Abstract
To define the necessity of calcineurin (Cn) signaling for cardiac maturation and function, the postnatal phenotype of mice with cardiac-specific targeted ablation of the Cn B1 regulatory subunit (Ppp3r1) gene (csCnb1−/− mice) was characterized. csCnb1−/− mice develop a lethal cardiomyopathy, characterized by impaired postnatal growth of the heart and combined systolic and diastolic relaxation abnormalities, despite a lack of structural derangements. Notably, the csCnb1−/− hearts did not exhibit diastolic dilatation, despite the severe functional phenotype. Myocytes isolated from the mutant mice exhibited reduced rates of contraction/relaxation and abnormalities in calcium transients, consistent with altered sarcoplasmic reticulum loading. Levels of sarco(endo) plasmic reticulum Ca-ATPase 2a (Atp2a2) and phospholamban were normal, but phospholamban phosphorylation was markedly reduced at Ser16 and Thr17. In addition, levels of the Na/Ca exchanger (Slc8a1) were modestly reduced. These results define a novel mouse model of cardiac-specific Cn deficiency and demonstrate novel links between Cn signaling, postnatal growth of the heart, pathological ventricular remodeling, and excitation-contraction coupling.
Keywords: calcium signaling, restrictive cardiomyopathy, cardiac hypertrophy, excitation-contraction coupling, cardiac mitochondria
derangements in cardiac myocyte calcium (Ca2+) homeostasis are linked to the development of heart failure (4). In addition to its role in excitation-contraction coupling, Ca2+ serves important functions in cellular signaling and metabolism. The serine-threonine phosphatase, calcineurin (Cn), is an important transducer of Ca2+ to cellular signaling events. The active Cn holoenzyme consists of a complex, including calmodulin and the Cn A and B subunits. This holoenzyme is activated by sustained elevation of intracellular Ca2+ levels, such as occurs in working striated muscle (37), and acts to regulate transcription of genes via activation of the nuclear factor of activated T cells family of transcription factors (9, 16). Cn has been implicated in the programs controlling pathological cardiac hypertrophic growth and remodeling (14, 42), skeletal muscle fiber-type determination (31), myocyte excitation-contraction coupling (7), and mitochondrial energy metabolism (40).
A role for Cn in modulation of cardiac hypertrophic growth has been the subject of numerous studies. Cardiac-specific overexpression of constitutively active CnA (Cn*) in mice causes profound cardiac hypertrophy, dysfunction, and death (28). Cardiac hypertrophy in response to pressure overload is attenuated following inhibition of Cn, whether pharmacologically (20) or by overexpression of Cn inhibiting proteins (10, 36). As a downstream effector of Ca2+ signaling, Cn also acts to regulate the activity of proteins involved in excitation-contraction coupling. Cn has been reported to modulate the activity of sarco(endo)plasmic reticulum Ca-ATPase (SERCA2a) (29), the ryanodine receptor (7), and phospholamban (PLN) (22).
The role of Cn in normal physiological processes in the heart and its downstream targets has not been fully elucidated (27, 42). Loss-of-function studies are necessary to delineate the biological roles of Cn signaling in vivo. Global Cn loss of function, via deletion of the CnB regulatory subunit, led to embryonic lethality due to failure of vascular patterning in the mouse embryo (13). To avoid this problem, Cn loss-of-function mouse models, in which either the CnAα or the CnAβ subunit has been deleted, have been generated (6, 45). The CnA subunit has three isoforms, α, β and γ, of which α and β are ubiquitously expressed. Deletion of CnAα had no reported cardiac phenotype (34, 45). CnAβ−/− mice also did not exhibit an overt cardiac phenotype at baseline (6). Thus, in both cases, the presence of the other CnA isoform was sufficient to prevent the embryonic lethality seen in the global CnB deletion. However, the CnAβ−/− mice were unable to mount a response to pathological hypertrophic stimuli (6). Additionally, when crossed with a muscular dystrophy mouse model, CnAβ−/− mice restored cardiac function, providing additional support for a role for Cn signaling in pathological cardiac remodeling (32).
To further define the biological function of Cn signaling in the postnatal heart, we sought to develop mice with cardiac-specific Cn deficiency. To this end, we developed an independent line of mice with cardiac-restricted deletion of the Cnb1 gene using Cre recombinase driven by the α-myosin heavy chain gene promoter [csCnb1−/− mice (40)]. This strategy targets the Cnb1 subunit, which does not have functionally redundant isoform relatives in heart. Gene expression studies in these mice conducted in the early postnatal period revealed that genes involved in myocyte fatty acid oxidation were downregulated, consistent with the known role of Cn as an activator of the transcriptional coactivator peroxisome proliferator-activated receptor coactivator-1α (PGC-1α), a master regulator of cellular energy metabolism (40). In this study, we have conducted rigorous cardiac phenotyping studies of csCnb1−/− mice from neonatal to adult stages. We found that Cn signaling is critical for normal postnatal cardiac growth and function, as well as survival. Specifically, loss of Cn signaling results in reduced postnatal heart growth and a severe cardiomyopathy, with some features characteristic of restrictive physiology. The contractile phenotype of the Cn-deficient mice is associated with derangements in excitation-contraction coupling related to altered activity and expression of several key cellular Ca2+ pumps. These results demonstrate the importance of Cn signaling in postnatal heart maturation and in the regulation of myocyte Ca2+ homeostasis. The csCnb1−/− mice should prove a useful model of Cn signaling in the adult myocardium.
MATERIALS AND METHODS
Transgenic mice.
Transgenic mice expressing Cre-recombinase under control of the α-myosin heavy chain promoter (1) were crossed with mice in which exons 3–5 of the gene encoding the regulatory subunit of Cn (Ppp3r1) are flanked by loxp sites (30) to generate cardiac-specific Cn bl null (csCnb1−/−) mice. The final breeding resulted in the experimental mice, which all possessed the floxed allele (homozygous), but only 50% possessed Cre. Control mice possessed the floxed (Ppp3r1) gene (homozygous), but lacked the Cre transgene. Mice possessing the Cre transgene alone were also included in initial studies to ensure that expression of Cre did not confer a cardiac phenotype. Mice were euthanized by carbon dioxide inhalation, and the hearts were harvested for subsequent analysis. All animal experiments and euthanasia protocols were conducted in accordance with the National Institutes of Health guidelines for humane treatment of laboratory animals and were reviewed and approved by the Institutional Animal Care and Use Committee of Washington University School of Medicine.
mRNA analysis.
