Abstract
Our previous studies have shown that nitric oxide (NO) synthase (NOS)-containing neurons in the rostral ventrolateral medulla (rVLM) are activated during cardiac sympathoexcitatory reflexes (Refs. 12 and 13). However, the precise function of NO in the rVLM in regulation of these reflexes has not been defined. Three isoforms of NOS, including neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS), are located in the rVLM. We explored the role of NO, derived from different NOS isoforms in the rVLM, in processing cardiac-sympathetic reflexes using whole animal reflex and electrophysiological approaches. We found that, in anesthetized cats, increased mean arterial blood pressure and renal sympathetic nerve activity elicited by epicardial application of bradykinin (BK; 1–10 μg/ml, 50 μl) were significantly attenuated following unilateral rVLM microinjection of the nonselective NOS inhibitor, Nω-nitro-l-arginine methyl ester (50 nmol/50 nl), or a specific nNOS inhibitor, 7-nitroindazole (7-NI; 5–10 pmol/50 nl; both P < 0.05). In contrast, the responses of mean arterial blood pressure and renal sympathetic nerve activity to cardiac BK stimulation were unchanged by unilateral rVLM microinjection of Nω-nitro-d-arginine methyl ester (inactive isomer of Nω-nitro-l-arginine methyl ester, 50 nmol/50 nl), 3–6% methanol (7-NI vehicle), N6-(1-iminoethyl)-l-lysine (250 pmol/50 nl; iNOS inhibitor), or N5-(1-iminoethyl)-l-ornithine (250 nmol/50 nl; eNOS inhibitor). Furthermore, in separate cats, we noted that iontophoresis of 7-NI (0.1 mM) reduced the increased discharge of cardiovascular sympathoexcitatory rVLM neurons in response to cardiac stimulation with BK (P < 0.05). These neurons were characterized by their responses to inputs from baroreceptors, and their cardiac rhythmicity was determined through frequency and time domain analyses, correlating their discharge to arterial blood pressure and cardiac sympathetic efferent nerve activity. These data suggest that NO, specifically nNOS, mediates sympathetic cardiac-cardiovascular responses through its action in the rVLM.
Keywords: heart, cardiovascular reflex, nitric oxide synthase
metabolites produced during myocardial ischemia, such as bradykinin (BK), stimulate cardiac sympathetic afferents and evoke excitatory cardiovascular reflexes, including acute hypertension and tachyarrhythmias, which can result in significant patient morbidity and mortality (18, 26, 33). The precise mechanisms underlying the control of these reflexes in the central nervous system are unknown. The rostral ventrolateral medulla (rVLM) is a critical site of the brain for controlling presympathetic vasomotor outflow and forms a necessary central component of sympathetic reflexes (15). There is now considerable evidence showing that nitric oxide (NO) in the rVLM regulates sympathetic outflow to the cardiovascular system (7, 19, 23). NO is formed from the amino acid, l-arginine, by NO synthase (NOS). Previous studies have suggested that NO in the brain exerts an overall excitatory effect on cardiac sympathoexcitatory responses (34). Recently, we observed that NO-producing neurons in the rVLM are activated during excitatory cardiac-cardiovascular reflexes (12). These results imply that endogenous NO in the rVLM contributes to the regulation of excitatory cardiac-cardiovascular reflexes.
Three isoforms of NOS in the rVLM have been identified, including neuronal NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS) (6, 9). While eNOS is located primarily in blood vessels, nNOS and iNOS are present in rVLM neurons (6). All three NOS isoforms are synthesized in the rVLM under physiological conditions (6), but they produce different effects on basal sympathetic vasomotor outflow. In this regard, nNOS or iNOS in the rVLM may cause sympathoexcitation or sympathoinhibition (4, 5). In addition, eNOS in the rVLM does not appear to contribute to maintenance of basal sympathetic vasomotor tone (6) but has been shown to be involved in angiotensin II-induced depression of the cardiac baroreflex in the nucleus tractus solitarii (27). Moreover, the prevalence of nNOS over iNOS activity in the rVLM suggests that nNOS is responsible for maintenance of neurogenic vasomotor tone by endogenous NO (5). The three NOS isoforms in the rVLM also are involved in cardiovascular responses associated with a number of physiological and pathological processes (5, 7, 20, 25). In this respect, activation of nNOS contributes to the pressor response to pulsatile compression of the rVLM (24). Downregulation of iNOS in the rVLM is responsible for augmented sympathetic vasomotor tone in spontaneously hypertensive rats (3). Overexpression of eNOS in the rVLM induces hypotension and bradycardia (20). Thus the role of NO within the rVLM in cardiovascular regulation appears to be highly complex and variable among the different NOS isoforms (5, 20, 25). Despite this wealth of information, the importance of these NOS isoforms in the rVLM in cardiac sympathoexcitatory reflexes has not been defined.
