Abstract
Tumor necrosis factor-α (TNF-α) upregulates the expression of monocyte chemoattractant protein-1 (MCP-1) and adhesion molecules in type 2 diabetes. We hypothesized that TNF-α and MCP-1 may interact to contribute to the evolution of vascular inflammation and endothelial dysfunction in coronary arterioles in type 2 diabetes. To test this hypothesis, we administered anti-MCP-1 to block MCP-1 signaling in genetically modified mice with type 2 diabetes (Leprdb) and in heterozygote (m Leprdb) lean control. Anti-MCP-1 partially restored vasodilation to the endothelium-dependent vasodilator acetylcholine in isolated, cannulated, and pressurized coronary arterioles in Leprdb mice but did not affect vasodilation in m Leprdb mice. Anti-MCP-1 attenuated superoxide production and the protein expression of nitrotyrosine, which is an indicator of peroxynitrite production, in isolated coronary arterioles of Leprdb mice. Immunostaining results showed that the expression of MCP-1 and vascular cellular adhesion molecule-1 is colocalized with endothelial cells and macrophages. Anti-TNF-α or anti-MCP-1 markedly reduced macrophage infiltration and the number of MCP-1-positive endothelium in Leprdb mice. The neutralization of TNF-α or anti-MCP-1 reduced the expression of adhesion molecules, suggesting that proinflammatory cytokines interact to amplify the signaling process that leads to vascular dysfunction. These findings demonstrate that the endothelial dysfunction occurring in type 2 diabetes is the result of the effects of the inflammatory cytokine TNF-α and TNF-α-related signaling, including the expression of MCP-1 and adhesion molecules, which further exacerbates vessel inflammation and oxidative stress.
Keywords: coronary microcirculation, cytokines, inflammation, vasodilation
diabetes is accompanied by a maturing inflammatory response that consists of increased tumor necrosis factor-α (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) expression in adipocytes and the lumen of vessels (20). At the tissue level, diabetic inflammation enhances the expression of adhesion molecules, which facilitates macrophage infiltration (4, 14, 17). We previously found that the increases in TNF-α expression induces the activation of NAD(P)H oxidase and the production of reactive oxygen species (ROS), leading to endothelial dysfunction in type 2 diabetes (6). Additionally, TNF-α is reported to enhance MCP-1 expression (16); thus, the interaction between these systems may contribute significantly to the evolution of vascular disease in diabetes. MCP-1 is expressed by a variety of activated cells (e.g., endothelial cells, monocytes, and smooth muscle cells) and is a major component of adipose tissue that contributes to abnormal lipid metabolism (9). Recent evidence suggests that MCP-1 plays a detrimental role in the development of vascular disease through the recruitment of monocytes that accumulate in/on the vascular endothelium and may augment vascular disease at the inflammatory site (27). Monocytes that migrate from the peripheral vasculature into the myocardium undergo maturation to macrophages, which then release proteolytic enzymes and damage the myocardium (8). The action of MCP-1 during monocyte recruitment (rolling and/or adhesion) is associated with the activation of specific cell surface proteins. In this regard, adhesion molecules, e.g., vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), and E-selectin as well as MCP-1, are responsible for myocardial injury and cardiac contractile dysfunction (15).
Thus the goal of this study was to document the cellular and molecular mechanisms involved with MCP-1 in the coronary microcirculation in TNF-α-induced endothelial dysfunction in type 2 diabetes. We examined the role of MCP-1 in type 2 diabetes and tested whether the interaction of TNF-α and MCP-1 amplified the signaling of adhesion molecules and macrophage infiltration. Endothelium-dependent vascular function and the production of ROS were determined in isolated coronary arterioles (40–100 μm) from genetically modified mice with type 2 diabetes (Leprdb) with and without treatment of the MCP-1 neutralizing antibody. We also tested the colocalization of MCP-1, CD68 (monocyte/macrophage maker), and adhesion molecules in the hearts of type 2 diabetic mice using immunohistochemical analysis.
MATERIALS AND METHODS
Animals.
The procedures followed were approved by and in accordance with the guidelines set by the Laboratory Animal Care and Use Committee at Texas A&M University. Heterozygous controls [BKS.Cg-m+/+ Leprdb/J (m Leprdb)], homozygous type 2 diabetes [BKS.Cg-m+/+ Leprdb/J (Leprdb)], and Leprdb null for TNF-α (dbTNF−/dbTNF−) mice were purchased from Jackson Laboratory and maintained on a normal rodent chow diet. Heterozygotes (m Leprdb) are normal in body weight, blood glucose, and plasma insulin. Our studies used 12–16-wk-old, 20–30-g m Leprdb and 40–60 g Leprdb and dbTNF−/dbTNF− mice of either sex. The cross of Leprdb with TNF knockout is heterozygous for Leprdb and homozygous for TNF knockout (TNF−/−). These dbTNF−/dbTNF− mice show the phenotypes of hyperglycemia and obesity, the diabetic phenotype that is consistent with the penetrance of the leptin receptor mutation (6). The obese mice from the second round of breeding of Leprdb and TNF−/− mice were used in experimentation. We also investigated the homozygous wild-type (C57BLKS/J, WT) control for the Leprdb diabetic mouse. We include both WT and m Leprdb as the controls in this study: heterozygous m Leprdb mice do not show an intermediate phenotype, but the results obtained from homozygous WT and heterozygous m Leprdb mice were identical (6).
Measurement of blood parameters: blood glucose, cholesterol, and insulin level.
