Abstract
We examined the impact of coexpressing the inwardly rectifying potassium channel, Kir2.3, with the scaffolding protein, synapse-associated protein (SAP) 97, and determined that coexpression of these proteins caused an approximately twofold increase in current density. A combination of techniques was used to determine if the SAP97-induced increase in Kir2.3 whole cell currents resulted from changes in the number of channels in the cell membrane, unitary channel conductance, or channel open probability. In the absence of SAP97, Kir2.3 was found predominantly in a cytoplasmic, vesicular compartment with relatively little Kir2.3 localized to the plasma membrane. The introduction of SAP97 caused a redistribution of Kir2.3, leading to prominent colocalization of Kir2.3 and SAP97 and a modest increase in cell surface Kir2.3. The median Kir2.3 single channel conductance in the absence of SAP97 was ∼13 pS, whereas coexpression of SAP97 led to a wide distribution of channel events with three distinct peaks centered at 16, 29, and 42 pS. These changes occurred without altering channel open probability, current rectification properties, or pH sensitivity. Thus association of Kir2.3 with SAP97 in HEK293 cells increased channel cell surface expression and unitary channel conductance. However, changes in single channel conductance play the major role in determining whole cell currents in this model system. We further suggest that the SAP97 effect results from SAP97 binding to the Kir2.3 COOH-terminal domain and altering channel conformation.
Keywords: inward rectifier, postsynaptic density protein domain, channelosome, membrane cytoskeleton
in the heart, the inwardly rectifying potassium current, IK1, is responsible for stabilizing the resting membrane potential, determining excitation threshold, and for initiating the final repolarization process of the cardiac action potential (18). Three isoforms, Kir2.1, Kir2.2, and Kir2.3, can contribute to IK1 in the myocardium. Despite a high degree of amino acid identity, each isoform retains distinct regulatory and biophysical properties (4, 35). It has been shown that, in canine, sheep, and human myocardium, the Kir2.3 isoform is a predominant component of the atrial inward rectification mechanism and also contributes to ventricular IK1 (4, 20, 37). Moreover, although Kir2.1 is found primarily at the T tubules in canine ventricular cardiomyocytes, Kir2.3 preferentially localizes to the intercalated disc (20). Consequently, the response of Kir2.x channels to stimuli such as changes in extracellular potassium or pH in each cellular microdomain may differ because of the distinct biophysical and regulatory properties of Kir2.1 and Kir2.3.
In a native cell environment, ion channel proteins are often found in specific membranous domains as macromolecular complexes with scaffolding proteins. Members of the Kir2 subfamily (Kir2.1, Kir2.2, and Kir2.3; Kir2.x) have a COOH-terminal motif that enables interaction with PDZ domain-containing proteins (Fig. 1A). PDZ stands for PSD95 (postsynaptic density protein), discs-large (the Drosophila septate junction protein), and zona occludens-1 (the epithelial tight junction protein) (2). The subfamily of PDZ proteins called the membrane-associated guanylate kinase homologs (MAGUK) has been suggested to play important roles in regulating ion channels (19, 32), and synapse-associated protein 97 (SAP97; Fig. 1B), a cardiac MAGUK protein, has been shown to bind the COOH-terminal domain of Kir2.x (14, 15). However, for the most part, the mechanism(s) by which SAP97 might impact Kir2.3 channels is unknown.
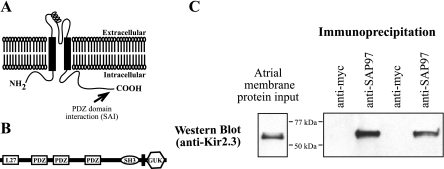
Fig. 1.
Synapse-associated protein (SAP) 97 and inwardly rectifying potassium channel (Kir) 2.3 coimmunoprecipitate from sheep atrial membranes. A: the PDZ binding domain of Kir2.3, residue SAI, is found at the extreme COOH-terminal end of the polypeptide. B: SAP97 is a member of the membrane-associated guanylate kinase homolog (MAGUK) family of scaffolding proteins and contains multiple protein-protein interaction domains such as L27, PDZ, and src homology 3 (SH3). GUK, guanylate kinase. C: sheep atrial membrane preparations were incubated with antibody (monoclonal anti-SAP97 or anti-myc as a control), antibody-protein complexes were isolated using protein G-Sepharose, and the resulting preparations were subjected to Western blot analysis with the anti-Kir2.3 antibody.
Association of MAGUK proteins with ion channels has been demonstrated to alter ion channel trafficking within the cell (8, 9, 17), and it has been suggested that binding to these proteins is important for anchoring the Kir2.x channels at the plasma membrane (15). The cellular localization of MAGUK proteins also varies, and ion channel-MAGUK protein interactions may be an important mechanism for determining the subcellular distribution of ion channels (22, 26). SAP97 is highly concentrated in the intercalated disc region of cardiac myocytes, although it is also found at the T tubules (16, 22), suggesting that it could play a role in Kir2.3 channel localization either by itself or in conjunction with additional scaffolding proteins.
The biophysical properties of ion channels also may change when bound to MAGUK proteins (24). Coexpression of Kir2.3 with Veli (Lin7) increased macroscopic current activity, and it was suggested that this resulted from decreased channel endocytosis (26). In contrast, whole cell currents were inhibited, and the unitary conductance was decreased when Kir2.3 channels were coexpressed with PSD95, another MAGUK protein (24). However, despite evidence that Kir2.3 and SAP97 colocalize in cardiac myocytes and bind to each other in vitro, it is not known if the interaction of Kir2.3 and SAP97 alters the properties of these channels. In this study, we examined the impact of coexpressing Kir2.3 with the scaffolding protein SAP97 and demonstrated that coexpression of these two proteins results in a twofold increase in whole cell currents. Further investigation determined that this increase in whole cell current was the result of modestly increased cell surface expression of Kir2.3 and a robust increase in unitary conductance.