In the present study, we examined the role of endogenous rVLM NO in the regulation of excitatory cardiac cardiovascular reflexes. The following hypotheses were tested: 1) endogenous NO in the rVLM mediates sympathoexcitatory cardiac-cardiovascular reflexes; and 2) nNOS, but not iNOS nor eNOS, in the rVLM mainly contributes to processing sympathoexcitatory cardiac-cardiovascular reflexes.
METHODS
Animal Preparation
Adult cats of either sex (3–4 kg) were used for this study. All procedures were carried out in accordance with the National Institutes of Health guidelines. Surgical and experimental protocols of this study were approved by the animal use and care committee at the University of California, Irvine. The minimum possible numbers of cats were used to obtain reproducible and statistically significant results. Throughout the study, steps were taken to minimize discomfort of the animals. The cats were anesthetized initially with ketamine. A femoral vein was cannulated for intravenous injection of drugs. Anesthesia was maintained with α-chloralose (40–50 mg/kg iv). Adequate depth of anesthesia was verified by the absence of responses to noxious pinch of the paw. Supplemental doses of α-chloralose (20–30 mg/kg iv) were applied to maintain an appropriate level of anesthesia. A femoral artery was cannulated to allow measurement of arterial blood pressure (BP). After intubation of the trachea, respiration was maintained artificially (Harvard pump, model 661). Arterial blood gases was analyzed frequently (Radiometer, model ABL-3) and were maintained within physiological limits by adjusting the ventilator or intravenously injecting 1 M sodium bicarbonate. Body temperature was maintained between 36 and 38°C with a water-perfused heating pad. A median sternotomy was used to expose the anterior surface of the left ventricle. Excitatory cardiac reflexes were induced by epicardial application of BK, as described in our previous studies (14, 34). BK is a particularly relevant chemical stimulus, since it is produced during myocardial ischemia and activates cardiac sympathetic afferents (26, 33). BP and heart rate (HR) were monitored throughout the experiment and were recorded during application of BK (Spike2 Version 5, Cambridge Electronic Design).
Exposure of Ventral Medulla Oblongata
The animal was mounted on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) in the spine position. The head was held rigidly by steel plugs introduced through the auditory meatus and by bars holding the upper jaw. The ventral surface of the medulla was exposed by a partial occipital craniotomy, as our laboratory has described previously (40). Briefly, the trachea and esophagus were retracted. After detaching the prevertebral muscles from the basal of the skull, the basal occipital bone was removed. The craniotomy was extended for ∼4.5 mm on each side of the midline over the surface of the medulla. The dura was cut and cerebrospinal fluid removed. The surface of the medulla was covered immediately with warm normal saline solution.
Microinjection into the rVLM
In pilot experiments, we noted that consistent repetitive pressor responses induced by epicardial application of BK (1–10 μg/ml, 50 μl) could be obtained after placing glass micropipette(s) unilaterally but not bilaterally into the rVLM (two animals for each approach). Moreover, unilateral microinjection of a nonselective NOS inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME, 50 nmol/50 nl), into the rVLM significantly attenuated BK-induced cardiac pressor reflexes (n = 2). Thus unilateral microinjection into rVLM was used in the present study. Microinjection was performed unilaterally with a glass micropipette (tip diameter: 10∼30 μm) positioned by a micromanipulator (Kopf Instruments). Through a fluorinated ethylene propylene tubing (0.12-mm inner diameter) and tubing adaptors, a micropipette was connected to a microsyringe fastened to microdialysis pump (CMA/102, North Chelmsford, MA). The injection was carried out at a rate of 0.6 μl/min over a 5-s period. The total volume of injection was 50 nl. Injections were located 1.5∼2.5 mm caudal to the trapezoid body, 2.8∼3.6 mm lateral to the midline, at depths of 1.0∼2.0 mm below the ventral surface, as we described previously (40). The rVLM was identified by a pressor response to glutamate (2 nmol, 50 nl) and later confirmed by histological examination of stain from 0.5% pontamine sky blue, which was injected along with the chemicals tested (7). After fixation in 10% paraformaldehyde, the medulla oblongata was cut into 60-μm coronal sections using a cryostat and stained subsequently with neutral red. The site of injection was identified by the sky blue marker (40).