Total RNA was isolated from left ventricular (LV) tissue of 3-mo-old male mice by the RNAzol method (Tel-Test, Friendswood, TX), as described previously (18). RNA was quantified spectrophotometrically, reverse transcription was performed, and quantitative PCR was carried out on a 7500 Sequence Detector (Applied Biosystems, Foster City, CA) using the mouse-specific primer-probe sets shown in Supplemental Table 1. (The online version of this article contains supplemental data.)
Protein analysis.
Protein extracts from cardiac tissue of 3-mo-old male mice were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Western blotting was performed using antibodies against SERCA2 (Affinity Bioreagents, Golden, CO), Na/Ca exchanger (Swant, Bellinzona, Switzerland), PLN (Zymed, Carlsbad, CA), phospho-PLN (Cyclacel, Dundee, UK), and actin (Sigma Chemicals, St. Louis, MO). Detection was performed by measuring chemiluminescent signal, as assayed by enhanced chemiluminescence (Amersham, Pittsburgh, PA).
Histological analysis.
After death of 2- and 4-mo-old male mice, ventricular and atrial tissue were removed, fixed in 10% formalin overnight, dehydrated in graded concentrations of alcohol, and embedded in paraffin from which 5-μm sections were prepared. Tissue sections were stained with hematoxylin-eosin or Masson's trichrome by the Morphology Core at Washington University School of Medicine run by the Digestive Diseases Research Core Center.
Echocardiography and pressure catheter analysis.
Serial transthoracic echocardiography and cardiac catheterization were performed, as described previously (35). For echocardiography, 1-, 2-, and 4-mo-old male mice were anesthetized with an intraperitoneal injection of 0.01 ml of 2.5% Avertin per gram of body weight (BW). Tissue Doppler imaging (TDI) was used to assess LV diastolic function. Transmitral flow velocity was obtained from a modified apical four-chamber view with the pulse-wave Doppler sample volume positioned at the ventricular side of the mitral valve leaflets. Mitral annulus velocity measurement was performed from the same four-chamber view by placing the TDI sample volume on the lateral portion of the mitral annulus. Digitally acquired echocardiographic images were analyzed offline by two observers blinded to the genotype of the animals.
For cardiac catheterization, 3-mo-old male mice were anesthetized with a mixture of xylazine (16 mg/kg) and ketamine (80 mg/kg). Closed-chest cardiac catheterization was performed by identifying and cannulating the right carotid artery and advancing a 1.4F Millar catheter into the ascending aorta, where it was secured. Hemodynamic measurements were then recorded. After acquisition of baseline data, dobutamine was serially infused at rates of 2, 4, 8, 16, and 32 ng·g BW−1·min−1. Echocardiography and catheterization were performed by the Mouse Cardiac Phenotyping Core in the Center for Cardiovascular Research at Washington University School of Medicine.
Mitochondrial respiration.
Mitochondria were isolated from both ventricles of 3-mo-old mice using a trypsin digestion procedure, as previously described (38). Briefly, tissue was minced, washed, and suspended in isolation medium (300 mM sucrose, 0.2 mM EDTA, 10 mM HEPES, pH = 7.4). Following digestion, tissues were gently homogenized with a Teflon-glass homogenizer (Eberbach, Ann Arbor, MI). Following centrifugation and washes, isolated mitochondria were resuspended in suspension buffer (230 mM mannitol, 70 mM sucrose, 0.02 mM EDTA, 20 mM Tris·HCl, 5 mM K2HPO4, pH = 7.4). Total protein was quantified by a bicinchoninic acid assay (Pierce, Rockford, IL), mitochondria (300 μg protein/ml) were placed in respiration media (125 mM KCl, 20 mM HEPES, 3 mM Mg-acetate, 0.4 mM EGTA, 0.3 mM dithiothreitol, 5 mM KH2PO4, 0.2% BSA, pH = 7.1) containing malate (5 mM) and either palmitoylcarnitine or pyruvate (10 mM), and respiration was determined as previously described (43) at 25°C using an optical oxygen sensor (FOXY Probe, Ocean Optics, Dunedin, FL). Following measurement of basal respiration, maximal (ADP-stimulated) respiration was determined by adding 1 mM ADP. Uncoupled respiration was evaluated following addition of oligomycin (1 μg/ml). The solubility of oxygen in the respiration buffer at 25°C was taken as 246.87 nmol O2/ml. Respiration rates were expressed as nanomoles of O2 per minute per milligram of protein.
Measurement of myocyte contractility and Ca2+ transients.
Ventricular myocytes were isolated from 3-mo-old female mice, and single-cell contraction and Ca2+ transients were measured as described (12, 19). Briefly, unloaded cells were maintained with normal Tyrode solution and stimulated with bipolar stimulating electrodes. A video edge detection system (Crescent Electronics, Sandy, UT) was used to measure myocyte length, and data were collected and analyzed using Axon Instruments (Molecular Devices, Sunnyvale, CA). Isolated myocytes were incubated 20 min at room temperature (24 ± 2°C) with 10 μM Fluo-LOJO (a leakage-resistant version of Fluo-4 acetoxymethyl ester, TefLabs, Austin, TX). Cells were superfused with 0.9 mM Ca2+ normal Tyrode solution for 20 min to wash out excess indicator and allow deesterification. Cells were field stimulated at 0.5 Hz until steady state, followed by a rapid application of 10 mM caffeine to release sarcoplasmic reticulum (SR) Ca2+ and to measure Ca2+ extrusion via Na/Ca exchanger (3). Fluo-LOJO was excited at 490 ± 3 nm and emitted fluorescence measured at 530 ± 20 nm. Background fluorescence (F) was subtracted before F/F0 was calculated (where F0 is diastolic F under control conditions). Intracellular Ca concentration ([Ca]) decline is based on single-exponential (τ) curve fits.
Statistical analysis.