Blood was obtained from the vena cava after anesthesia with pentobarbital sodium (50 mg/kg ip) and exposure of the vein. A whole blood sample was spun in at 3,000 rpm for 10 min, and the serum was stored at −80°C until analysis. We used an Accu-check compact glucometer (Roche Diagnostic, Mannheim, Germany) for measuring blood glucose in m Leprdb, Leprdb, and Leprdb mice treated with anti-MCP-1 at the same time (8:00 am–10:00 am) every time (note: food was provided ad libitum because the absence of a functional leptin receptor in Leprdb mice makes food deprivation stressful; this precluded a fasting protocol). The serum cholesterol level was measured with the Cholesterol/Cholesteryl Ester Quantitation kit (Biovision), and insulin was measured with the use of a commercial kit, Insulin (Mouse) Ultrasensitive EIA (ALPCO Diagnostics) with the use of spectrophotometry (Multiskan MCC, Fisher Scientific).
Measurement of superoxide by electron paramagnetic resonance spectroscopy.
Superoxide (O2•−) quantitation from the electron paramagnetic resonance (EPR) spectra was determined in the homogenate (4–6 isolated coronary arterioles) by double integration of the peaks, with reference to a standard curve from horseradish peroxidase generation of the anion from standard solutions of hydrogen peroxide, using p-acetamidophenol as the cosubstrate (22, 29) and then normalized to the mean value of lean control (m Leprdb).
Treatment with TNF-α or MCP-1 neutralization.
The neutralizing antibody to TNF-α (12) is 2E2 monoclonal antibody (2E2 MAb 94021402, NCI Biological Resources Branch). At 12–14 wk of age, all mice received the neutralizing anti-TNF-α (2E2 MAb, 0.625 mg·ml−1·kg−1·day−1 ip for 3 days); the dosage was based on our estimates of TNF-α expression (in the low ng or pg range), and this amount is able to neutralize 10–100-fold more than the estimated levels of TNF-α. All mice were treated with the neutralizing antibody to MCP-1 (anti-MCP-1; 200 or 400 μg·kg−1·day−1 ip for 3 days, Murine; Biovision) to block MCP-1 signaling.
Functional assessment of isolated coronary arterioles.
The techniques for the identification and isolation of coronary microvessels were described in detail previously (11, 29). Coronary arterioles (40–100 μm in diameter) from mouse hearts were carefully dissected for in vitro study. To determine whether MCP-1 played a role in the vascular dysfunction in type 2 diabetes, the endothelium-dependent vasodilator acetylcholine (ACh, 0.1 nmol/l to 10 μmol/l)-induced vasodilation was assessed in isolated coronary arterioles in m Leprdb, Leprdb, and Leprdb mice treated with anti-MCP-1 (200 or 400 μg·kg−1·day−1 ip for 3 days).
Immunohistochemical analysis.
The mouse heart was perfused with phosphate-buffered saline (PBS) and then perfused again with 4% paraformaldehyde. Isolated heart tissue was fixed in 4% paraformaldehyde diluted in 0.1 mol/l PBS (overnight at 4°C). Fixed heart tissue was perfused in 20% sucrose diluted in 0.1 mol/l PBS (overnight at 4°C). Once the heart was completely perfused, the tissue was embedded in optimum cutting temperature compound tissue tek, snap frozen at −60°C, and stored at −80°C. Frozen tissues were sectioned at 5 μm thick using cryostat (Leica), and the slides were allowed to dry at room temperature; the tissues were then incubated in 0.3% Triton X-100 for 30 min and washed three times in PBS. Goat serum (5%) was used for 30 min to block nonspecific binding. Anti-rat first primary MCP-1 and VCAM-1 antibodies (Abcam) were incubated overnight at 4°C, followed by an incubation with Texas red goat anti-rat IgG. Anti-rabbit second primary α-actin, von Willebrand factor, and anti-mouse second primary CD68 (Abcam) as an monocyte/macrophage marker were incubated sequentially overnight at 4°C, followed by an incubation with FITC goat anti-rabbit or anti-mouse IgG. Sections were washed three times in PBS. The appropriate secondary FITC (green color)- and Texas red (red color)-conjugated antibodies were incubated with sections for 4 h and washed off. The sections were finally mounted in an antifading agent (Slowfade gold with 4',6-diamidino-2-phenylindole, Molecular Probes), and the slides were then observed and analyzed with the use of a fluorescence microscope (Zeiss Axioplan microscope with a ×40 objective or a Nikon microscope with a ×63 objective).
Protein expression by Western blot analyses.
Coronary arterioles (4–6 vessels per sample) were separately homogenized and sonicated in lysis buffer (Cellytic MT Mammalian Tissue Lysis/Extraction Reagent, Sigma). The equal amounts of protein [10 μg for nitrotyrosine (N-Tyr), 20 μg for adhesion molecules, and 40 μg for TNF-α and MCP-1] were separated by SDS-PAGE and transferred to nitrocellulose membranes. Protein concentrations were assessed with a BCA Protein Assay kit (Pierce), and equal amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose (Hybond, Amersham). N-Tyr [Abcam, an indicator for peroxynitrite (ONOO−)-mediated tissue injury], TNF-α (Santa Cruz), MCP-1 (Abcam), VCAM-1 (Santa Cruz), ICAM-1 (Santa Cruz), E-selectin (Santa Cruz), IκB (Santa Cruz), phospho-IκB (Santa Cruz), and NF-κB (Abcam) were determined with m Leprdb, Leprdb, and Leprdb mice treated with anti-TNF-α (0.625 mg·ml−1·kg−1·day−1 ip for 3 days) or anti-MCP-1 (200 or 400 μg·kg−1·day−1 ip for 3 days). Signals were visualized by enhanced chemiluminescence (Santa Cruz), scanned with a Fuji LAS3000 densitometer, and quantified by Multigauge software (Fujifilm).
Data analysis.
At the end of each experiment, the vessel was relaxed with 100 μmol/l SNP to obtain its maximal diameter at 60 cmH2O intraluminal pressure (11). All diameter changes in response to agonists were normalized to the vasodilation in response to 100 μmol/l SNP and expressed as percentages of maximal dilation. All data are presented as means ± SE or means ± SD. Statistical comparisons of vasomotor responses under various treatments were performed with two-way ANOVA. Significance was accepted at P < 0.05.