MATERIALS AND METHODS
Cell culture and transfection.
Human embryonic kidney (HEK293) cells were obtained from the American Type Tissue Collection (Manassas, VA). HEK293 cells stably expressing guinea pig Kir2.3 were cultured as described previously (21). Cells were transiently transfected using Effectene (Qiagen, Hilden, Germany) following the manufacturer's protocols.
Plasmids.
A plasmid encoding a myc-tagged rat SAP97 was provided by Dr. Morgan Shen. SAP97-IRES-EGFP was provided by Dr. Nathalie Neyroud. PCR-based mutagenesis (Strategene QuickchangeII) was used to introduce an influenza hemagglutinin antigen (HA) epitope tag (YPYDVPYA) at codon 88 in guinea pig Kir2.3 (forward primer, 5′-cacggtgacctggagTACCCCTACGACGTGCCCGACTACGCCgccagcgcggcggtg-3′; reverse primer, 5′-caccgccgcgctggcggcgtagtcgggcacgtcgtaggggtactccaggtcaccgtg-3′) or to delete the three COOH-terminal codons (ΔSAI) in guinea pig Kir2.3 (forward primer, 5′-CTACCGCAGGGAATGAGCCATCTGA-3′; reverse primer, 5′-TCAGATGGCTCATTCCCTGCGGTAG-3′).
Antibodies.
Rabbit anti-Kir 2.3 polyclonal antibody was obtained from Alomone Labs (Jerusalem, Israel). Phycoerythrin (PE)-conjugated mouse anti-HA, and PE-conjugated isotype control were from Miltenyi Biotec. Monoclonal antibodies, obtained from the Developmental Studies Hybridoma Bank under the auspices of the National Institute of Child Health and Human Development, included: anti-human c-myc, 9E10, developed by J. Michael Bishop; anti-RhoB, 56.4H7, developed by Thomas Jessel; and anti-LAMP1, H4A3, developed by J. Thomas August and James E. K. Hidreth. The monoclonal anti-58K Golgi protein antibody was from Abcam (Cambridge, MA). Normal donkey serum and all secondary antibodies were from Jackson ImmunoResearch Laboratories. 4,6-Diamidino-2-phenylindole, dihydrochloride (DAPI) was from Invitrogen (Carlsbad, CA).
Immunocytochemistry.
Cells were fixed with 4% formaldehyde/PBS for 5 min, permeabilized with 0.1% Triton X/PBS (2 min), and then incubated with blocking buffer (10% normal donkey serum-0.05% Tween 20-PBS) for 1 h at room temperature. Preparations were sequentially incubated in primary antibody diluted in blocking buffer, washed with 0.05% Tween/PBS and then incubated with secondary antibodies. Cover slips were washed as above, briefly incubated with 300 nmol/l DAPI, and then mounted with gelvatol containing 0.2 mg/ml 1,4-diazobicyclo[2.2.2]octane. Images were obtained on a Zeiss Axioplan 2e imaging microscope using widefield fluorescent microscopy or optical sectioning with structured illumination (Apotome) (38).
Western blot.
Cells were washed with PBS, lysed in buffer (20 mmol/l Tris·HCl, 250 mmol/l NaCl, 3 mmol/l EDTA, pH 7.4, 1% Triton X-100, and 1% protease inhibitor mixture), and sonicated. Protein concentrations were determined using a modified Lowry assay (Bio-Rad). Cell lysates were separated on 7.5% SDS-PAGE gels and transferred to nitrocellulose. Nonspecific protein binding was blocked by incubation with 10% nonfat dry milk in PBS, and the blots were incubated with 0.5 μg/ml rabbit anti-Kir2.3 antibody (Alomone Laboratories) diluted in 5% BSA-0.025% NaN3-PBS. Following washing in PBS-0.05% Tween 20, the membranes were incubated with peroxidase-conjugated goat anti-rabbit antibody. Antigen complexes were visualized using enhanced chemiluminescence (Pierce).
Flow cytometry.
Cultures were washed with PBS, and cells were lifted from the dish using cold enzyme-free cell dissociation buffer (GIBCO-Invitrogen). Following washing with PBS (addition of 3 ml PBS followed by centrifugation at 500 g for 3 min), 1 × 106 cells were incubated on ice in blocking solution (5% IgG-free BSA) for 15 min. PE-conjugated anti-HA antibody, PE-congugated mouse IgG1, or buffer was added at the manufacturer's recommended concentrations. Cells were incubated on ice for 30 min, and then preparations were washed two times with PBS. Following the final wash, cell pellets were resuspended in 2% formaldehyde in PBS and analyzed (>10,000 events) with BD LSRII or FACSCalibur flow cytometers.
Immunoprecipitation.
Immunoprecipitation of sheep atrial membrane proteins was performed essentially as described (16). Purified mouse anti-SAP-97 antibody or mouse anti-myc (1 μg each) were used for immunoprecipitations using 400 mg of sheep atrial membrane proteins. Protein-antibody complexes were recovered using recombinant protein A/G-Sepharose (Pierce) and subjected to Western blot analysis as described above.
Electrophysiolgy.