The data are presented as means ± SE. One-way analysis of variance was used to evaluate heart mass and isolated cell mechanics. Individual mean differences were assessed using the Student-Newman-Keuls method. Unpaired t-tests were used for evaluating the effect of Cre-recombinase on gene expression, echocardiographic analysis, or mitochondrial respiration. Analysis of covariance was used to evaluate the effect of genotype and dobutamine infusion for cardiac catheterization studies. An α-level of 0.05 was used to indicate statistical significance.
RESULTS
Abnormal postnatal cardiac growth and progressive cardiomyopathy in csCnb1−/− mice.
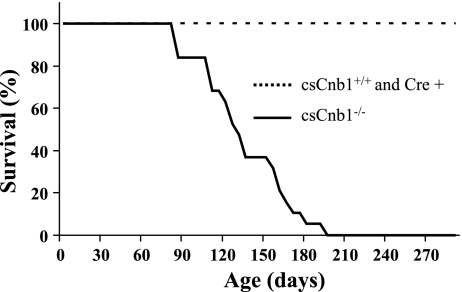
Mice with cardiac-specific disruption of the gene encoding the Cn regulatory subunit B1 (csCnb1−/− mice) were generated by crossing mice possessing two floxed Ppp3r1 alleles with mice expressing Cre recombinase under control of the α-myosin heavy chain (MHC) promoter (40). Our initial analysis of the csCnb1−/− mice involved phenotypic characterization within the first 2 mo of life (40). By 3 wk of age, Cnb1 expression is reduced to ∼15% of normal in whole hearts of the csCnb1−/− mice. At 2 mo of age, csCnb1−/− mice appeared grossly normal without evidence of neonatal lethality, based on Mendelian ratios (Ref. 40 and data not shown). Beginning at 2½ mo of age, the csCnb1−/− mice exhibited increased postnatal mortality compared with mice homozygous for the floxed allele, but lacking Cre (Cnb1+/+ mice) or MHC-Cre control mice (Fig. 1). Approximately 50% of the csCnb1−/− mice died by 4 mo of age, with 100% mortality by age 7 mo (Fig. 1). Additionally, of 14 female csCnb1−/− breeders, none survived beyond 2 wk postpartum. In those few animals in which we observed mortality, death occurred following a period (<1 day) of lethargy. None of the MHC-Cre mice exhibited death or cardiac dysfunction by echocardiography (data not shown), and there was no increase in mortality during the study period (Fig. 1). Continuous telemetric electrocardiographic monitoring revealed no significant rhythm disturbances (data not shown).
Fig. 1.
csCnb1−/− mice [mice with cardiac-restricted deletion of the calcineurin (Cn) b1 gene; solid line, n = 19] exhibit increased mortality compared with control mice [dashed line; csCnb1+/+, n = 5; myosin heavy chain-Cre+ (Cre+) n = 5]. Graph represents percent survival through 9 mo of life. Early mortality begins at 2½ mo of age, and by 6 mo of age nearly all csCnb1−/− mice died.
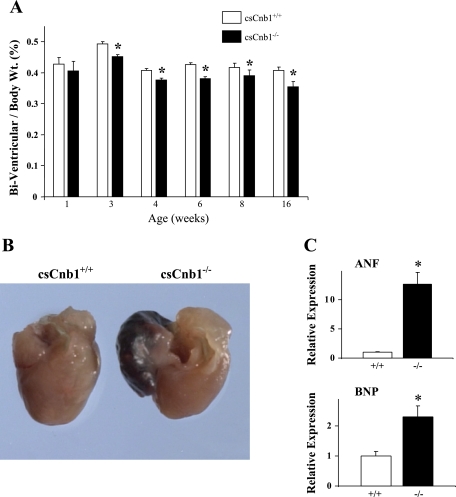
Cardiac biventricular (BV) to BW ratios (BV/BW) were normal in 1-wk-old csCnb1−/− mice, consistent with the maintenance of Cnb1 expression during the first week of life. However, on loss of Cnb1 protein expression, and throughout the remainder of life, the mean BV/BW in csCnb1−/− mice was ∼10% less than that of littermate csCnb1+/+ controls (Fig. 2A). Total BW was not different among the groups (data not shown). The gross appearance of hearts from 3- to 6-wk-old csCnb1−/− mice was normal, except that the ventricles of the mutant mice were slightly smaller (data not shown). However, beginning at 2–3 mo of age, the atria became massively dilated in the absence of ventricular dilatation (Fig. 2B). Although ventricular weights were less than normal, expression of the ANF and BNP genes was elevated in the ventricles of csCnb1−/− mice (Fig. 2C).
Fig. 2.
Hearts of csCnb1−/− mice exhibit postnatal ventricular growth retardation with enlarged atria. A: mean biventricular-to-body weight ratios following birth for csCnb1+/+ (open bars, n = 10) and csCnb1−/− (solid bars, n = 12) mice. B: representative photos of hearts from csCnb1−/− mice and age-matched controls. Beginning at 3–4 mo of age, enlarged atria are seen. C: mRNA levels for atrial natriuretic factor (ANF; top) and brain natriuretic peptide (BNP; bottom), markers of the fetal gene program, in 2-mo-old csCnb1−/− (−/−) and csCnb1+/+ (+/+) controls (n = 9). Bars represent mean mRNA expression (±SE), normalized to 18S signal using real-time quantitative PCR. *P < 0.05 compared with controls.
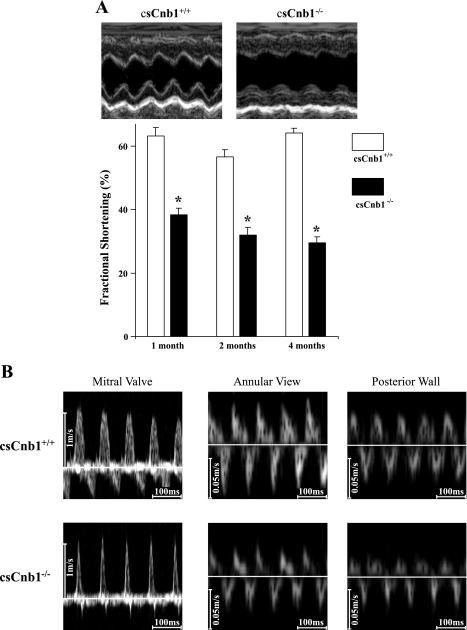
Echocardiographic analysis revealed marked reduction in systolic function and a decrease in relative wall thickness in csCnb1−/− mice as young as 1 mo of age (Fig. 3A and Supplemental Table 2). Strikingly, however, the ventricles of the mutant mice did not exhibit diastolic dilatation, even at 4 mo of age (Supplemental Table 2). Doppler recordings of transmitral blood flow velocities and TDI of mitral annular velocities revealed characteristic patterns of restrictive physiology with elevated LV filling pressure, including decreased E wave deceleration times, diminished Ea velocity (peak velocity of early diastolic mitral annular flow), and an increased ratio of transmitral E to mitral annular Ea (Fig. 3B and Supplemental Table 3).