RESULTS
Body weight, abdominal girth, serum concentration of glucose, cholesterol, and insulin level.
Serum parameters were measured at 12–16 wk in different strains of mice (Table 1). Table 1 shows the evaluations of the diabetic conditions in m Leprdb, Leprdb, and Leprdb mice treated with anti-MCP-1.
Table 1.
Baseline serum parameters
| m Leprdb | Leprdb | Leprdb + anti-MCP-1 | |
|---|---|---|---|
| Body weight, g | 25.82±1.5 | 48.45±2.3* | 49.33±2.7* |
| Glucose, mg/dl (nonfasting) | 139±17.26 | 437.5±44.5* | 446.12±35.2* |
| Abdominal girth, cm | 8.05±0.5 | 11.9±0.4* | 11.8±0.39* |
| Cholesterol level, μg/μl | 1.12±0.23 | 1.8±0.4* | 1.57±0.4* |
| Insulin, ng/ml (nonfasting) | 2.234±0.73 | 2.58±0.52 | 2.59±0.488 |
Values are means ± SD; n = 8 animals. Glucose and cholesterol level were higher in genetically modified mice with type 2 diabetes (Leprdb) and Leprdb mice treated with anti-monocyte chemoattractant protein-1 (MCP-1) than in heterozygote (m Leprdb) lean control mice. Abdominal girth was higher in Leprdb and Leprdb mice treated with anti-MCP-1 vs. m Leprdb on the day of surgery.
P < 0.05 vs. m Leprdb.
TNF-α and MCP-1 amplification of signaling in coronary arterioles in type 2 diabetes.
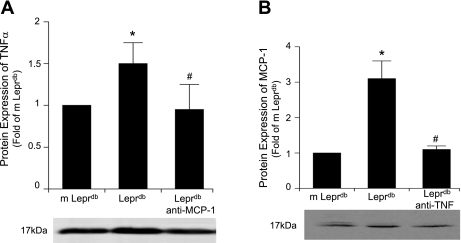
We determined whether TNF-α and MCP-1 interact to induce their protein expressions. The protein expression of TNF-α and MCP-1 from isolated coronary arterioles was analyzed in m Leprdb, Leprdb, and Leprdb mice treated with anti-TNF-α or anti-MCP-1. Western blot analysis (Fig. 1) revealed that MCP-1 expression was decreased in anti-TNF-α-treated Leprdb mice and similarly TNF-α expression was decreased in anti-MCP-1-treated Leprdb mice, indicating that there is an association between MCP-1 and TNF-α signaling.
Fig. 1.
Interaction between TNF-α and monocyte chemoattractant protein-1 (MCP-1). A: protein expression of TNF-α from isolated coronary arterioles was higher in genetically modified mice with type 2 diabetes (Leprdb) vs. heterozygote (m Leprdb) lean control mice, but anti-MCP-1 (200 μg·kg−1·day−1) attenuated TNF-α expression in Leprdb mice. B: MCP-1 expression was elevated in Leprdb vs. m Leprdb mice, but anti-TNF-α (0.625 mg·ml−1·kg−1·day−1 ip for 3 days) attenuated MCP-1 expression in Leprdb mice. Data represent means ± SD; n = 4 separate experiments. *P < 0.05 vs. m Leprdb; #P < 0.05 vs. Leprdb.
Cellular source of MCP-1 expression in type 2 diabetes.
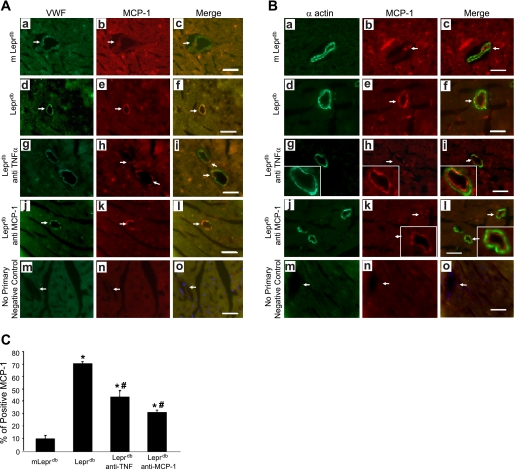
Immunostaining (Fig. 2) showed that MCP-1 protein expression (red) was present in endothelial cells but not in vascular smooth muscle cells. Additionally, MCP-1 expression was higher in Leprdb than in m Leprdb mice, but anti-TNF-α or anti-MCP-1 decreased MCP-1 expression in Leprdb mice. Leprdb mouse heart exhibited 71.23% MCP-1-positive staining in the endothelium, followed by Leprdb mice treated with anti-TNF-α at 43.42%, anti-MCP-1 at 30.71%, and m Leprdb mice at 10.54% (Fig. 2). We quantified the MCP-1 expression in vessels by counting the specific stained MCP-1 and calculated a percent increase of MCP-1-expressed vessels in total vessels per heart section (Fig. 2C). Experiments were performed without the primary antibodies to test whether or not staining was related to the nonspecific binding of the secondary antibodies, which showed no staining in heart sections, indicating that the signals were due to specific binding of the primary antibody.
Fig. 2.