Single-unit Extracellular Recording and Iontophoresis of Chemicals in the rVLM
A three-barrel micropipette for combination recording and iontophoresis was used for extracellular neuronal recordings in the rVLM, as we have described previously (30, 32). The three barrels contained a recording electrode merged in Chicago blue, a testing chemical, and 4 M NaCl to balance the current. Chemicals loaded in micropipette barrels were administered by iontophoresis, as described below (31). The pipette was inserted into the rVLM through a ventral approach, according to stereotaxic coordinates noted above for microinjection. Location of the pipette tip in the rVLM was identified preliminarily by the pressor responses following microinjection of glutamate (2 nmol, 50 nl) and further confirmed histologically, as described above. The three-barrel micropipette was advanced slowly until cellular electrical activity was recorded. Neuronal activity was amplified, filtered, and delivered to an oscilloscope, audio monitor, and chart recorder, and transferred to a computer for analysis (EGAA, RC Electronics or Spike2 Version 5, Cambridge Electronic Design). Procedures for recording and analyzing cellular activity in the rVLM were analogous to those described in examples provided in Fig 5. Neurons in the rVLM were classified as cardiovascular sympathoexcitatory, if they responded to manipulation of baroreceptors and displayed concordance with cardiovascular pulse and/or cardiac sympathetic efferent activity using time and frequency domain analysis (pulse- or spike-triggered averaging and coherence) (30, 31).
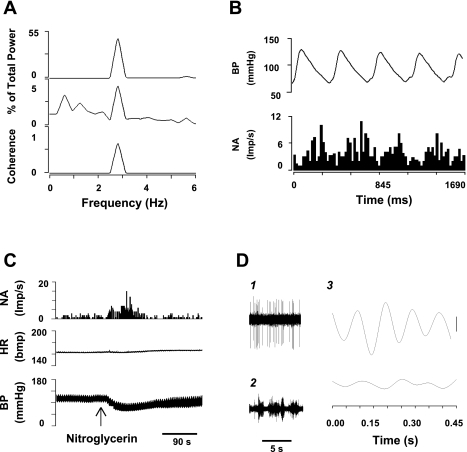
Fig. 5.
Characteristics of a cardiac sympathoexcitatory neuron in the rVLM. A: autospectra (AS) of arterial BP (top trace), rVLM neuronal activity (NA) (middle trace) and coherence (bottom trace). B: arterial pulse-triggered analysis of NA. Frequency domain analysis shows a significant coherence between BP and rVLM activity (0.69; A), while time domain analysis using pulse-triggered averaging demonstrated a relationship between BP and rVLM activity (B). C: changes in BP, heart rate (HR), and NA after intravenous administration of nitroglycerin (300 μg/kg). D: spike-triggered analysis of NA and inferior cardiac sympathetic nerve discharge (SND) suggests a relationship between NA and SND. 1, tracings of NA; 2, tracings of SND; 3, spike-triggered (top) and dummy-triggered (bottom) averages of SND. Vertical calibration is 10 counts.
Iontophoresis.
To apply small quantities of drugs in the close neighborhood of neurons in the rVLM, as our laboratory has described previously (30, 31), we used the technique of iontophoresis, in which a current was passed through the iontophoresis pipette using a current-balancing system from a constant-current source (28). Retention current for balancing is +25 to −10 nA. The current pump monitors the voltage applied to the pipette and was observed constantly to monitor blockage of the tip, signaled by an abrupt increase in the pipette's resistance. The drugs were injected over a 2-min period with currents between 10 and 120 nA, as described in the experimental protocol below.
Pulse and spike-triggered averaging.
As our laboratory and others have described previously (30, 31), time domain analysis was used to evaluate the relationship in time between rVLM neuronal activity and arterial BP or cardiac sympathetic nerve activity (SNA) (1, 21). Activity of rVLM neurons tightly related in time to pressure pulse or cardiac SNA provide a measure of the time relationship between these neurons and cardiovascular/cardiac sympathetic activity. For time domain analysis, arterial pulse- or cardiac sympathetic spike-triggered averaging methods used a threshold that was set at the systolic phase of the arterial pulse or cardiac sympathetic nerve discharge. We used spike height discrimination and waveform recognition to sort action potentials during the 300-s period of evaluation. Averages of the arterial pulse and histograms of cardiac SNA and rVLM neuronal activity were constructed.
Coherence assessment.
Frequency domain analysis was used to assess coherence of rVLM neuronal activity with arterial BP or cardiac SNA, as described previously (1, 21, 30, 31), and as shown in Fig. 5. Original data were recorded with a sampling rate of 10,000 Hz. Reconstructed data utilized every 10th sample and included assessment of the mean and peak amplitudes, as well as the maximum and minimum slopes of the original spike, to be certain that all action potentials were preserved. Action potentials were sorted and identified with a window discriminator to construct histograms before coherence analysis. The number of data sections (15–20, each lasting for 12.8 s) was chosen to determine the average histogram. Autospectra of rVLM discharge and arterial BP were generated with a fast Fourier transform. Thus coherence was generated with seven overlapping windows, each with a length of 12.8 s, consisting of 256 bins with bin widths of 50 ms. The autospectral analysis was generated using contiguous segments of 256 beats with 50% overlap between the segments. The frequency resolution was 1/12 s or 0.08 Hz. The coherence function (normalized cross spectrum) provided a measure of the strength of linear correlation of rVLM neuronal activity and cardiac SNA or BP at each frequency. Coherence of ≥0.5 indicates a statistically significant relationship between the frequencies of two variables.