Fig. 3.
Ventricular systolic function is impaired in csCnb1−/− mice. A: mean ventricular percent fractional shortening is depressed in csCnb1−/− mice (n = 5) compared with csCnb1+/+ (n = 4) from an early age. Top: representative M-mode left ventricular echocardiographic images of csCnb1+/+ and csCnb1−/− mice at 2 mo of age. Bottom: bars represent mean percent fractional shortening (±SE). *P < 0.05 compared with controls at the same time point. B: representative Doppler measurements of transmitral flow (left) and tissue Doppler imaging based on annular and posterior wall orientations (middle and right).
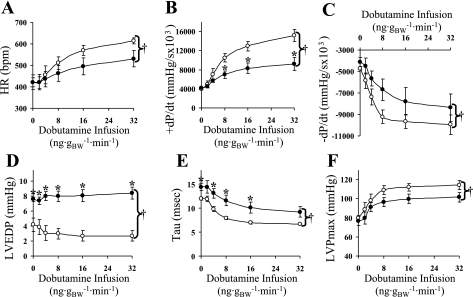
To further investigate the cardiac phenotype of the csCnb1−/− mice, hemodynamics were obtained on 3-mo-old mice via closed-chest cardiac catheterization at baseline and following an infusion of the β-adrenergic agonist dobutamine. At baseline, LV end-diastolic pressure was significantly higher in the mutant mice compared with the controls. Following administration of dobutamine, heart rate and the rates of pressure development and relaxation failed to increase appropriately in the csCnb1−/− mice (ANOVA, P < 0.05). Tau, a separate measure of ventricular relaxation, decreased significantly less in csCnb1−/− mice than controls during the dobutamine infusion, while the elevated LV end-diastolic pressure was maintained. Additionally, the dobutamine-induced rise in maximal LV pressure was blunted in the csCnb1−/− mice during the dobutamine infusion (Fig. 4). Taken together with the morphological and echocardiographic data, these results indicate that the csCnb1−/− mice exhibit both systolic and diastolic ventricular dysfunction and features of severe restrictive physiology.
Fig. 4.
Ventricular contraction and relaxation are impaired in csCnb1−/− mice. Graphical representation of functional data (means ± SE) was obtained from control and csCnb1−/− mice during closed-chest cardiac catheterization, measured at baseline and with increasing dosage of infused dobutamine. A: heart rate (HR). B: rate of pressure development (+dP/dt). C: rate of pressure relaxation (−dP/dt). D: left ventricular end diastolic pressure (LVEDP). E: tau (time constant). F: maximal left ventricular pressure (LVPmax). Control mice (n = 4) are indicated by open circles; csCnb1−/− mice (n = 4) by solid circles. †P < 0.05, analysis of covariance comparing genotypes. *P < 0.05, for pairwise comparisons between genotypes at the same dobutamine dosage. BW, body weight; bpm, beats/min.
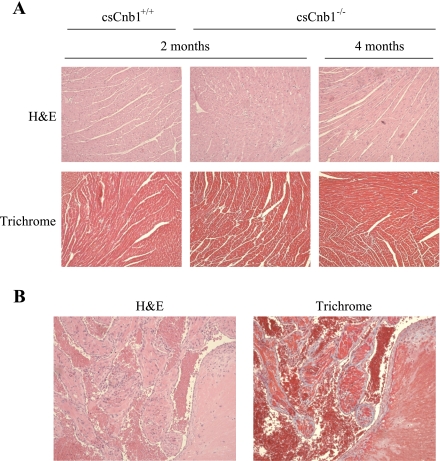
Cardiac ventricles of csCnb1−/− mice do not exhibit histological abnormalities or evidence of cell death.
Despite the dramatic cardiac functional phenotype, the histological appearance of the ventricles of csCnb1−/− mice was similar to that of control mice at both 2 and 4 mo of age. Specifically, there was no evidence of infiltrate, cellular disarray, or fibrosis (Fig. 5A). Moreover, staining for activated caspase-3 was remarkable for a lack of evidence for an apoptotic process (data not shown). These results strongly suggested that the cardiomyopathy of csCnb1−/− mice was not due to major alterations in the extracellular matrix or significant myocyte death. In striking contrast, atrial sections from 4-mo-old csCnb1−/− mice showed marked fibrosis and organized thrombosis, likely related to chronically elevated atrial pressure related to the noncompliant ventricles (Fig. 5B). Thus the ventricular dysfunction preceded any sign of atrial abnormality by at least 2 mo. Of note, there was evidence of pulmonary edema, but no parenchymal damage or pulmonary vascular changes in 3-mo-old mice (data not shown).
Fig. 5.
Histological analysis of ventricular and atrial sections. A: representative ventricular sections stained with hematoxylin-eosin (H&E) or trichrome from control and csCnb1−/− mice at 2 and 4 mo of age reveals no evidence for cellular disarray or fibrosis in the ventricles. B: thrombus and fibrosis are apparent in sections prepared from the atria of 4-mo-old csCnb1−/− mice.
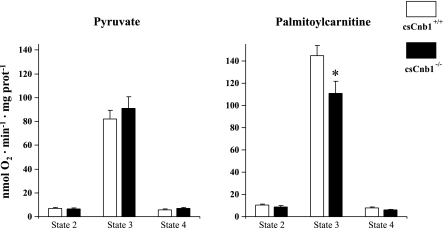
Mild impairment of fatty acid oxidation in mitochondria isolated from ventricles of csCnb1−/− mice.