Whole cell and single channel recordings were obtained as previously described (4). In the HEK293 cells stably expressing Kir2.3, transient expression of SAP97 was identified by the green fluorescent protein (SAP97-IRES-GFP). When Kir2.3 and SAP97 were transiently coexpressed, they were identified, respectively, by GFP and DsRED in the IRES vectors. Whole cell recordings were carried out at 35°C, and single channel recordings were carried out at room temperature (21–22°C). Pipette resistance was 1.5–2.5 MΩ for whole cell recordings and 8–12 MΩ for single-channel recordings. Data acquisition and analysis were carried out using the Axopatch 200B amplifier and the pCLAMP version 9 suite of programs (Axon Instruments, Union City, CA). To study whole cell Kir2.3 currents, voltage-clamp ramps (ramp rate = 25 mV/s) were applied from a test potential of −100 to 0 mV. Membrane potential was corrected for liquid-junction potential as previously described (4), and current-voltage plots were generated to reflect the command potential from the clamp amplifier (4). Currents were analyzed as BaCl2 (1 mmol/l)-sensitive currents to eliminate potential contamination by endogenous currents in HEK293 cells.
The degree of rectification for the Kir2.3 current was estimated as the relative chord conductance (Gc) in accordance with previous studies (4, 34). Gc was calculated as the ratio of the actual current and current predicted by assuming a linear unblocked current. The Gc vs. voltage relationship was fitted by the sum of two Boltzmann equations described by:
where V is the membrane potential, V1,2 are parameters representing the voltage at midpoint of rectification for the two components, and the sum of their respective amplitudes A1 and A2 are normalized to 1.0, i.e., A1 + A2 = 1.0. λ represents the slope factor and is equal to zF/RT, where z stands for the effective valency or steepness of rectification, F is Faraday constant, R is the gas constant, and T is the absolute temperature. We have previously demonstrated that the steepness of rectification is relatively shallow in Kir2.3 channels compared with the Kir2.1 isoform and that the effective valency can be described by two components (z1, z2) with relatively different extents of steepness (4). Single channel currents were recorded in the cell-attached configuration as previously published (4). Events were measured from and to baseline. To construct event (conductance) histograms, an approximately equal number of events were taken from each patch, thus ensuring that events from a particular patch do not skew the overall distribution of unitary events. Single channel traces (durations of 15–20 s) were used for estimating channel open probability (Po), using the equation: Po = ∑ i = 0N N(i·ti)/(T·N), where i is the number of unitary current levels displayed, t is the time at each current level, T is the total recording time, and N is the total number of current levels.
Solutions.
Bath solution for whole cell recordings contained (in mmol/l) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.33 NaH2PO4, and 5.0 HEPES; pH 7.4 (NaOH). The pipette filling solution for whole cell recordings contained (in mmol/l) 20 KCl, 90 potassium aspartate, 10 KH2PO4, 5.0 EDTA, 1.9 K2ATP, 5.0 HEPES, and 7.9 Mg2+; pH 7.2 (KOH). With the above concentration of EDTA, Mg2+ concentration is expected to be 1.1 mmol/l. The bath solution for cell-attached recordings contained (in mmol/l) 140 KCl, 1.8 CaCl2, 5 HEPES, and 0.33 NaH2PO4; pH 7.4 (KOH). The pipette solution for cell-attached recordings contained (in mmol/l) 140 KCl, 1 CaCl2, and 5 HEPES; pH 7.4 (KOH). For experiments involving acidification, the pH of the extracellular solution (pH = 6) was adjusted using 2-(N-morpholino) ethanesulfonic acid buffer (5 mM) as previously described (27).
Statistical analysis.
Differences between groups were compared with the Student's t-test, z-test or one-way ANOVA as appropriate. Differences were considered to be statistically significant when P < 0.05. Data are expressed as means ± SE.
RESULTS
Kir2.3 and SAP97 interact in sheep atrial myocardium.
Both Kir2.3 and SAP97 preferentially localize to the intercalated disc in atrial myocardium (16, 20, 22), and the COOH terminus of Kir2.3 binds to SAP97 in vitro (14, 16). To test whether these proteins physically interact in atrial tissue (directly or indirectly), we purified membrane proteins from sheep atria, immunoprecipitated SAP97 from this membrane preparation, and then determined if Kir2.3 copurified with SAP97. As shown in Fig. 1C, Kir2.3 copurified with SAP97, but the channel was absent when an irrelevant antibody (anti-myc tag) was used for the immunoprecipitation.
Kir2.3 whole cell currents increase in SAP97-expressing cells.
We next examined whole cell Kir2.3 currents following coexpression with SAP97 to determine the biophysical and regulatory consequences of their interaction. Whole cell Kir2.3 current density was recorded in the Kir2.3-expressing cell line after transient transfection of SAP97 or empty vector demonstrating that expression of SAP97 in the Kir2.3-expressing cells caused an approximately twofold increase in current density (Fig. 2). Average control peak inward current density was −12.42 ± 2.6 pA/pF in the Kir2.3-expressing cells, and coexpression of SAP97 increased current density to −29.32 ± 3.6 pA/pF (Fig. 2B). A double Boltzmann fit of Kir2.3 currents (Fig. 2C) showed that SAP97 did not significantly change current rectification [z1 = 7.21 ± 0.83 and z2 = 1.29 ± 0.21 for Kir2.3 (n = 5); z1 = 5.97 ± 0.29 and z2 = 1.2 ± 0.08 for Kir2.3 with SAP97 (n = 6)]. Moreover, the pH sensitivity of Kir2.3 (21) was unchanged in the presence of SAP97 [Fig. 2D; see also pH sensitivity of unitary Kir2.3 channels in the presence of SAP97, presented in Supplemental Fig. 1 (Supplemental material for this article may be found on the American Journal of Physiology: Heart and Circulatory Physiology website)].