Sympathetic Nerve Activity
Multiunit SNA of cardiac or renal sympathetic nerves was recorded, as described previously (10, 32). Briefly, using a left retroperitoneal approach, a branch of the renal nerve was separated from the renal plexus and surrounding connective tissue with the aid of an operating microscope (Zeiss, German). For cardiac nerve recording, the left inferior cardiac branch was isolated through a sternotomy approach. The renal or cardiac nerve was cut distally to eliminate afferent discharge. The central end of the nerve was placed across a stainless steel electrode. The site then was covered with mineral oil. The signal from the electrodes was amplified and filtered (100–3,000 Hz) with a preamplifier (model P511K, Grass Instrument), displayed on an oscilloscope and monitored with an audio amplifier (model AM8, Grass Instrument). Data were fed into a Cambridge Electronics Design (CED) 1401 laboratory interface coupled to a Dell Pentium 4 computer. Data were digitized and recorded using Spike 2 (CED) software for storage and subsequent offline quantitative analysis. The quality of renal or cardiac SNA was assessed by pulse synchronous rhythmicity and decreased magnitude of firing in response to sinoaortic baroreceptor loading induced by intravenous administration of phenylephrine (1–4 μg). At the end of the experiment, the baseline noise level was established by quantifying the remaining electrical signal after administration of 2% lidocaine to the renal or cardiac nerve and crushing the nerve proximal to the recording electrode. Spikes were considered to represent renal or cardiac nerve discharge when they exceeded a threshold set by placing a low-threshold cursor just above background electrical noise. The nerve discharge signals were rectified and counted (10).
Drug Administration
Test agents used in this study included a nonselective NOS inhibitor, l-NAME; an inactive isomer of l-NAME, Nω-nitro-d-arginine methyl ester (d-NAME); a specific nNOS inhibitor, 7-nitroindazole (7-NI); a selective iNOS inhibitor, N6-(1-iminoethyl)-l-lysine (l-NIL); and a potent eNOS inhibitor, N5-(1-iminoethyl)-l-ornithine (l-NIO; both from A. G. Scientific). All other chemicals were obtained from Sigma Chemical (St Louis, MO). Chemical and drug solutions were prepared fresh daily. They were dissolved in artificial cerebrospinal fluid (aCSF, pH 7.4) containing 0.5% pontamine sky blue for subsequent histological site verification (7). The vehicle for 7-NI included 3–6% methanol. Fifty nanoliters of each agent were injected. Possible volume effect of microinjection was assessed by injecting the same amount of aCSF or the appropriate solvent. To avoid confounding effects of drug interactions, each animal received only one test agent or vehicle, which was microinjected into the rVLM 30 min after the completion of glutamate (Sigma) application to determine the location of the pipette in a pressor region (7). BK (1–10 μg/ml, Sigma) was dissolved in 0.9% NaCl and was applied directly to the surface of the heart.
Experimental Protocols
Cardiac reflexes studies.
After the sternotomy, craniotomy, placement of the renal nerve electrode, and obtaining an appropriate pattern for renal sympathetic nerve discharge, a micropipette was placed unilaterally in the rVLM. Repeated epicardial application of BK was performed to induce excitatory cardiac-cardiovascular reflexes every 30 min for three or four successive applications. Microinjection of drugs or vehicles for the drugs into the rVLM was performed 5 min before the second application of BK. BP, HR, and SNA were recorded during repeated application of BK to the epicardial surface. Our laboratory has observed in several studies, as a time control, that the cardiovascular response to epicardial BK is consistent for at least six repeated applications, spaced 20–30 min apart (12, 14, 34).
Animals were randomly divided into the following seven groups: 1) a treatment group involving l-NAME (50 nmol/50 nl); 2) a treatment group using d-NAME (50 nmol/50 nl); 3) a treatment group employing the inhibitor of nNOS, 7-NI (5–10 pmol/50 nl); 4) a vehicle control group for the inhibitor of nNOS, 3–6% methanol; 5) a treatment group applying the inhibitor of iNOS, l-NIL (250 pmol/50 nl); 6) a treatment group using the inhibitor of eNOS, l-NIO (250 nmol/50 nl); 7) a vehicle control group for the inhibitors of iNOS and eNOS, aCSF. The effective concentrations of these NOS inhibitors have been determined by their influence on the cardiovascular system in other previous studies (4–6) and in our pilot experiments. In this respect, other studies have shown that bilateral microinjection of 7-NI (5 pmol/50 nl) or l-NIL (250 pmol/50 nl) into the rVLM of the rat decreases or increases, respectively, arterial BP (5). Bilateral application of l-NIO (92 nmol/50 nl) to the rVLM does not change arterial BP in the rat (6), although a similar dose of l-NIO has been shown to cause contraction of aorta in vitro and to increase arterial BP following intravenous administration (29). To be sure, we achieved adequate blockade; we administered dosages of l-NIO that were more than twofold higher than those used in those previous investigations.