The lack of structural abnormalities in the cardiac ventricles of the csCnb1−/− mice suggested that the functional phenotype could be due to a primary metabolic abnormality. We and others have shown that the Cn signaling pathway activates the expression of the nuclear receptor peroxisome proliferator-activated receptor (PPAR)-α and its coactivator, PGC-1α, key transcriptional regulators of cardiac mitochondrial energy metabolism (40). To determine whether derangements in mitochondrial energy production caused the cardiomyopathic phenotype, respiratory function studies were performed on mitochondria isolated from ventricles of 3-mo-old control and csCnb1−/− using malate and either pyruvate or palmitoylcarnitine as substrate. State 2 (ADP-limited) and 3 (maximal) respiration rates were similar in csCnb1−/− and control mitochondria when using pyruvate as a substrate (Fig. 6). With palmitoylcarnitine, state 2 rates were normal, but state 3 rates were modestly but significantly diminished in the mitochondria isolated from the csCnb1−/− mice (Fig. 6). Uncoupled respiration was not different between the groups with either substrate (Fig. 6). These results were consistent with our previous observation that the expression of PGC-1α and its downstream targets involved in mitochondrial fatty acid oxidation are modestly but significantly reduced in csCnb1−/− mice (40). Whereas the impairment of maximal rates of palmitoylcarnitine-driven respiration in the csCnb1−/− cardiac mitochondria is consistent with the known role of Cn in the regulation of the PPARα/PGC-1α pathway, this relatively minimal impairment is highly unlikely to account for the observed mortality and severe cardiac phenotype. In support of this conclusion, mRNA expression of genes encoding citrate synthase, cytochrome oxidase IV, and ATP synthase-β is maintained in the hearts of the csCnb1−/− mice (data not shown).
Fig. 6.
Mild reduction in mitochondrial respiration in csCnb1−/− mice using palmitoylcarnitine (but not pyruvate) as a substrate. Mean respiratory rates (±SE) were measured with mitochondria isolated from ventricles of control (csCnb1+/+, n = 8) and csCnb1−/− mice (n = 8) under the following conditions: state 2 (basal), state 3 (ADP stimulated), and following oligomycin treatment (state 4). *P < 0.05 compared with controls.
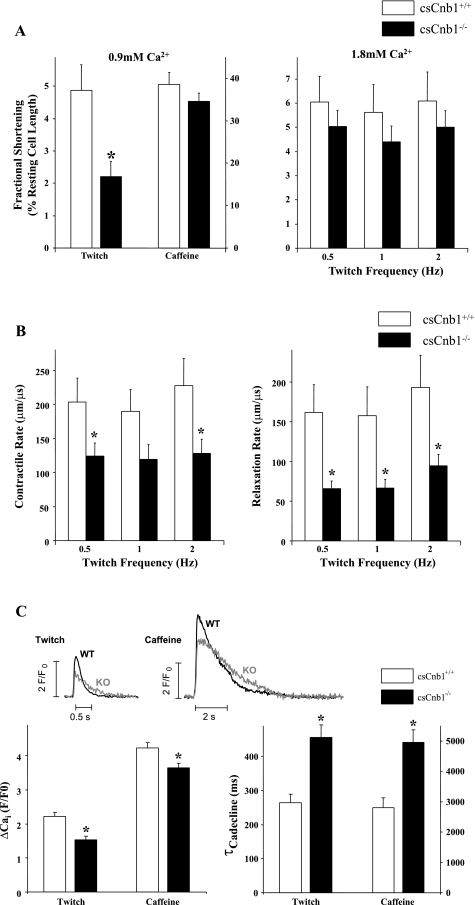
Impaired contractility and excitation-contraction coupling in cardiac myocytes isolated from csCnb1−/− mice.
Cardiac myocytes were isolated from 3-mo-old female csCnb1−/− mice and littermate csCnb1+/+ controls to further assess contractile function. csCnb1−/− myocytes exhibited a markedly reduced fractional shortening when stimulated at 0.5 Hz in the presence of 0.9 mM Ca2+ (near normal physiological level in the mouse; Fig. 7A). However, caffeine-induced contractures, indicative of SR Ca2+ content, were not significantly altered in csCnb1−/− myocytes (Fig. 7A). When extracellular [Ca2+] was increased from 0.9 to 1.8 mM (supra-physiological for mouse, and a [Ca2+] near which contraction is maximal), there was not a significant decrease in contraction amplitude when stimulated at 0.5–2.0 Hz (Fig. 7A). However, the rates of contraction and relaxation, based on changes in measured length per time during maximal contractions (measured at 1.8 mM [Ca2+]), were significantly lower in csCnb1−/− vs. control myocytes (Fig. 7B). Thus excitation-contraction coupling is defective in the csCnb1−/− myocytes, possibly due to altered Ca2+ handling.
Fig. 7.
Altered contractile parameters of isolated adult cardiac myocytes from csCnb1−/− mice. A: mean contraction amplitudes as a percentage of total cell length of cardiac myocytes (n = 13 cells per group) isolated from csCnb1+/+ and csCnb1−/− ventricles measured at 1 Hz and 0.9 mM Ca2+ (left) or 0.5–2 Hz and 1.8 mM Ca2+ (right). B: mean rates of contraction (left) and relaxation (right) of cardiac myocytes maintained in 1.8 mM Ca2+ and stimulated at 0.5–2 Hz. C: Ca2+ transients induced by electrical stimulation (0.5 Hz) or rapid exposure to 10 mM caffeine (0.9 mM Ca2+ concentration). Amplitudes (left) and time constants (τ) of intracellular Ca2+ (Cai) concentration decline (right) are shown. *P < 0.05 compared with controls at the same contraction frequency. WT, wild type; KO, knockout; F/F0, ratio of background fluorescence to diastolic fluorescence under control conditions.
We then measured myocyte Ca2+ transients. At 0.5 Hz, Ca2+ transient amplitude was lower in csCnb1−/− compared with control, and this was also true for caffeine-induced Ca2+ transients, indicating that SR Ca2+ content is reduced in csCnb1−/− myocytes (Fig. 7C). The small decrease in SR Ca2+ content could explain the larger reduction of twitch-induced Ca2+ transients, because of the steep relationship between SR Ca2+ content and fractional release (41). The rate of decline of twitch and caffeine-induced Ca2+ transients provides information about SERCA and Na/Ca exchanger Ca2+ transport rates, respectively (19). The results are consistent with the conclusion that both SERCA2a and Na/Ca exchanger transport Ca2+ more slowly in csCnb1−/− myocytes. The large reduction in SR Ca2+ uptake rate would be expected to reduce SR Ca2+ content, but this may be partly mitigated by the reduced Na/Ca exchange function (which would slow Ca2+ extrusion from the cell, allowing more SR Ca2+ uptake).