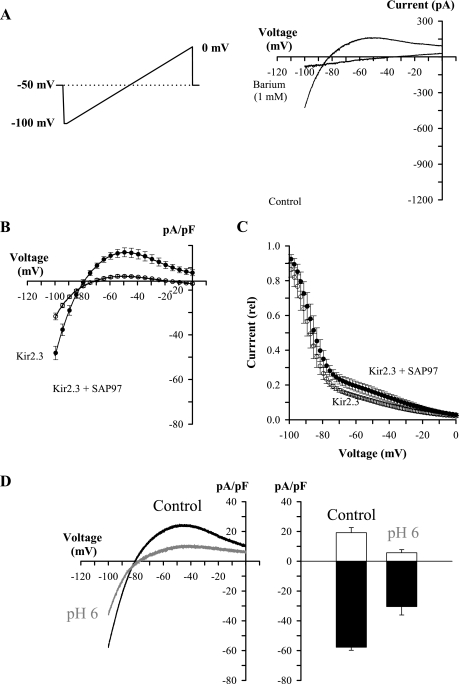
Fig. 2.
SAP97 increases whole cell Kir2.3 currents in HEK293 cells without changing rectification or pH sensitivity. A: Kir2.3 currents were measured as barium (1 mM)-sensitive currents (control current − barium current), and membrane potentials reflect command voltages of the amplifier. Inset: voltage protocol. B: Kir2.3 currents measured in the Kir2.3 stable cell line with or without coexpression with SAP97 (Kir2.3, N = 5; Kir2.3 + SAP97, N = 6). C: rectification of Kir2.3 current in control and following coexpression with SAP97 (Kir2.3, N = 5; Kir2.3 + SAP97, N = 6). D: Kir2.3 current in the presence of SAP97 is inhibited by decreased pHo. Left: current density in control and at pHo = 6.0. Right: average peak inward (filled bars) and outward (open bars) current density in control and at pHo = 6.0 (N = 3).
SAP97 interacts with Kir2.x by binding to a COOH-terminal motif (SE/AI) on the channel protein (14, 15). To test if SAP97's impact on Kir2.3 current required the COOH-terminal PDZ binding motif, we transiently transfected wild-type Kir2.3 and Kir2.3ΔSAI channels in HEK. As shown in Fig. 3A, mutant (Kir2.3ΔSAI) channels exhibited properties similar to wild-type channels. Similar to the results in Fig. 2, SAP97 increased Kir2.3 whole cell currents in cells expressing wild-type channels (Fig. 2B), but not Kir2.3ΔSAI channels (Fig. 2C). Moreover, neither SAP97 nor GFP induced any currents in HEK cells when expressed alone (Fig. 3D). To rule out the possibility of SAP97-induced current transients, these experiments were performed using voltage-clamp steps. The histograms in Fig. 3E summarize SAP97 effects on peak inward and peak outward current density in the experiments shown in Fig. 3, B–D.
Fig. 3.
Increase in Kir2.3 current requires SAP97 binding motif on channel COOH terminal. A: current density-voltage relationship of transiently transfected Kir2.3 wild-type and mutant (Kir2.3 ΔSAI) channels in HEK cells (Kir2.3 wild type N = 6; Kir2.3 ΔSAI N = 7). B: SAP97-induced increase in current wild-type Kir2.3 channels (Kir2.3 wild type N = 6; Kir2.3 wild type + SAP97 N = 5). C: SAP97 does not increase whole cell currents in Kir2.3 ΔSAI channels (Kir2.3 ΔSAI N = 7; Kir2.3 ΔSAI + SAP97 N = 5). D: transfecting SAP97 does not induce Kir2.3-like currents in HEK cells [green fluorescent protein (GFP) N = 4; GFP + SAP97 N = 6]. E: SAP97 effects on peak inward and peak outward current density from data presented in B–D.
The SAP97-induced increase in Kir2.3 whole cell currents could have resulted from changes in one or more of the following factors: number of functional channels in the cell membrane (N), unitary channel conductance (γ), and channel Po. Each of these factors has been examined in turn to determine which mechanism(s) is responsible for the increased whole cell current in the SAP97-expressing cells.
Kir2.3 is localized primarily in a vesicular compartment in the absence of SAP97.
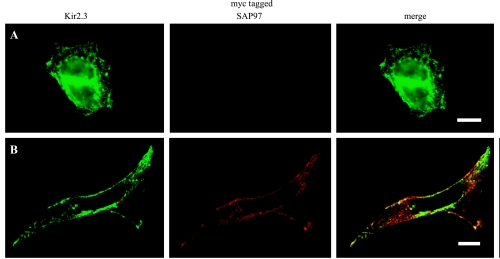
The work of others has demonstrated that binding to scaffolding proteins of the MAGUK family, such as SAP97, may alter the subcellular targeting of the ion channel proteins (13, 26, 36). Thus, coexpression of SAP97 could lead to more efficient targeting of Kir2.3 to the cell membrane or stabilization of Kir2.3 channels at the membrane with a resulting increase in the number of functional Kir2.3 channels. As a first step to address this, we used immunocytochemistry to determine the subcellular localization of Kir2.3 protein in the absence of SAP97. Although Kir2.3 channel protein could be detected at the cell periphery and regions of cell-cell contact, most channel protein in the Kir2.3 stable cell line was found throughout the cytoplasm in a punctate pattern that was reminiscent of vesicular staining (Fig. 4). These results demonstrate that, although Kir2.3 protein stably expressed in HEK293 cells is able to traffic correctly to the cell membrane (Fig. 2), at steady state most of the Kir2.3 protein is not at the cell surface. Using markers for early endosomes (early endosome antigen 1, EEA1), late/recyling endosomes (RhoB), and lysosomes (LAMP1), we have determined that in our model system Kir2.3 can be found in all three vesicular compartments (Fig. 4). In contrast, when SAP97 was introduced into the Kir2.3 stable cells, there was a dramatic redistribution of the Kir2.3 protein, leading to an almost complete loss of the punctate localization pattern and a prominent colocalization of the Kir2.3 and SAP97 proteins (Fig. 5). In some cells coexpressing SAP97, Kir2.3 expression at the cell periphery was increased. However, the targeting of SAP-97 and Kir2.3 to the plasma membrane was inefficient in this model system, with the vast majority of both proteins remaining distributed throughout the cytoplasm. Although partial overlap was seen with markers for Golgi and endoplasmic reticulum (data not shown), it did not appear that the Kir2.3 protein was localized to a cytoplasmic membranous compartment when coexpressed with SAP97 in HEK293 cells. The pattern most likely reflects endoplasmic reticulum localization and is consistent with the localization of SAP97 that has been described in neurons (31).