Colocalization and regulation of MCP-1 expression in microvessels. Dual fluorescence combining MCP-1 and endothelial cells [von Willebrand factor (VWF); A] or vascular smooth muscle cells (α-actin; B) with use of specific antibodies were followed by a fluorescent-labeled secondary antibody. a–c: Dual labeling of VWF or α-actin (green) and MCP-1 (red) in control heart tissue. d–f: Dual labeling of VWF or α-actin (green) and MCP-1 (red) in diabetic heart tissue. g–i: Dual labeling of VWF or α-actin (green) and MCP-1 (red) in diabetic mouse heart tissue treated with anti-TNF-α. j–l: Dual labeling of VWF or α-actin (green) and MCP-1 (red) in diabetic mouse heart tissue treated with anti-MCP-1 (200 μg·kg−1·day−1). The 2 signals coincide in endothelial cells but do not coincide in smooth muscle cells. MCP-1 protein expression in microvessels was downregulated by anti-TNF-α or anti-MCP-1 treatment. m–o: Negative control shows an absence of staining in vessels with the secondary antibodies. Arrows show colocalization of MCP-1 in endothelial cells but not in smooth muscle cells. MCP-1 positive-stained microvessels were counted per section (C). Bar size is 50 μm. Original magnification, ×40. Data shown are representative of 4 separate experiments. *P < 0.05 vs. m Leprdb; #P < 0.05 vs. Leprdb.
Role of MCP-1 in type 2 diabetes-induced vascular dysfunction.
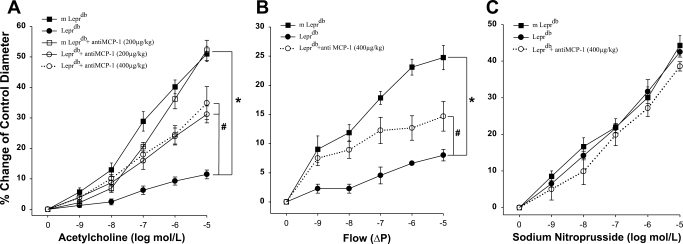
Vasodilation to the endothelium-dependent vasodilator ACh was impaired in Leprdb mice compared with m Leprdb mice (Fig. 3A). Conversely, the neutralizing antibody to MCP-1 (200 μg·kg−1·day−1) partially restored ACh-induced vasodilation in isolated coronary arterioles in Leprdb mice. To test whether MCP-1 is totally secondary to TNF-α in this setting, we administered a higher concentration of MCP-1 antibody (400 μg·kg−1·day−1) in Leprdb mice, which restored endothelial vasodilation in a similar manner with a lower dose of anti-MCP-1 (200 μg·kg−1·day−1, Fig. 3B). Similarly, anti-MCP-1 also restored flow-induced vasodilation in Leprdb mice, but SNP induced equal vasodilation in m Leprdb, Leprdb, and Leprdb mice. Additionally, anti-MCP-1 did not further increase the vasodilation in dbTNF−/dbTNF− mice (data not shown), which demonstrates that MCP-1 did not produce additional detrimental effects in dbTNF−/dbTNF− mice.
Fig. 3.
Isolated mouse coronary arterioles dilated in a concentration-dependent manner to ACh. ACh-induced vasodilation was blunted in coronary arterioles from Leprdb mice (n = 7). A lower (200 μg·kg−1·day−1, n = 7 animals) and a higher (400 μg·kg−1·day−1, n = 4 animals) dose of neutralizing antibodies to MCP-1 restored nitric oxide-mediated coronary arteriolar dilation to ACh in Leprdb mice in a similar manner (A). Flow-induced vasodilation (B) was impaired in Leprdb mice coronary arterioles (n = 6 animals) compared with m Leprdb (n = 6 animals), but it was restored with neutralizing antibodies to MCP-1 (400 μg·kg−1·day−1, n = 5 animals). ΔP, difference in pressure. Sodium nitroprusside-induced, endothelium-independent vasodilation (C) was identical in m Leprdb, Leprdb, and Leprdb treated with anti-MCP-1 (400 μg·kg−1·day−1). *P < 0.05 vs. m Leprdb; #P < 0.05 vs. Leprdb.
Role of ROS in type 2 diabetes-induced vascular dysfunction.
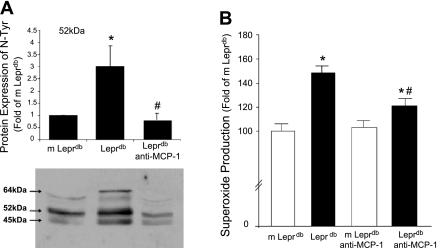
To address whether the overexpression of MCP-1 influences enhanced oxidative stress in Leprdb mice, we analyzed the protein expression of N-Tyr (Fig. 4A) to infer the concentration of ONOO− and measured O2•− production using EPR (Fig. 4B). Multiple bands revealed that the protein expression of N-Tyr was higher in Leprdb mice versus m Leprdb mice, but the neutralization of MCP-1 attenuated the protein expression of N-Tyr in Leprdb mice. The EPR result showed that O2•− production was elevated and neutralization of MCP-1 decreased O2•− production in Leprdb mice.
Fig. 4.
Effects of neutralizing antibody to MCP-1 in Leprdb on oxidative stress. A: measurement of ONOO−-mediated protein damage with nitration of the amino acid tyrosine has been tested using specific antibodies probed against nitrotyrosine (N-Tyr). Protein expression of N-Tyr from coronary arterioles was determined. The results show N-Tyr bands migrating at 52 and 45 kDa, respectively. Both 52- and 45-kDa bands of N-Tyr were higher in Leprdb mice than in m Leprdb mice and Leprdb mice treated with anti-MCP-1 (200 μg·kg−1·day−1, n = 4 separate experiments). B: electron paramagnetic resonance spectroscopy revealed that O2•− production was higher in Leprdb mice than in m Leprdb and that anti-MCP-1 reduced O2•− production in Leprdb mice (n = 6 animals). *P < 0.05 vs. m Leprdb; #P < 0.05 vs. Leprdb.