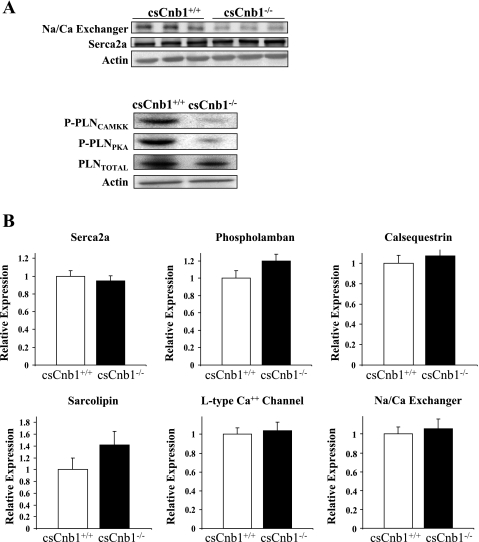
Altered expression of Na/Ca exchanger and PLN in the hearts of csCnB1−/− mice.
As an initial step toward identifying the mechanistic basis for the observed abnormality in myocyte Ca2+ transients in the csCnb1−/− mice, we quantified the expression of proteins involved in Ca2+ release and re-uptake. Western blot studies demonstrated that the levels of the Na/Ca exchanger were significantly lower in the csCnb1−/− hearts (Fig. 8A). Expression of SERCA2a was not different in the csCnb1−/− mice compared with control (Fig. 8A). Additionally, no difference was seen in the levels of PLN protein expression. However, the ability of PLN to inhibit SERCA2a depends on its phosphorylation state. Therefore, we also determined the amount of phospho-PLN using antibodies specific to sites Ser16 and Thr17. Both antibodies demonstrated that the phosphorylation state of PLN was markedly lower in the csCnb1−/− hearts (Fig. 8A). No difference was detected in the mRNA expression of any target Ca2+ handling genes, indicating that, although Cn is known to play a role in excitation-transcriptional coupling, expression of these genes is not downregulated by loss of Cn activity (Fig. 8B).
Fig. 8.
Altered levels and phosphorylation status of proteins involved in myocyte calcium release and re-uptake in csCnb1−/− mice. A: representative Western blots for candidate proteins (abbreviations defined in text). The signal obtained with actin is shown as a control (n = 4 per group). B: bars represent mean mRNA levels normalized to 18S signal. Control mice (csCnb1+/+) are indicated by open bars (n = 9); csCnb1−/− mice by solid bars (n = 9).
DISCUSSION
Previous studies using mouse systems to overexpress Cn* or Cn inhibitory proteins have revealed that Cn signaling plays a critical role in the transduction of cardiac myocyte Ca2+ signals to pathological hypertrophic growth and to regulation of normal energy metabolism (40, 42, 44). Targeted gene disruption strategies in mice have confirmed a role for Cn signaling in pathological cardiac remodeling in response to pathophysiological stress, such as pressure overload (32, 33). However, the minimal baseline phenotype of previous CnA gene loss-of-function lines (α or β) has not provided a complete understanding of the biological necessity of this signaling pathway in postnatal heart, presumably due to compensation by the other CnA isoform. Recently, we generated mice with cardiac-specific deficiency of the Cn B1 regulatory subunit (csCnb1−/−) and found that they survived following birth, despite modest alterations in the expression of genes involved in mitochondrial fatty acid oxidation due to derangements in PGC-1α/PPAR-α signaling (40). As described here, the severe adult phenotype of the csCnb1−/− mice provides new information about the importance of Cn signaling in postnatal cardiac growth, ventricular contractility, and myocyte excitation-contraction coupling.
csCnb1−/− mice die of heart failure due to a cardiac phenotype that includes a postnatal growth defect and an unusual cardiomyopathy that exhibits systolic ventricular dysfunction, combined with diastolic abnormalities and restrictive features. It is possible, given the surprising lack of ventricular dilatation, that the cardiomyopathy of the csCnbl−/− mice reflects, in part, the observed growth defect. Cardiac mass is normal in 1-wk-old csCnb1−/− mice, but, thereafter, is significantly lower than that in wild-type controls. These results are consistent with the known role of Cn signaling in programs directing cardiac hypertrophy (10, 24, 28, 36) and indicates that Cn signaling is necessary for normal physiological growth of the postnatal heart, as well as pathological forms of hypertrophy. Given the known role of Cn in driving hypertrophy, and the fact that the phenotype is mainly manifest in the postnatal period, it is likely that the reduced heart mass is due to size reduction of individual cells, rather than a loss in cell number. This conclusion is further supported by the absence of markers of increased cell death. However, our results do not exclude the possibility that reduced cell number does not contribute to the smaller heart size. It should also be noted that, due to the limitations of Cre-recombinase targeting approaches, we cannot be sure that a small residual amount of CnA remains in the csCnb1−/− mice.
Beginning at 2 mo of age, the atria of the csCnb1−/− mice become massively dilated in the absence of ventricular dilatation, also consistent with severe ventricular diastolic dysfunction. Doppler recordings of transmitral blood flow velocities, TDI of mitral annular velocities, and invasive hemodynamic measurements confirmed characteristic patterns of diastolic ventricular dysfunction and restrictive physiology in the mutant mice (with the exception of structural abnormality). Two-dimensional echocardiographic analysis revealed marked reduction in systolic function and a decrease in relative wall thickness in csCnb1−/− mice, yet the ventricles did not exhibit diastolic dilatation.