Fig. 4.
Prominent endosomal localization of channel protein is seen in the Kir 2.3 stable cell line. The colocalization of channel protein (green) in the Kir 2.3 cell line with antigen markers (red) for early endosomes [early endosome antigen 1 (EEA1; A), middle and recycling endosomes (RhoB; B), and lysosomes (LAMP1; C)] was determined using immunocytochemistry. Merged images show colocalization of a subset of Kir2.3 positive vesicles with each vesicle subcompartment (see arrows). The nucleus is counterstained with 4,6-diamidino-2-phenylindole, dihydrochloride (blue). Bar = 20 μm.
Fig. 5.
Transfection of myc-tagged SAP97 into Kir2.3-expressing cells results in decreased vesicular localization of the channel protein. The distribution of Kir2.3 was determined in Kir2.3 stable cells after the transient transfection of empty plasmid (A) or myc-tagged SAP97 (B). Bar = 10 μm. Optical sections (0.6 μm thick) were obtained using structured illumination microscopy.
Coexpression of SAP97 increases the cell surface expression of Kir2.3 channels.
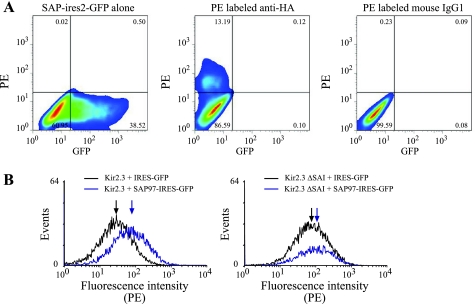
To determine if increased SAP97 expression altered cell surface abundance of Kir2.3, we used flow cytometry to analyze cell surface expression of HA-tagged Kir2.3 in HEK293 cells with or without cotransfection of SAP97. As shown in Fig. 6, coexpression of Kir2.3 and SAP97 results in a rightward shift in the fluorescence intensity, indicating a modest increase in cell surface expression of the channel protein. As expected, the SAP97-induced increase in Kir2.3 cell surface expression was attenuated when the COOH-terminal SAI-motif was deleted (Fig. 6).
Fig. 6.
Introduction of SAP97 in Kir2.3-expressing HEK cells increases channel protein abundance and cell surface expression. A: HEK cells were transfected with SAP97-IRES2-GFP or hemagglutinin antigen (HA)-tagged Kir2.3. Left: cells expressing SAP97-IRES2-GFP. Cells transfected with HA-tagged Kir2.3 were stained with either a PE-conjugated anti-HA antibody (middle) or a PE-conjugated isotype control antibody (right). The percentages of cells in each of the four quadrant gates is indicated. B: cell surface expression of Kir2.3 in HEK cells transiently transfected with HA-tagged Kir2.3 or HA-tagged Kir2.3ΔSAI and pIRES-GFP (black) or pSAP97-IRES-GFP (blue). Surface expression of Kir2.3 was detected using an PE-conjugated anti-HA antibody. Gating parameters were adjusted to display the surface expression of Kir2.3 in the GFP positive cells. The median fluorescence intensity for each population is indicated by an arrow. The data shown are representative of two separate experiments. Removal of the COOH-terminal PDZ-binding motif (HA-tagged Kir2.3ΔSAI) attenuated the SAP97-induced increase in cell surface expression in transiently transfected cells.
SAP97 increases the unitary conductance of Kir2.3 channels.
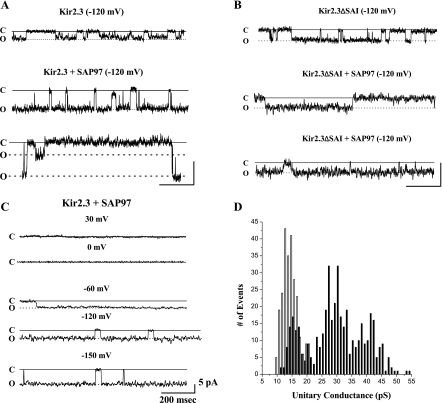
To determine if coexpression of SAP97 also altered the unitary conductance of Kir2.3 channels, we examined the single channel current properties of Kir2.3 channels coexpressed with SAP97. Figure 7A shows representative traces of cell-attached single channel events in patches from control cells (top) and in two different patches from cells in which Kir2.3 is coexpressed with SAP97 (traces in middle and bottom). The traces in Fig. 7A, middle, from SAP97-expressing cells show unitary conductance values of ∼30 pS, whereas the trace in Fig. 7A, bottom, shows two distinct current levels, suggesting that SAP97's effect on Kir2.3 channels is dynamic rather than static. Figure 7B shows unitary events in a patch from a cell expressing truncated Kir2.3 channel (top), and in two different patches from cells coexpressing the truncated channel and SAP97. Average unitary conductance of the truncated Kir2.3 channel was 16 ± 0.4 pS (n = 117), and was not significantly changed when coexpressed with SAP97 [18 ± 0.6 pS (n = 25)], suggesting that the deleted residues were important for the SAP97-induced effect on the channel. The large unitary conductance induced by coexpression of wild-type channel and SAP97 had voltage-dependent rectification properties typical of inward-rectifier channels (Fig. 7C). Figure 7D is a histogram for single channel events in the absence [no. of patches (N) = 6, number of events (n) = 326] and in the presence of SAP97 (N = 5, n = 371). In contrast to the single peak centered at 13 pS in the absence of SAP97, coexpression of SAP97 leads to a wide distribution of channel events from ∼14 to ∼50 pS, with three distinct peaks centered at 16, 29, and 42 pS. In another set of analyses, we examined whether SAP97 altered the Po of Kir2.3 channels. In control traces, each of ∼15–20 s duration, the mean Po value of 0.67 ± 0.10 (n = 3) is similar to previously reported values for this channel (40). In the presence of SAP97, measurements in four 20-s traces without burst activity gave mean Po values of 0.88 ± 0.03 (n = 4). Thus the SAP97-induced increase in Kir2.3 current did not significantly alter channel Po.