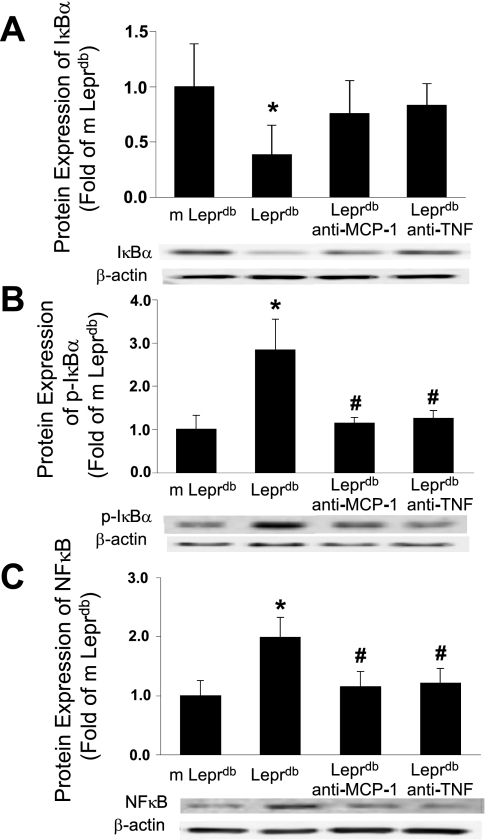
Protein expression of IκB, Ser32 phosphorylation of IκB, and NF-κB.
The protein expression of IκB from isolated coronary arterioles was decreased in Leprdb mice versus m Leprdb mice. IκB protein expression in anti-TNF-α- or anti-MCP-1-treated Leprdb mice was not statistically different from either m Leprdb or Leprdb mice (Fig. 5). Ser32 phosphorylation of IκB was elevated in Leprdb mice, but the administration of anti-TNF-α or anti-MCP-1 reduced IκB phosphorylation. The protein expression of NF-κB p65 was increased in Leprdb mice and was attenuated by either anti-TNF-α or anti-MCP-1 treatment.
Fig. 5.
Anti-TNF-α or anti-MCP-1 inhibits IκB phosphorylation and NF-κB p65 expression. A: protein expression of IκB from isolated coronary arterioles was decreased in Leprdb mice vs. m Leprdb mice. IκB protein expression in anti-TNF-α- or anti-MCP-1 (400 μg·kg−1·day−1)-treated Leprdb mice was not statistically different from m Leprdb mice or Leprdb mice. B: Ser32 phosphorylation of IκB was elevated in Leprdb mice, but administration of anti-TNF-α or anti-MCP-1 reduced IκB phosphorylation. p-IκBα, phospho-IκBα. C: protein expression of NF-κB p65 was increased in Leprdb mice, which was attenuated by either anti-TNF-α or anti-MCP-1 treatment. *P < 0.05 vs. m Leprdb; #P < 0.05 vs. Leprdb; n = 4 separate experiments.
TNF-α and MCP-1-induced activation of macrophage.
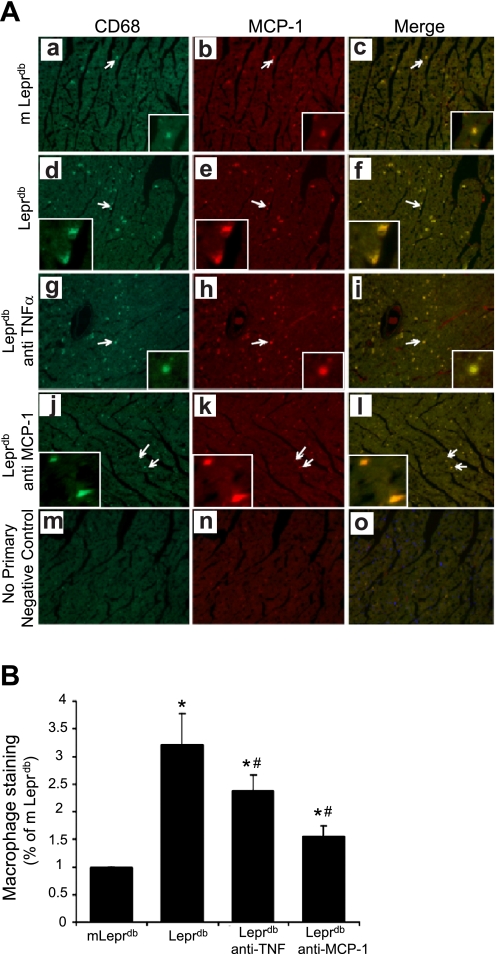
We further investigated the biological function of MCP-1, which is responsible for the chemoattraction of circulating monocyte-derived macrophages. Immunostaining analysis revealed the coexpression of the monocyte/macrophage marker CD68, and MCP-1 was 3.23-fold higher in Leprdb versus m Leprdb mice; anti-TNF-α or anti-MCP-1 treatment significantly reduced the infiltration of macrophages in Leprdb mice to express at 2.37- and 1.52-fold, respectively (Fig. 6), which suggests that coronary arteriolar dysfunction is associated with macrophage influx. We quantified macrophage infiltration by counting the specifically stained macrophages per equal unit area and normalizing to the mean value of m Leprdb mice (Fig. 6B).
Fig. 6.
A: lean control (m Leprdb) heart (a–c), Leprdb mice (d–f), Leprdb mice with anti-TNF-α treatment (g–i), and Leprdb mice with anti-MCP-1 treatment (j–l). Macrophage infiltration was significantly increased in Leprdb mice compared with m Leprdb mice and decreased after anti-TNF-α and anti-MCP-1 treatment (200 μg·kg−1·day−1) to Leprdb mice. Negative control (m–o) shows an absence of staining in vessels containing secondary antibodies. Arrows show colocalization of MCP-1 in macrophages. Specific stained macrophages were counted per equal unit area by blind test and normalized to mean value of lean control (m Leprdb) (B). Original magnification, ×16. Data shown are representative of 4 separate experiments. *P < 0.05 vs. m Leprdb; #P < 0.05 vs. Leprdb.
TNF-α and MCP-1-induced adhesion molecules and macrophage infiltration.