Studies of rVLM neuronal activity.
Cats were instrumented for electrophysiological recordings and iontophoresis in the rVLM, as mentioned above. An electrode also was placed around the left inferior cardiac nerve. After identifying a neuron that responded to epicardial BK, nNOS inhibition was achieved through iontophoresis of 7-NI (pH 6.5) and the neuronal response to BK was reevaluated. A control group of additional animals was assessed in an identical manner using repetitive stimulation with BK, with the exception that the vehicle, 3% methanol, was substituted for the inhibitor. A negative current of 10–120 nA was used for iontophoresis of 7-NI for 2 min. A positive current of 5–10 nA was used to prevent leakage of the drugs. The balancing current was achieved through 4 M NaCl solution, as described in methods. The rVLM neuronal response to cardiac stimulation with BK was evaluated before and after ionotophoretic application of 7-NI (0.1 mM). A series of maneuvers were used to classify the neuronal phenotype before application of 7-NI. The response to baroreceptor stimulation/inhibition was assessed to determine whether the neuron was sympathoexcitatory. A 5-min period of recording allowed frequency domain analysis of the coherence of neuronal discharge activity with BP and time domain analysis using pulse-triggered averaging. Finally, we used spike-triggered analysis to compare the relationship between rVLM and cardiac sympathetic efferent activity.
Statistical Analyses
Animals were included for statistical analysis, if the site for microinjection or neuronal recording was found to be in the rVLM, as determined by subsequent histological examination. Values are expressed as means ± SE and were considered to be significantly different when P < 0.05. A statistical software package, SigmaStat (version 3.0, Jandel Scientific), was used for these analyses. Mean BP, HR, and RSNA were compared in each group by using a one-way repeated-measures analysis of variance followed by a Tukey post hoc test. If data were not normally distributed, as determined by the Kolmogorov-Smirnov test, they were compared using the Friedman repeated-measures ANOVA on ranks with a Dunnett's post hoc test. Medullary neuronal discharge activity was evaluated in relation to BP and/or cardiac SNA using coherence analysis and pulse- or spike-triggered averaging, in addition to responses to baroreceptor unloading/stimulation (Fig. 5). Paired responses of rVLM neurons to BK stimulation on the heart were examined using a paired Student's t-test.
RESULTS
Epicardial BK-induced Increases in BP and RSNA Following Application of Nonspecific NOS Inhibitor in the rVLM
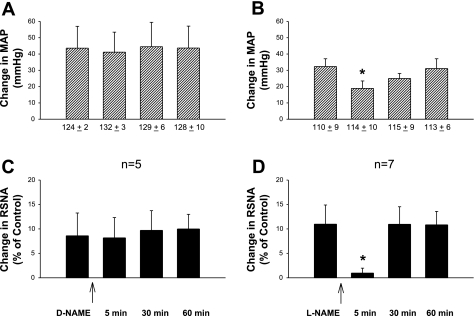
Similar to previous observations (14, 34), in the present study, we found that repetitive epicardial application of BK (1–10 μg/ml, 50 μl) caused very consistent increases in mean arterial BP (MAP) and RSNA, but not HR. These increased changes were not influenced by unilateral microinjection of d-NAME (50 nmol, 50 nl), an inactive isomer of l-NAME, into the rVLM (Fig. 1) of five cats. However, 5 min after unilateral microinjection of l-NAME (50 nmol, 50 nl), a nonselective NOS inhibitor into the rVLM, the enhanced MAP and RSNA induced by epicardial application of BK were significantly attenuated in seven animals (both P < 0.05). The BP and RSNA responses to BK stimulation returned to the control levels 30 and 60 min after application of l-NAME (Fig. 1). Application of l-NAME did not alter resting MAP (Fig. 1) and RSNA.
Fig. 1.
Changes in increased mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) induced by epicardial application of bradykinin (BK) following unilateral rostral ventrolateral medulla (rVLM) microinjection of a nonselective nitric oxide synthase (NOS) inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME; 50 nmol/50 nl; B and D), or Nω-nitro-d-arginine methyl ester (d-NAME), an inactive isomer of l-NAME (50 nmol/50 nl; A and C). Values under bars in A and B represent MAP before application of BK. *P < 0.05, compared with control before rVLM microinjection.