Fig. 7.
SAP97 increases the unitary conductance of Kir2.3 channels. A: representative trace of cell-attached single channel events from a control cell (top) and from two patches in cells in which Kir2.3 was coexpressed with SAP97 (middle and bottom). Scale bars 200 ms, 5 pA. B: unitary current events in a patch from a cell expressing truncated Kir2.3 channel (top), and from patches in two cells coexpressing the truncated channel and SAP97. Scale bars 400 ms, 5 pA. C: SAP97-induced unitary currents display voltage-dependent rectification similar to other Kir2 channels. D: histogram for single channel events in the Kir2.3 stable cell line (open bars) and in these cells following transient transfection of SAP97 (filled bars). Pipette potential (Vp) = +120 mV [Kir2.3: no. of patches (N) = 6, no. of unitary events (n) = 326 and Kir2.3 + SAP97: N = 5, n = 371].
DISCUSSION
The inwardly rectifying potassium channels, Kir2.1, Kir2.2, and Kir2.3, have a COOH-terminal PDZ domain binding motif (15), and numerous studies have shown that ion channel properties can be modulated by interactions with PDZ-containing MAGUK proteins (7, 22, 24, 26, 36). However, there are relatively few studies on the functional impact of Kir2.x channel association with these proteins. In this study, we have examined the impact of coexpressing Kir2.3 and SAP97, both of which are found in cardiac myocytes. We have demonstrated that the association of Kir2.3 with SAP97 in HEK293 cells results in increased whole cell current due to modestly increased cell surface expression of the channel and robustly increased unitary conductance.
Localization of ion channels to specific membrane domains.
Macromolecular complexes containing a different combination of ion channel/PDZ domain proteins have the potential to recruit or retain ion channels in specific cellular microenvironments. Kir2.3 localizes to the basolateral membrane in MDCK cells, a polarized epithelial cell line (13, 26), and the COOH-terminal PDZ-binding motif is required for this basolateral localization (26). Kir2.2 also localizes to the basolateral membrane in MDCK cells, and removal of its COOH-terminal PDZ-binding domain or coexpression of a dominant-negative PDZ domain protein are both sufficient to perturb basolateral localization (15). We have demonstrated that Kir2.3 and SAP97 interact in sheep atrial myocardium and that the coexpression of these two proteins in HEK293 cells alters the cellular distribution of Kir2.3 and increased cell surface expression of Kir2.3. Evidence from other potassium channels suggests that coexpression of SAP97 may help to stabilize ion channels at the cell surface, possibly by tethering the channel to the membrane cytoskeleton. When Kir4.1 was coexpressed with PSD95 or SAP97, whole cell currents increased two- to threefold without changes in either unitary conductance or channel Po, suggesting an increase in the number of functional channels in the membrane (7). Similar results were also reported for a voltage-gated potassium channel (Kv1.5) (22). For Kir2.x channels, direct association of the channels with the cortical cytoskeleton also has an impact on the number of functional channels in the membrane. Association of Kir2.1 with the actin-binding protein filamin increases whole cell current without changing Po or unitary conductance (30). In addition, forcing Kir2.1 interaction with the cortical cytoskeleton through the introduction of a heterologous protein 14-3-3 binding domain is sufficient to increase cell surface expression (33).
In total, these data suggest that tethering of Kir2.x channels to the membrane cytoskeleton, either through direct binding to cytoskeletal proteins or indirectly through interactions with PDZ domain proteins, may represent an important mechanism for regulating channel number and location. However, in the HEK293 cell expression system used in this study, increased Kir2.3 cell surface expression in the presence of SAP97 does not appear to be the major mechanism underlying the increased current in Kir2.3 cells coexpressing SAP97. We suggest that this may be due to the relatively inefficient targeting of SAP97 itself to the cell surface in HEK293 cells compared with that seen in polarized epithelial cells or cardiac myocytes in situ. Coexpression of additional MAGUK proteins, such as CASK-1, may be required for efficient cell surface localization of SAP97 in HEK293 cells (12).
Physical interactions at the COOH terminus may regulate channel properties.
In general, members of the Kir family are composed of four subunits, and each subunit has two transmembrane domains (M1 and M2), cytoplasmic NH2 and COOH termini, and a pore-forming structure between M1 and M2 (11). A very significant portion (∼70%) of the channel protein forms a cytoplasmic pore important for intracellular channel regulation and rectification (35). The structure of the cytoplasmic NH2- and COOH-terminal domains of murine Kir2.1 was recently described and placed on the KirBac1.1 structure (27). This structure demonstrated that the extreme COOH terminus of Kir2.1 (last 57 residues), which contains the PDZ binding motif, is disordered and protrudes from the rest of the cytoplasmic aspect of the channel. Moreover, it was suggested that binding to cytoplasmic factors may stabilize this extreme COOH-terminal domain (27). The high degree of amino acid homology between Kir2.1 and Kir2.3 (78% homology overall, 82% over the entire COOH-terminal domain) suggests that the extreme COOH terminus of Kir2.3 might also protrude from the rest of the cytoplasmic domain.