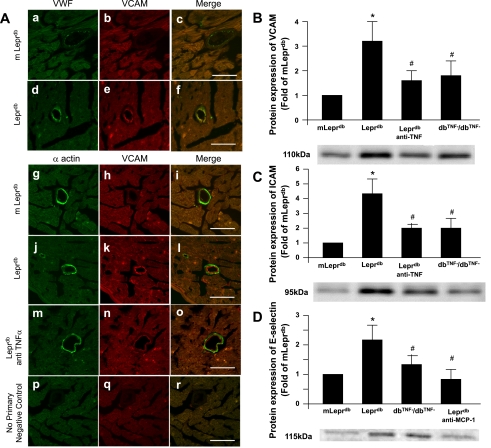
Figure 7A shows increased protein expression of VCAM-1 (red) in the heart of Leprdb versus m Leprdb mice. The result is consistent with our Western blot analysis (Fig. 7, B–D), which showed an elevated protein expression in Leprdb mice. To test whether the adhesion molecules are correlated with the increased expression of cytokines and chemokines (e.g., TNF-α and MCP-1) in diabetes, we analyzed the protein expression of VCAM-1 (Fig. 7B), ICAM-1 (Fig. 7C), and E-selectin (Fig. 7D) in m Leprdb, Leprdb, dbTNF−/dbTNF−, Leprdb mice treated with anti-TNF-α, or Leprdb mice treated with anti-MCP-1. The protein expression of these adhesion molecules was significantly elevated in Leprdb mice. Neutralizing antibodies to TNF-α attenuated the protein expression of VCAM-1 and ICAM-1 in Leprdb mice, and anti-MCP-1 attenuated the E-selectin expression in Leprdb versus m Leprdb mice. Additionally, VCAM-1, ICAM-1, and E-selectin protein expression was attenuated in dbTNF−/dbTNF− versus Leprdb mice.
Fig. 7.
A: dual fluorescence combining VCAM-1 with markers for endothelial cells (VWF; a–f), vascular smooth muscle cells (α-actin; g–o) using specific antibodies followed by a fluorescent-labeled secondary antibody. Arrows show colocalization of VCAM in endothelial cells but not in smooth muscle cells. No primary negative control (p–r) shows an absence of staining in vessels with secondary antibodies. Bar size is 50 μm. Original magnification, ×63. Data shown are representative of 4 separate experiments. B: TNF-α or MCP-1 induced upregulation of adhesion molecules. Protein expression of VCAM, ICAM (C), and E-selectin (D) from isolated coronary arterioles were elevated in Leprdb mice vs. m Leprdb mice, but administration of anti-TNF-α or Leprdb null for TNF-α (dbTNF−/dbTNF−) or anti-MCP-1 (200 μg·kg−1·day−1) attenuated VCAM, ICAM, or E-selectin protein expression, respectively (n = 4 separate experiments). *P < 0.05 vs. m Leprdb; #P < 0.05 vs. Leprdb.
DISCUSSION
Our results suggest that MCP-1 interacts with TNF-α to amplify adhesion molecules and oxidative stress, thereby contributing to vascular dysfunction in coronary microcirculation in type 2 diabetes. Our molecular evidence indicated that the expression of TNF-α and MCP-1 were significantly increased in Leprdb mice, but anti-MCP-1 decreased TNF-α protein expression and anti-TNF-α attenuated MCP-1 expression. Antibody neutralization of MCP-1 attenuated ONOO− and O2•− generation and formation of N-Tyr in Leprdb mice and prevented coronary endothelial dysfunction but could not further improve endothelial function in dbTNF−/dbTNF− (data not shown). These results demonstrate that MCP-1 is secondary to TNF-α in this setting in type 2 diabetes. The administration of anti-TNF-α or anti-MCP-1 to Leprdb mice resulted in attenuating cardiac macrophage accumulation comparable with that in the control, suggesting that an interaction of MCP-1 and TNF-α may accentuate the process of macrophage accumulation. Most importantly, the blockade of TNF-α or MCP-1 attenuated the expression of adhesion molecules (e.g., VCAM, ICAM, and E-selectin) in isolated coronary vessels in diabetes. Interactions of TNF-α and MCP-1 signaling may contribute to oxidative stress by increasing inflammatory cell accumulation and induce endothelial dysfunction in coronary arterioles in diabetic Leprdb mice.
Roles of TNF-α and MCP-1 in type 2 diabetes.
The important manifestation of diabetes-induced microvascular injury in the heart is a diminution in coronary blood flow. This is emphasized by clinical data showing that ∼25% of patients with acute myocardial infarction do not have an adequate restoration of myocardial tissue perfusion in the infarct region, despite coronary artery recanalization (10). The endothelium-derived nitric oxide (NO) is an important endogenous vasodilator that regulates microvascular tone (7). We previously found that increases in TNF-α expression induces the activation of NAD(P)H oxidase and the production of ROS, leading to endothelial dysfunction in type 2 diabetes (6). Consistent with our previous studies, our present study shows that the neutralization of MCP-1 prevented coronary endothelial dysfunction in Leprdb mice. Western blot results indicated that the expression of TNF-α and MCP-1 were significantly increased in Leprdb mice, but anti-MCP-1 decreased TNF-α protein expression and anti-TNF-α attenuated MCP-1 expression. Western blot analysis (Fig. 1) revealed that there may be an association between MCP-1 and TNF-α signaling. To support this conclusion, we also showed the classical TNF-α signaling members IκB phosphorylation and NF-κB p65 expression. The results indicate that anti-TNF-α or anti-MCP-1 inhibits IκB phosphorylation and NF-κB p65 expression (Fig. 5). Immunostaining in Leprdb mice showed that MCP-1 was colocalized in endothelial cells but not in vascular smooth muscle cells. A 6.76-fold-plus expression of MCP-1 occurred in endothelial cells in Leprdb diabetic mice versus m Leprdb control mice, and anti-TNF-α or anti-MCP-1 significantly attenuated MCP-1 expression in endothelial cells in Leprdb mice. There is no significant difference in the insulin level between Leprdb diabetic mice and m Leprdb control mice in the nonfasting state (Table 1). Because of the significant increase in lipid and insulin after food ingestion, the variability in lipid and insulin measurements is larger in the nonfasting state. However, postprandial lipid levels were more strongly associated with atherothrombosis than fasting concentrations in both the cerebral and coronary circulations. Postprandial lipid and insulin may be a more potent predictor of risk (18). Despite the similarities in glucose, body weight, cholesterol level, insulin level, and abdominal girth in diabetic animals with or without anti-MCP-1 treatment, the endothelial function was improved in Leprdb mice treated with anti-MCP-1. Thus the results suggest that 1) MCP-1 signaling plays a pivotal role in attenuating TNF-α protein expression, 2) MCP-1 is secondary to TNF-α in this setting in type 2 diabetes, and 3) TNF-α is the key cytokine that induces endothelial dysfunction in type 2 diabetes (6).