Surprisingly, despite evidence of a severe cardiomyopathy, we did not find structural or cellular derangements, including fibrosis, cell death, or infiltration in the myocardium of the csCnb1−/− mice. These findings led us to explore a metabolic basis for the cardiomyopathy. Recently, we found reduced expression of transcriptional activators (PGC-1α, PPAR-α) and downstream target genes involved in mitochondrial energy metabolism in the hearts of young csCnb1−/− mice (40). However, other than a modest impairment in maximal rates of palmitoylcarnitine-driven respiration, mitochondrial function was normal in the hearts of the csCnb1−/− mice, making energy metabolic derangements as the primary cause of the cardiomyopathy unlikely. Therefore, we assessed cardiac myocyte excitation-contraction properties in the mutant mice. csCnb1−/− myocytes exhibited a markedly reduced fractional shortening, which was improved by increasing the [Ca2+] in the media, yet rates of contraction and relaxation (changes in measured length per time) remained abnormal, despite improved Ca2+ transient amplitudes. These results suggested a defect in myocyte Ca2+ transients, which was confirmed. csCnb1−/− myocyte Ca2+ transients exhibited reduced amplitude compared with control, and the SR Ca2+ content was diminished, as evidenced by reduced caffeine-induced Ca2+ transients, consistent with a reduced SR Ca2+ content. The rate of decline of twitch and caffeine-induced Ca2+ transients suggested functional impairment of both SERCA2a and Na/Ca exchanger in csCnb1−/− myocytes. Indeed, we found that the levels of the Na/Ca exchanger were reduced in the hearts of the mutant mice. In addition, although both SERCA2a and its negative modulator, PLN, were expressed at normal levels in the csCnb1−/− mice, the phosphorylation state of PLN was greatly diminished. PLN, an integral membrane protein of the SR, functions as a modulator of the SERCA2a Ca2+ pump. In its dephosphorylated state, PLN binds to SERCA2a and inhibits pump function (22). By dephosphorylating PLN, Cn acts to enhance the kinetics of Ca2+ handling, as demonstrated in mice overexpressing activated Cn (8).
Phosphorylation of PLN at Ser16 and Thr17 occurs in response to β-adrenergic signaling via PKA and Ca2+-triggered events through CAMKII, respectively (11, 23). We found that both sites are equally and nearly completely dephosphorylated in the csCnb1−/− mice, indicating that Cn is necessary for the activity of these signaling pathways. In the absence of Cn signaling, SERCA2a is subject to inhibition by PLN, contributing to cardiac dysfunction. The specific function for phosphorylation at each site (2, 5, 26, 46) and their interaction is an area of active investigation (23). The lack of Cn, a phosphatase, cannot directly explain the lower phosphorylation, so the effect must be indirect. This indirect pathway must also differ from that in the failing heart, where Cn is activated and PLN phosphorylation reduced (29). Phosphatases other than Cn (especially PP1) are the primary determinants of the phosphorylation state of PLN. Direct links between Cn and the PKA and CAMKII circuits have not been fully defined. In fact, Cn has been shown to oppose both CAMKII (by dephosphorylation of Thr17) and PKA (by opposing the inhibition of PP1 by PKA) (23). It is possible that, in the context of chronic ablation of Cn action, the actions of PP1 or other relevant phosphatases become dominant in this system. However, the hearts of mice overexpressing Cn* exhibit increased phosphorylation of PLN (8), consistent with our results. In addition, others have suggested that interactions between Cn and MAPK signaling cascade (39) can modulate PLN phosphorylation. Further studies will be necessary to define the links between Cn signaling and PLN.
In addition to SERCA2a, Ca2+ is removed from the cytosol by the Na/Ca exchanger (Slc8a1), extruding Ca2+ across the plasma membrane. We found that the expression of the Slc8a1 Na/Ca exchanger is reduced at the protein level in the csCnb1−/− mice, although mRNA levels are unchanged. Thus loss of Cn activity likely alters either translation and/or stability of this exchanger. Loss of Na/Ca exchanger activity is consistent with the slowed intracellular [Ca2+] decline during caffeine exposure, as the SERCA pumps are inactivated and would tend to limit the decline in SR Ca2+ content (3, 19, 41). However, cardiac-specific Na/Ca exchanger gene ablation in mice is associated with very limited impairment of contractility and no observed mortality (15). Thus the slightly reduced Na/Ca exchanger expression and function is unlikely to be causative of depressed cardiac function in the csCnbl−/− mice. Rather, the reduction of Na/Ca exchanger activity may be compensatory in these animals, as the dramatic reduction in SERCA2a pump activity would be expected to deplete SR Ca2+ stores with normal Na/Ca exchanger function.
Reduction in SERCA2a activity via PLN-mediated inhibition is a likely contribution to the impaired ventricular systolic and diastolic function in the csCnb1−/− mice. The impact of PLN on SERCA2a activity and cardiac function has been nicely demonstrated using gene ablation and overexpression strategies in mice. PLN loss of function results in heightened myocyte contractility and increased rates of contraction and relaxation (21). Conversely, overexpression of PLN, causes decreased diastolic and systolic ventricular function (17), a phenotype similar to that of the csCnb1−/− mice. Transgenic mice overexpressing glycogen synthase kinase-3β, which opposes Cn action, exhibit diastolic dysfunction with impaired Ca2+ kinetics (25). The precise link between CnA signaling and PLN phosphorylation has not been established previously or by our results. It is possible that CnA acts within the PKA and CAMKII circuits. Alternatively, CnA could control a postnatal developmental maturation program that is required for proper control of PLN.
In summary, we conclude that loss of Cn signaling in the postnatal heart results in an impairment in postnatal growth and chronic PLN-mediated inhibition of SERCA2a, leading to a cardiomyopathy with features of impaired ventricular relaxation and systolic function, in the absence of ventricular remodeling.
GRANTS
This work was supported by National Institutes of Health Grants F32 AR48758 and RO1 HL058427, the Digestive Diseases Research Core Center (P30 DK052574), and the Clinical Nutrition Research Unit Core Center (P30 DK56341).
Supplementary Material
ACKNOWLEDGMENTS
We thank Mary Wingate and Shonna Hyde for expert assistance in the preparation of the manuscript, Jefferson Gomes for assistance in the preparation of adult mouse cardiac myocytes, and Deanna Loseke for assistance with the Na/Ca exchanger and Serca2a protein immunoblotting experiments. We also thank Dr. Gerald Crabtree for providing the Cnb1 “floxed” mice.