Results from our electrophysiological experiments show that whole cell and unitary properties of Kir2.3 were modified by SAP97 and that the effect on whole cell current was blunted when the PDZ-binding motif on the channel was removed. Thus, consistent with an increase in macroscopic Kir2.3 current, the microscopic conductance increased in the presence of SAP97, with relatively little effect on channel Po. Although Kir2.3 channels exhibited “burst” opening in the presence of SAP97, a mode unusual for wild-type Kir2 channels (35, 40), the rectification properties of Kir2.3 current and pH sensitivity were not changed by SAP97 (Fig. 2, C and D). In addition, no endogenous currents were activated when HEK293 cells were transfected with SAP97 (Fig. 3). We have demonstrated that coexpression of SAP97 and Kir2.3 in HEK293 cells increases the unitary conductance of the channel. In contrast, coexpression of PSD95, another PSD domain protein, with Kir2.3 channels decreased unitary conductance (24). This suggests that tethering the Kir2.3 COOH-terminal domain through binding to MAGUK proteins can alter ion flux through the channel pore but whether conductance increases or decreases may depend on the protein bound. Although PSD95 and SAP97 have highly conserved primary sequence, they form distinctly different structures in solution. Although PSD95 forms a horseshoe-shaped monomer with dimensions of ∼100 × 60 Å, SAP97 is preferentially found in an extended conformation that can oligomerize via its NH2-terminal L27 domain (23). There is ample evidence that the geometry of an ion channel vestibule and charge density can influence ion permeation (conductance and selectivity) through the channel pore (3, 6, 10), and it is tempting to speculate that the SAP97 effect that we report results from SAP97 binding to the Kir2.3 COOH terminus and altering channel conformation. Thus binding of PSD95 to the extreme COOH terminus of Kir2.3 may be sufficient to impede the flux of K+ out of the cytoplasmic vestibule. On the other hand, we predict that binding of the elongated SAP97 to the channel would be less likely to restrict the flow of ions from the cytoplasmic pore and may stabilize in a more “open” configuration either the cytoplasmic vestibule or the girdle formed by the G-loops. Future studies can be designed to test this prediction. Taken together, results from our experiments would suggest that there are multiple levels for the regulation of Kir2.x channels, in addition to the distinct properties of the individual isoforms (i.e., pH sensitivity and rectification). All of these mechanisms may exist to determine how the classical inward-rectifier channel current responds to changes in the cellular environment in normal and pathophysiological conditions.
Physiological significance.
The role of IK1 in maintaining the resting membrane potential, determining excitation threshold, and initiating the final repolarization process of the cardiac action potential has suggested that perturbations altering this current could be arrhythmogenic. In fact, increased IK1 in a transgenic mouse model leads to very stable episodes of ventricular fibrillation (25), and gain of function mutations in Kir2.1 have been identified for short QT syndrome 3 (28) and familial atrial fibrillation (39). Potentially, any modification that sufficiently increases (or decreases) IK1 also could be arrhythmogenic. Modification of Kir2.x channel-scaffolding protein interactions may modulate channel retention time in the membrane, or directly modify one or more biophysical properties of the channel such as its conductance. We speculate that these modifications may occur dynamically under conditions that lead to protein kinase A activation (16) or chronically during the ionic remodeling that occurs during heart failure (29) or chronic atrial fibrillation (5). It is noteworthy that SAP97 has been suggested to similarly modulate another potassium channel (Kv1.5) in cardiac myocytes (1). These observations clearly justify the need to better understand how individual components of the Kir2.x macromolecular complex contribute to ion channel function.
Limitations of the study.
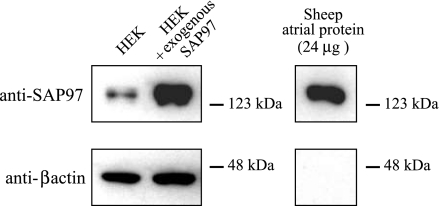
We have used an HEK293 cell expression system to examine the functional impact of the association of Kir2.3 with SAP97. The absence of any significant expression of endogenous inwardly rectifying currents and the relatively low levels of endogenous SAP97 render these cells a useful heterologous expression system into which specific combinations of channel and scaffolding protein can be introduced. In the HEK293 cell system, the proteins of interest are expressed at extremely high levels (see Ref. 21 and Fig. 8), resulting in a highly skewed stoichiometry between the expressed ion channel and any endogenous scaffolding proteins. Although this does not negate the impact of any endogenous scaffolding proteins, it does minimize their impact. The heterologous expression system simplifies the study of individual components of the much more complex macromolecular complexes found in cardiac myocytes. However, it clearly does not reproduce the cellular environment of the cardiac myocyte, and care must be taken in extrapolating data obtained using HEK293 to the native cell environment.
Fig. 8.
Total amount of SAP98 in SAP97-transfected HEK cells was compared with the endogenous SAP97 levels in this cell line using Western blot analysis, with sheep atrial homogenate serving as a positive control. In all samples, the anti-SAP97 antibody detected a single band of the appropriate size. As expected, SAP97-transfected HEK cells expressed substantially more of the scaffolding protein than the nontransfected cells, with expression levels comparable to those seen in 24 μg of sheep atrial homogenate. For this amount of sheep atrial proteins, β-actin immunoreactivity was not detectable at the exposure used for the HEK cell samples.