The role of MCP-1 in ROS production in coronary arterioles in type 2 diabetes.
The link between diabetes and inflammation leading to vascular disease is associated with enhanced oxidative stress (6). Tyrosine nitration is caused by ONOO−, a ROS formed by the reaction of NO with O2•−, which further consumes NO and increases oxidative stress (1). ONOO− has a very short half-life. The measurement of nitration of protein tyrosine residues is frequently used as an assay for ONOO− in tissues, blood, and perfusates (26). The presence of N-Tyr has been used as a marker of ONOO−-dependent damage in vivo (2). The index of its formation of N-Tyr (an indicator for ONOO−-mediated tissue injury) was measured by performing Western Blot analyses.
Numerous studies have noted that the nitration of tyrosine occurs at high levels of ONOO− during the pathogenesis of human atherosclerosis (3, 21). In particular, a decrease in expression of constitutive NO synthase and an inactivation of NO has been implicated in the endothelial dysfunction seen in type 2 diabetes (21). The interaction between activated macrophages and endothelial cells produces oxidative stress that affects the activation state of transcription factor NF-κB (19). To test whether MCP-1 enhances macrophage infiltration and induces ROS, macrophages derived from circulating monocytes were characterized by CD68 staining, which specifically labels macrophages and monocytes. Our immunostaining results showed that Leprdb mice expressed MCP-1 in macrophage (over 3-fold in heart sections), but anti-TNF-α attenuated MCP-1 expression (2-fold in heart sections), which suggests that TNF-α upregulates MCP-1 expression in macrophages. Antibody neutralization of MCP-1 reduced O2•− generation and formation of N-Tyr in Leprdb mice. This result suggests that TNF-α initiates the interaction of macrophages and endothelial cells via the upregulation of MCP-1 signaling, which results in macrophage infiltration and the production of ROS.
One of the major signs of endothelial dysfunction is vascular inflammation, which is associated with impaired vascular control. It appears that MCP-1 residing in the endothelium contributes to the regulation of NO-mediated vasodilation under diabetic conditions because anti-MCP-1 significantly enhanced endothelium-dependent NO-mediated coronary arteriolar dilations and decreased O2•− production in isolated blood vessels from diabetic mice. Our results support an endogenous role of vascular MCP-1 in regulating the NO-mediated dilation of the coronary microcirculation in type 2 diabetes. Consistently, our molecular results showed that anti-MCP-1 decreased TNF-α protein expression, anti-TNF-α attenuated MCP-1 expression, and antibody neutralization of MCP-1 attenuated ONOO− and O2•− generation and formation of N-Tyr in Leprdb mice.
Enhancement of adhesion molecules by MCP-1 and TNF-α in endothelial cells in diabetes.
Endothelial cells play a major role in maintaining vessel homeostasis. Recent studies suggest that MCP-1 also plays a role in the evolution of vascular disease through inducing adhesion molecule expression on/in the endothelium and recruiting monocytes/macrophages at the inflammatory site (5, 19, 27). Elevated adhesion molecules in the vessels have been thought to be significantly associated with an increase in the risk of coronary arterial disease (20). E-selectin is important in monocyte arrest or the transition from slow rolling to firm adhesion. VCAM and ICAM are critical for the firm attachment of monocytes in the recruitment cascade (14). The modification of the pattern of gene expression in the endothelial cells is a critical step during the progression of vascular disease. In endothelial cells, NF-κB regulates the inducible expression of genes encoding chemokines and adhesion molecules (21). Previous work detailed the role of TNF-α, NF-κB, and ROS in inflamed heart and vessels and suggested that they trigger NF-κB activation to induce TNF-α protein expression (6, 28). In our studies, the inflammatory cytokine TNF-α has been explored as a factor in the initiation or progression of vascular disease (6, 29). Inflammatory mediators, MCP-1 and adhesion molecules, have also been implicated in the inflammatory processes related to TNF-α in the development of vascular disease in type 2 diabetes (13). Importantly, anti-TNF-α or anti-MCP-1 inhibits IκB phosphorylation and NF-κB p65 expression. We postulated that if anti-MCP-1 attenuates TNF-α signaling, then the neutralization of MCP-1 may affect TNF-α-induced NF-κB gene products and adhesion molecules (e.g., VCAM, ICAM, and E-selectin). Immunostaining data showed that endothelial cells express adhesion molecules. The actions of adhesion molecules are favored in small vessels (∼50 μm) compared with those seen in the large coronary arteries under normal conditions because of the lower flow and shear stress in diabetes. Western blot analysis revealed that TNF-α and MCP-1 increased the expression of adhesion molecules, which suggests that the elevated expression of adhesion molecules is the result of the release of TNF-α and MCP-1 from endothelial cells and macrophages in Leprdb mice. Moreover, anti-MCP-1 treatment appeared to reduce the E-selectin level by attenuating TNF-α-induced signaling. Based on the evidence that NF-κB binding site, a critical element for the transcription of MCP-1 gene, is important for TNF-α-induced enhancer activities (23, 25), TNF-α may enhance NF-κB activity to induce MCP-1 gene expression in endothelial cells, thereby possibly inducing adhesion molecule expression leading to vascular disease.