Present addresses: P. J. Schaeffer, Department of Zoology, Miami University, Oxford, OH 45056; T. C. Leone and D. P. Kelly, Burnham Institute for Medical Research, Orlando, FL 32827.
REFERENCES
- 1.Agah R, Frenkel PA, French BAMLH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bassani RA, Bassani JW, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol 453: 591–608, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res 93: 487–490, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Brixius K, Wollmer A, Bölck B, Mehlhorn U, Schwinger RHG. Ser 16-, but not Thr 17-phosphorylation influences frequency-dependent force generation in human myocardium. Pflügers Arch 447: 150–157, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in calcineurin A beta-deficient mice. Proc Natl Acad Sci USA 99: 4586–4591, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bultynck G, Vermassen E, Szlufcki K, DeSmet P, Fisore RA, Callewaert G, Missiaen L, DeSmedt H, Parys JB. Calcineurin and intracellular Ca2+-release channels: regulation or association? Biochem Biophys Res Commun 311: 1181–1193, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chu G, Carr AN, Young KB, Lester JW, Yatani A, Sanbe A, Colbert MC, Schwartz SM, Frank KF, Lampe PD, Robbins J, Molkentin JD, Kranias EG. Enhanced myocyte contractility and Ca2+ handling in a calcineurin transgenic model of heart failure. Cardiovasc Res 54: 105–116, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin and NF-AT. Cell 96: 611–614, 1999 [DOI] [PubMed] [Google Scholar]
- 10.De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, Glascock BJ, Kimball TF, del Monte F, Hajjar RJ, Molkentin JD. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 98: 3322–3327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drago GA, Colyer J. Discrimination between two sites of phosphorylation on adjacent amino acids by phosphorylation site-specific antibodies to phospholamban. J Biol Chem 269: 25073–25077, 1994 [PubMed] [Google Scholar]
- 12.Flagg TP, Charpentier F, Manning-Fox J, Remedi MS, Enkvetchakul D, Lopatin A, Koster J, Nichols C. Remodeling of excitation-contraction coupling in transgenic mice expressing ATP-insensitive sarcolemmal K ATP channels. Am J Physiol Heart Circ Physiol 286: H1361–H1369, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105: 863–875, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange. Cardiac-specific knockout of NCX1. Circ Res 95: 604–611, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest 97: 533–539, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol Heart Circ Physiol 274: H1335–H1347, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Lim HW, De Windt LJ, Steinber L, Taigen T, Witt SA, Kimball TR, Molkentin JD. Calcineurin expression, activation, and function in cardiac pressure-overload hypertrophy. Circulation 101: 2431–2437, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res 75: 401–409, 1994 [DOI] [PubMed] [Google Scholar]
- 22.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Mattiazzi A, Mundia-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phohpholamban phosphorylation on Thr 17 in cardiac physiological and pathological conditions. Cardiovasc Res 68: 366–375, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Meguro T, Hong C, Asai K, Takagi G, McKinsey TA, Olson EN, Vatner SF. Cyclosporine attenuates pressure-overload hypertrophy in mice while enhancing susceptibility to decompensation and heart failure. Circ Res 84: 735–740, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, Shao Z, Bhattacharya K, Kilter H, Huggins G, Andreucci M, Periasamy M, Solomon RN, Liao R, Patten R, Molkentin JD, Force T. Glycogen synthase kinase-3 beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem 279: 21383–21393, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mills GD, Kubo H, Harris DM, Berretta RM, Piacentino V, 3rd, Houser SR. Phosphorylation of phospholamban at threonine-17 reduces cardiac adrenergic contractile responsiveness in chronic pressure overload-induced hypertrophy. Am J Physiol Heart Circ Physiol 291: H61–H70, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Molkentin JD. Calcineurin and beyond-cardiac hypertrophic signaling. Circ Res 87: 731–738, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munch G, Bolck B, Karczewski P, Schwinger RH. Evidence for calcineurin-mediated regulation of SERCA 2a activity in human myocardium. J Mol Cell Cardiol 34: 321–334, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity 20: 255–2662004 [DOI] [PubMed] [Google Scholar]
- 31.Olson EN, Williams RS. Calcineurin signaling and muscle remodeling. Cell 101: 689–692, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Parsons SA, Millay DP, Sargent MA, Naya FJ, McNally EM, Sweeney HL, Molkentin JD. Genetic disruption of calcineurin improves skeletal muscle pathology and cardiac disease in a mouse model of limb-girdle muscular dystrophy. J Biol Chem 282: 10068–10078, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem 279: 26192–26200, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Parsons SA, Wilkins BJ, Bueno OF, Molkentin JD. Altered skeletal muscle phenotypes in calcineurin A alpha and A beta gene-targeted mice. Mol Cell Biol 23: 4331–4343, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers JH, Tamirisa P, Kovacs A, Weinheimer C, Courtois M, Blumer KJ, Kelly DP, Muslin AJ. RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload. J Clin Invest 104: 567–576, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 98: 3328–3333, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev 80: 1483–1521, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Saks VA, Kuznetsov AV, Kupriyanov VV, Miceli MV, Jacobus WE. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane- matrix preparation. J Biol Chem 260: 7757–7764, 1985 [PubMed] [Google Scholar]
- 39.Sanna B, Bueno OF, Dai YS, Wilkins BJ, Molkentin JD. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol 25: 865–878, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaeffer PJ, Wende AR, Magee CJ, Neilson JR, Leone TC, Chen F, Kelly DP. Calcineurin and calcium/calmodulin-dependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle. J Biol Chem 279: 39593–39603, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional SR Ca release by total and free intra-SR Ca concentration. Biophys J 78: 334–343, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vega RB, Bassel-Duby R, Olson EN. Control of cardiac growth and function by calcineurin signaling. J Biol Chem 278: 36981–36984, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator PGC-1α in muscle refueling. J Biol Chem 282: 36642–36651, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Zhang BW, Zimmer G, Chen J, Ladd D, Li E, Alt FW, Wiederrecht G, Cryan J, O'Neill EA, Seidman CE, Abbas AK, Seidman JG. T cell responses in calcineurin A alpha-deficient mice. J Exp Med 183: 413–420, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias E, Casadei B. Reduced phopholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res 102: 242–249, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.