GRANTS
The work was supported by National Heart, Lung, and Blood Institute Grants 1R01-GM-076608, 1R01HL-080159 and 2PO1HL-039707.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yijuan Lin for expert technical assistance.
Current addresses: K. L. Vikstrom, R. Vaidyanathan, and J. M. B. Anumonwo, Department of Internal Medicine, Center for Arrhythmia Research, University of Michigan, Ann Arbor, MI, 48108; and R. O'Connell, Department of Molecuar and Integrative Physologogy, University of Michigan, Ann Arbor, MI 48108.
REFERENCES
- 1.Abi-Char J, El Haou S, Balse E, Neyroud N, Vranckx R, Coulombe A, Hatem SN. The anchoring protein SAP97 retains Kv1.5 channels in the plasma membrane of cardiac myocytes. Am J Physiol Heart Circ Physiol 294: H1851–H1861, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Brone B, Eggermont J. PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am J Physiol Cell Physiol 288: C20–C29, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cai M, Jordan PC. How does vestibule surface charge affect ion conduction and toxin binding in a sodium channel? Biophys J 57: 883–891, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JMB. Unique Kir2. x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res 94: 1332–1339, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Dobrev D, Wettwer E, Kortner A, Knaut M, Schuler S, Ravens U. Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res 54: 397–404, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. J Biol Chem 272: 12885–12888, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Hruska-Hageman AM, Benson CJ, Leonard AS, Price MP, Welsh MJ. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+-gated current. J Biol Chem 279: 46962–46968, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Jugloff DGM, Khanna R, Schlichter LC, Jones OT. Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J Biol Chem 275: 1357–1364, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Khakh BS, Lester HA. Dynamic selectivity filters in ion channels. Neuron 23: 653–658, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300: 1922–1926, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Fan S, Makarova O, Straight S, Margolis B. A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol 22: 1778–1791, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Maout S, Welling PA, Brejon M, Olsen O, Merot J. Basolateral membrane expression of a K+ channel, Kir 2.3, is directed by a cytoplasmic COOH-terminal domain. Proc Natl Acad Sci USA 98: 10475–10480, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, III, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2. x)-associated proteins. J Biol Chem 279: 22331–22346, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA. A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels. J Biol Chem 279: 19051–19063, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Leonoudakis D, Mailliard W, Wingerd K, Clegg D, Vandenberg C. Inward rectifier potassium channel Kir2.2 is associated with synapse-associated protein SAP97. J Cell Sci 114: 987–998, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Skeberdis VA, Francesconi A, Bennett MVL, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci 24: 10138–10148, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopatin AN, Nichols CG. Inward rectifiers in the heart: an update on I(K1). J Mol Cell Cardiol 33: 625–638, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res 69: 798–807, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Melnyk P, Zhang L, Shrier A, Nattel S. Differential distribution of Kir2.1 and Kir23 subunits in canine atrium and ventricle. Am J Physiol Heart Circ Physiol 283: H1123–H1133, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Munoz V, Vaidyanathan R, Tolkacheva EG, Dhamoon AS, Taffet SM, Anumonwo JM. Kir2.3 isoform confers pH sensitivity to heteromeric Kir2.1/Kir23 channels in HEK293 cells. Heart Rhythm 4: 487–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata M, Buckett PD, Zhou J, Brunner M, Folco E, Koren G. SAP97 interacts with Kv1.5 in heterologous expression systems. Am J Physiol Heart Circ Physiol 281: H2575–H2584, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron 44: 453–467, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Nehring RB, Wischmeyer E, Doring F, Veh RW, Sheng M, Karschin A. Neuronal inwardly rectifying K(+) channels differentially couple to PDZ proteins of the PSD-95/SAP90 family. J Neurosci 20: 156–162, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noujaim SF, Pandit SV, Berenfeld O, Vikstrom K, Cerrone M, Mironov S, Zugermayr M, Lopatin AN, Jalife J. Up-regulation of the inward rectifier K+ current (I K1) in the mouse heart accelerates and stabilizes rotors. J Physiol 578: 315–326, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen O, Liu H, Wade JB, Merot J, Welling PA. Basolateral membrane expression of the Kir 2.3 channel is coordinated by PDZ interaction with Lin-7/CASK complex. Am J Physiol Cell Physiol 282: C183–C195, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. Cytoplasmic domain structures of Kir2.1 and Kir31 show sites for modulating gating and rectification. Nature Neurosci 8: 279–287, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 96: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, O'Rourke B, Kass DA, Marban E, Tomaselli GF. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol 288: H2077–H2087, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson LJ, Leyland ML, Dart C. Direct interaction between the actin-binding protein filamin-A and the inwardly rectifying potassium channel, Kir2.1. J Biol Chem 278: 41988–41997, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci 21: 7506–7516, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 24: 1–29, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Shikano S, Coblitz B, Sun H, Li M. Genetic isolation of transport signals directing cell surface expression. Nature Cell Biology 7: 985–992, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Shyng SL, Sha Q, Ferrigni T, Lopatin AN, Nichols CG. Depletion of intracellular polyamines relieves inward rectification of potassium channels. Proc Natl Acad Sci USA 93: 12014–12019, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanfield PR, Nakajima S, Nakajima Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir30. Rev Physiol Biochem Pharmacol 145: 47–179, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K+ channel surface expression and clustering. J Cell Biol 148: 147–158, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation 98: 2422–2428, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Weigel A, Schild D, Zeug A. Resolution in the ApoTome and the confocal laser scanning microscope: comparison. J Biomed Opt 14: 014022, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J, Chen YA. Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun 332: 1012–1019, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol (Lond) 516: 699–710, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.