Monocyte-derived macrophages are mainly inflammatory cells. Macrophages contribute to the oxidation of LDL, and oxidized LDL acts on mature macrophages to form macrophage foam cells (9, 24). The initial low expression of adhesion molecules results in a low affinity to ligands, and a signal from MCP-1 is essential to mobilize adhesion molecules for a firm adhesion (5). Inflammatory mediators such as MCP-1 and adhesion molecules have been implicated in the inflammatory processes related to TNF-α in the development of vascular disease in type 2 diabetes. A colocalization of MCP-1 and VCAM-1 with macrophages was observed in myocardium, suggesting that MCP-1 and cell adhesion molecules play a role in monocyte transmigration into the heart. ICAM-1 and E-selectin also coexist in endothelial cells and macrophages (data not shown). Taken together, MCP-1 may play a critical role in the inflammation-induced endothelial dysfunction in coronary arterioles in diabetes by increasing the adhesion molecule expression in endothelial cells, thereby inducing macrophage infiltration at the inflammatory site without influencing the development of obesity or insulin resistance.
In this study, we found that beyond monocyte recruitment, MCP-1 is critical in TNF-α-induced endothelial dysfunction in type 2 diabetes. The postulated mechanism is that MCP-1 induced the production of O2•− and ONOO− in vascular endothelial cells, which then attenuated NO bioavailability and reduced NO-mediated vasodilation in the coronary microcirculation in type 2 diabetes. Furthermore, inflammatory cell infiltration was enhanced via the overexpression of TNF-α- and MCP-1-induced upregulation of cell adhesion molecules in coronary arterioles in type 2 diabetes. Our findings demonstrate that the endothelial dysfunction occurring in type 2 diabetes is the result of the effects of the inflammatory cytokine TNF-α and TNF-α-related signaling, including the expression of MCP-1, which also furthers oxidative stress.
GRANTS
This study was supported by a Pfizer Atorvastatin Research Award 2004-37; an American Heart Association Scientist Development Grant 110350047A; and National Heart, Lung, and Blood Institute Grants RO1-HL-077566 and RO1-HL-085119 (to C. Zhang).
REFERENCES
- 1.Beckman JS. Oxidative damage and tyrosine nitration from peroxinitrite. Chem Res Toxicol 9: 836–844, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol 233: 229–240, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Beckman JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler 375: 81–88, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Carvalho D, Savage C. Cytokines, adhesion molecules, antiendothelial cell autonatibodies and vascular disease. Cardiovasc Pathol 6: 61–78, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Constantin G, Majeed M, Giagulli C, Piccio L, Laudanna C. Chemokines trigger immediate beta 2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity 13: 759–769, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian W, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Leprdb mice. Circulation 115: 245–254, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Hein TW, Kuo L. cAMP-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, and KATP channels. Circ Res 85: 634–642, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmeyer MR, Scalia R, Ross CR, Jones SP, Lefer DJ. PR-39, a potent neutrophil inhibitor, attenuates myocardial ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 279: H2824–H2828, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Okamura A, Iwakura K, Masuyama T. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation 93: 1993–1999, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol Heart Circ Physiol 255: H1558–H1562, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Lattime EC, Stutman O. Thymic lymphomas mediate non-MHC-restricted, TNF-dependent lysis of the murine sarcoma WEHI-164. Cell Immunol 136: 69–79, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Lefer AM, Ma XL. Cytokines and growth factors in endothelial dysfunction. Crit Care Med 21, Suppl 2: S9–S14, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res 32: 733–742, 1999 [PubMed] [Google Scholar]
- 15.Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, Siddiqui MA. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation 104: 325–329, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Murao K, Ohyama T, Imachi H. TNF-stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochem Biophys Res Commun 276: 791–796, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atheroctomy specimens from patients with diabetes mellitus. Circulation 102: 2180–2184, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM. Fasting versus nonfasting triglycerides and the prediction of cardiovascular risk: do we need to revisit the oral triglyceride tolerance test? Clin Chem 54: 11–13, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Rimbach G, Valacchi G, Canali R, Virgili F. Macrophages stimulated with IFN-γ activate NF-κB and induce MCP-1 gene expression in primary human endothelial cells. Mol Cell Biol Res Commun 3: 238–242, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Ross R. Atherosclerosis: and inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Russo G, Leopold JA, Loscalso J. Vasoactive substances: nitric oxide and endothelial dysfunction in atherosclerosis. Vascul Pharmacol 38: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Salvemini D, Cuzzocrea S. Superoxide, superoxide dismutase and ischemic injury. Curr Opin Investig Drugs 3: 886–895, 2002 [PubMed] [Google Scholar]
- 23.Shyy YJ, Li YS, Kolattakudy PE. Structure of human monocyte chemoattractic protein gene and its regulation by TPA. Biochem Biophys Res Commun 169: 346–351, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Tangirala RK, Murao K, Quehenberger O. Regulation of expression of the human monocyte chemotactic protein-1 receptor (hCCR2) by cytokines. J Biol Chem 272: 8050–8056, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol 153: 2052–2063, 1994 [PubMed] [Google Scholar]
- 26.Vinten-Johansen J. Physiological effects of peroxynitrite: potential products of environment. Circ Res 87: 170–172, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Wang GP, Deng ZD, Ni J, Qu ZL. Oxidized low density lipoprotein and very low density lipoprotein enhance expression of monocyte chemoattractant protein-1 in rabbit peritoneal exudate macrophage. Atherosclerosis 133: 31–36, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C. Feed-forward signaling of TNF-α and NF-κB via IKK-β pathway contributes insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 296: H1850–H1858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian MW. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 26: 475–480, 2006 [DOI] [PubMed] [Google Scholar]