BK-induced Increases in BP and RSNA Following nNOS, iNOS, or eNOS Inhibition in the rVLM
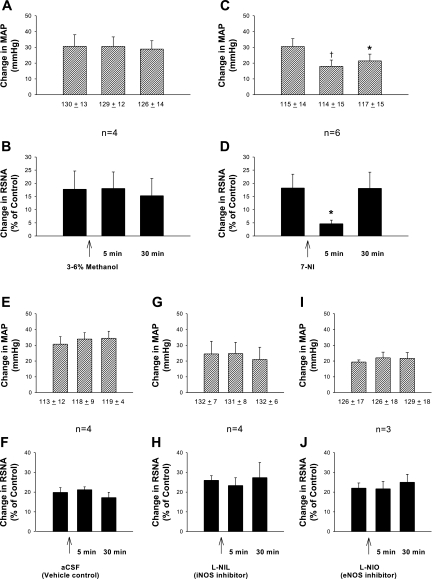
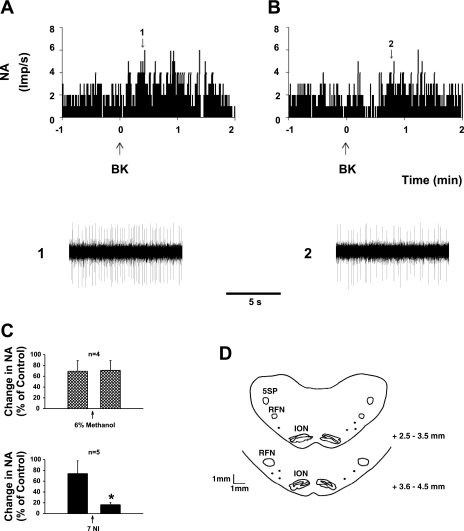
Similar to the responses mentioned above, three repeated epicardial applications of BK evoked consistent increases in MAP and RSNA, following unilateral rVLM injection of 3–6% methanol (50 nl), the vehicle for 7-NI 5 min before the second application of BK (n = 4; Fig. 2). Increases in MAP and RSNA induced by BK, however, were significantly reduced (both P < 0.05) compared with controls 5 min following unilateral microinjection of the nNOS inhibitor, 7-NI (5–10 pmol, 50 nl; n = 6; Fig. 2). Enhanced RSNA, but not MAP, returned to control levels 30 min after rVLM application of 7-NI. Resting MAP and RSNA were unchanged by application of 7-NI (Figs. 2 and 3). There was a similar effect of 7-NI in doses of 5 and 10 pmol (n = 4, and 2, respectively). Figure 3 displays original tracings of BP and RSNA responses before and after application of 7-NI on one animal.
Fig. 2.
Responses of MAP and RSNA induced by epicardial application of BK following unilateral rVLM microinjection of each specific inhibitor of the three NOS isoforms. A and B: vehicle for 7-nitroindazole (7-NI; 3–6% methanol, 50 nl); C and D: neuronal NOS (nNOS) inhibitor, 7-NI (5–10 pmol, 50 nl); E and F: vehicle, artificial cerebrospinal fluid (aCSF; 50 nl); G and H: inducible NOS (iNOS) inhibitor, N6-(1-iminoethyl)-l-lysine (l-NIL; 250 pmol, 50 nl); I and J: endothelial NOS (eNOS) inhibitor, N5-(1-iminoethyl)-l-ornithine (l-NIO; 250 nmol, 50 nl). Numbers under bars in the A, C, E, G, and I indicate MAP before application of BK. *P < 0.05, †P < 0.01, compared with control before rVLM microinjection.
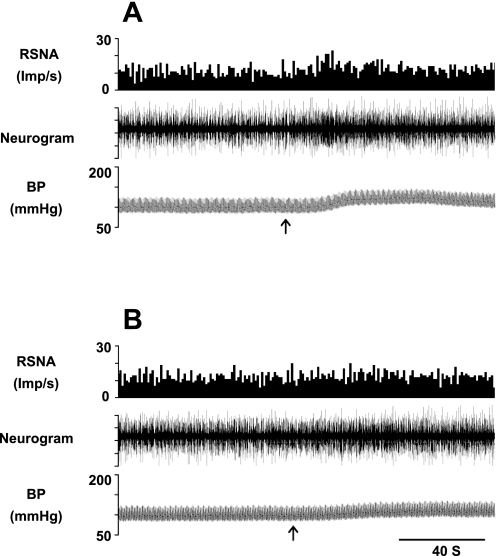
Fig. 3.
