suppl 4: S331–S341, 2002 [PubMed] [Google Scholar]
- 17.Hoch JR, Stark VK, Turnipseed WD. The temporal relationship between the development of vein graft intimal hyperplasia and growth factor gene expression. J Vasc Surg 22: 51–58, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS, Ozaki CK, Berceli SA. A novel vein graft model: adaptation to differential flow environments. Am J Physiol Heart Circ Physiol 286: H240–H245, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, Schultz GS, Ozaki CK, Berceli SA. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol 293: H482–H488, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol 24: 54–60, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kallenbach K, Salcher R, Heim A, Karck M, Mignatti P, Haverich A. Inhibition of smooth muscle cell migration and neointima formation in vein grafts by overexpression of matrix metalloproteinase-3. J Vasc Surg 49: 750–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent KC, Liu B. Intimal hyperplasia—still here after all these years! Ann Vasc Surg 18: 135–137, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Khan R, Agrotis A, Bobik A. Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovasc Res 74: 223–234, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res 65: 599–608, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Loftus IM, Porter K, Peterson M, Boyle J, London NJ, Bell PR, Thompson MM. MMP inhibition reduces intimal hyperplasia in a human vein graft stenosis model. Ann NY Acad Sci 878: 547–550, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Loftus IM, Thompson MM. The role of matrix metalloproteinases in vascular disease. Vasc Med 7: 117–133, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest 88: 904–910, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1: 169–178, 2000 [DOI] [PubMed] [Google Scholar]
- 29.McCaffrey TA, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, Bush HL., Jr Decreased type II/type I TGF-beta receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-beta1. J Clin Invest 96: 2667–2675, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabel EG, Shum L, Pompili VJ, Yang ZY, San H, Shu HB, Liptay S, Gold L, Gordon D, Derynck R. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci USA 90: 10759–10763, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev 85: 1–31, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 190: 300–309, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Pannu J, Gardner H, Shearstone JR, Smith E, Trojanowska M. Increased levels of transforming growth factor beta receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum 54: 3011–3021, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Pannu J, Gore-Hyer E, Yamanaka M, Smith EA, Rubinchik S, Dong JY, Jablonska S, Blaszczyk M, Trojanowska M. An increased transforming growth factor beta receptor type I:type II ratio contributes to elevated collagen protein synthesis that is resistant to inhibition via a kinase-deficient transforming growth factor beta receptor type II in scleroderma. Arthritis Rheum 50: 1566–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Pannu J, Nakerakanti S, Smith E, ten DP, Trojanowska M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem 282: 10405–10413, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Roulot D, Sevcsik AM, Coste T, Strosberg AD, Marullo S. Role of transforming growth factor beta type II receptor in hepatic fibrosis: studies of human chronic hepatitis C and experimental fibrosis in rats. Hepatology 29: 1730–1738, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res 74: 196–206, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz SM, deBlois D, O'Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res 77: 445–465, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Smith JD, Bryant SR, Couper LL, Vary CP, Gotwals PJ, Koteliansky VE, Lindner V. Soluble transforming growth factor-beta type II receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ Res 84: 1212–1222, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Sohn M, Tan Y, Wang B, Klein RL, Trojanowska M, Jaffa AA. Mechanisms of low-density lipoprotein-induced expression of connective tissue growth factor in human aortic endothelial cells. Am J Physiol Heart Circ Physiol 290: H1624–H1634, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Southgate KM, Mehta D, Izzat MB, Newby AC, Angelini GD. Increased secretion of basement membrane-degrading metalloproteinases in pig saphenous vein into carotid artery interposition grafts. Arterioscler Thromb Vasc Biol 19: 1640–1649, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Wehrle-Haller B, Imhof BA. Integrin-dependent pathologies. J Pathol 200: 481–487, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Westerband A, Mills JL, Marek JM, Heimark RL, Hunter GC, Williams SK. Immunocytochemical determination of cell type and proliferation rate in human vein graft stenoses. J Vasc Surg 25: 64–73, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Wolff RA, Ryomoto M, Stark VE, Malinowski R, Tomas JJ, Stinauer MA, Hullett DA, Hoch JR. Antisense to transforming growth factor-beta1 messenger RNA reduces vein graft intimal hyperplasia and monocyte chemotactic protein 1. J Vasc Surg 41: 498–508, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wong AP, Nili N, Jackson ZS, Qiang B, Leong-Poi H, Jaffe R, Raanani E, Connelly PW, Sparkes JD, Strauss BH. Expansive remodeling in venous bypass grafts: novel implications for vein graft disease. Atherosclerosis 196: 580–589, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Yao Y, Zebboudj AF, Torres A, Shao E, Bostrom K. Activin-like kinase receptor 1 (ALK1) in atherosclerotic lesions and vascular mesenchymal cells. Cardiovasc Res 74: 279–289, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Zhang WD, Bai HZ, Sawa Y, Yamakawa T, Kadoba K, Taniguchi K, Masuda J, Ogata J, Shirakura R, Matsuda H. Association of smooth muscle cell phenotypic modulation with extracellular matrix alterations during neointima formation in rabbit vein grafts. J Vasc Surg 30: 169–183, 1999 [DOI] [PubMed] [Google Scholar]