Representative examples of arterial blood pressure (BP) and RSNA responses to epicardial application of BK (10 μg/ml, 50 μl) before (A) and 5 min after (B) unilateral microinjection of 7-NI (5 pmol, 50 nl) into the rVLM of one cat. Top: neurohistogram of RSNA after rectification; middle: nerve tracings displaying raw RSNA; bottom: original recordings of BP. Arrows indicate time points when BK was applied.
In contrast to the action of 7-NI, increased MAP and RSNA elicited by epicardial application of BK were unchanged by unilateral rVLM microinjection of a specific inhibitor of either iNOS, l-NIL (250 pmol, 50 nl; n = 4), or eNOS, l-NIO (250 nmol, 50 nl; n = 3; Fig. 2), despite using doses that have been shown previously to be effective in inhibiting those two isoforms. The absence of responses to iNOS and eNOS blockade was similar to findings noted when aCSF (50 nl), the vehicle for both l-NIL and l-NIO, was injected unilaterally into the rVLM (n = 4; Fig. 2). In these three groups, consistent increases in MAP and RSNA were found following three repetitive epicardial applications of BK, despite treatment with any one of these three agents before the second application of BK (Fig. 2).
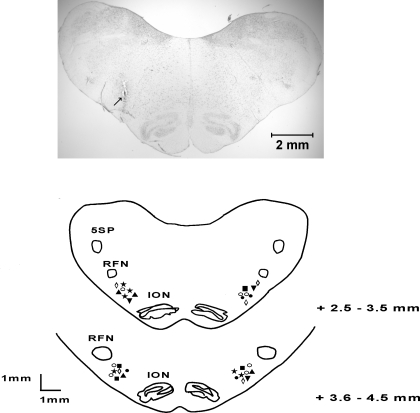
Figure 4 shows the microinjection sites of experimental agents in all groups, which were found to be in the rVLM, according to Berman's atlas for cat and in line with our previous studies (2, 14, 30, 40).
Fig. 4.
Top: original slide of the medulla oblongata showing actual microinjection site, indicated by arrow, in rVLM of a cat. Scale bar represents 2 mm. Bottom: composite maps of histologically verified sites of microinjection in rVLM. Sections represent combinations of medullary planes rostral to obex (11). Each symbol represents one site of injection of a drug or solution: ◊, d-NAME; ○, l-NAME; ▴, 3–6% methanol; ★, 7-NI; ■, aCSF; ●, l-NIL; ▾, l-NIO. 5SP, spinal trigeminal nucleus; ION, inferior olivary nucleus; RFN, retrofacial nucleus.
Effect of nNOS Inhibition on Response of BK-induced Increase in Cardiovascular Neuronal Activity
After identification of a cardiovascular-related sympathoexcitatory neuron described above (Fig. 5), the response to epicardial application of BK was examined (Fig. 6). We found that repetitive epicardial application of BK consistently increased extracellular activity of cardiovascular-related sympathoexcitatory neurons in the rVLM. These responses were not influenced by iontophoresis of 3% methanol, the vehicle for 7-NI into the rVLM (Fig. 6; n = 4). However, the increased rVLM neuronal discharge induced by epicardial application of BK was significantly reduced by rVLM iontophoresis of 7-NI (0.1 mM; n = 5, P < 0.05; Fig. 6). Basal discharge of rVLM neurons was not altered by application of 7-NI (Fig. 6). The sites of recording and iontophoresis were histologically confirmed in the rVLM, according to Berman's atlas for cat, and was shown to be in with the same region as our previous studies, as demonstrated in Fig. 6 (2, 14, 30, 40).
Fig. 6.
A and B: neurohistograms showing rVLM NA in response to epicardial application of BK (10 μg/ml) before (A) and after (B) iontophoresis of the nNOS inhibitor, 7-NI. 1 and 2: representative tracings of discharge activity at times indicated by the arrows above histograms. C: changes in NA of group of neurons responsive to cardiac BK stimulation before and after iontophoresis of 7-NI (bottom) or vehicle (top). *P < 0.05, compared with control before application of 7-NI. D: mapping sites of rVLM neuronal recordings in medullary planes rostral to obex.
DISCUSSION
In the present study, we found that cardiac sympathoexcitatory pressor responses are attenuated following unilateral inhibition of NOS, particularly nNOS in the rVLM. Moreover, inhibition of nNOS suppressed the increased activity of cardiovascular-related sympathoexcitatory neurons in the rVLM in response to chemical stimulation in the heart. Our data for the first time suggest a predominantly stimulatory role for NO in the rVLM in the regulation of sympathoexcitatory cardiovascular responses induced by cardiac stimulation.
Metabolites produced during myocardial ischemia stimulate cardiac sympathetic afferents to cause reflex hypertension and potentially lethal ventricular tachyarrhythmias (18, 33, 36). However, very few studies of central neural integration of cardiac-cardiovascular sympathoexcitatory reflexes have been conducted, and hence little is known about mechanisms underlying central regulation of these reflexes. Recently, we have located a number of regions in the brainstem that are activated during cardiac sensory stimulation (12, 14). In the present study, we evaluated the role of the rVLM in regulation of cardiac-cardiovascular sympathoexcitatory reflex responses, since this nucleus represents an important region that controls sympathetic outflow and hence cardiovascular function (15).
NO is formed by the action of NOS on l-arginine. There is growing evidence showing that NO adjusts sympathetic activity and BP in the rVLM (16, 25). However, past literature is mixed and frequently is internally inconsistent with respect to the modulatory influence of NO in the rVLM. For example, pharmacological studies in cats and other species show that NO interacts with both inhibitory, e.g., GABA, and excitatory neurotransmitters, including N-methyl-d-aspartate and α-amino- 3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in the rVLM, and, as such, may yield either cardiovascular inhibitory or excitatory responses (7, 35). Furthermore, NO donors administered into the rVLM can either stimulate or inhibit baseline BP in the absence of any reflex, depending on the dose (16, 25). Although the variable actions of NO in the rVLM observed in different species might be explained by interspecies variation in NOS density and distribution (16, 39), inconsistent results also have been shown within the same species (7, 25, 35). In these cases, opposite responses may be due to different experimental approaches. In this respect, Wu and coworkers (37) demonstrated, in cats, involvement of NO in glutamate-related excitation in the rVLM, whereas Zanzinger's group (38) observed inhibitory effect of NO on rVLM following application of NO donors and inhibitors in larger volumes (500 nl) than usual (50–100 nl). Such large volumes may result in diffusion of drugs to regions outside the rVLM, such as lateral tegmental field and medial depressor area, that contain different concentrations and hence ratios of sympathoinhibitory and sympathoexcitatory neurons (8, 11). In addition, some studies are internally inconsistent, showing no response of baseline BP and inhibition of glutamate-induced BP responses following both antagonism of NO production and administration of NO donors in the cat rVLM (7). In light of the variable results from previous studies, it has been unclear if NO exerts an inhibitory or a facilitatory influence on rVLM neurons that process cardiac sympathoexcitatory reflexes. In addition to their overall predominantly pharmacological orientation, many studies have relied on microinjection to investigate the action of NO in the rVLM, a method that does not distinguish between different types of neurons, for example, those that participate in central processing of cardiac-cardiovascular reflexes. There are few, if any, electrophysiological studies evaluating the influence of NO in the rVLM. To circumvent these problems, the present study evaluated the function of NO in processing cardiac sympathoexcitatory reflexes and its effects on cardiovascular-related sympathoexcitatory neurons in the rVLM using cardiovascular reflex and neuronal electrophysiological approaches that overcame limitations of previous studies.
Previously, we found that neurons containing NOS in the rVLM are activated by cardiac sympathoexcitatory reflexes (12). We speculated, therefore, that the rVLM processes excitatory reflexes from the heart, in part, through an NO mechanism. In the present study, we noted that nonspecific inhibition of rVLM NOS with l-NAME attenuates sympathoexcitatory cardiovascular reflex activation caused by cardiac stimulation with BK. This result strongly suggests an excitatory role for endogenous NO in the rVLM in regulation of cardiac sympathoexcitatory reflexes.
Three NOS isoforms, nNOS, iNOS, and eNOS, are located in the rVLM (6). While nNOS and iNOS are associated with nucleated cells, including neurons and glia, eNOS is associated with vascular endothelial cells (9). Variability in the role of NO within the rVLM in cardiovascular regulation exists among different NOS isoforms in various physiological and pathophysiological conditions. For example, nNOS is associated with sympathetic excitation, while iNOS is related to inhibition of sympathetic outflow (5). nNOS is more prevalent than iNOS, such that NO-related sympathetic excitation predominates and contributes to maintenance of basal sympathetic outflow and vasomotor tone (5). Inhibition of nNOS in the rVLM reduced pressor response to pulsatile compression of this area. Downregulation of iNOS in the rVLM augments sympathetic vasomotor tone in spontaneously hypertensive rats (3). eNOS contributes only minimally to basal BP, although its overexpression in the rVLM can lead to hypotension and bradycardia (5, 6, 20). We have found that global inhibition of brain nNOS attenuates the cardiovascular pressor responses to epicardial application of BK (34). However, there is no information on the specific roles of NOS isoforms in facilitating or inhibiting excitatory cardiac-cardiovascular responses. Interestingly, in the current study, we found that unilateral microinjection of specific inhibitors of nNOS into the rVLM attenuates sympathoexcitatory cardiac-cardiovascular reflexes. These reflexes were not changed following unilateral application of iNOS or eNOS inhibitors. These findings extend previous observations noted above, which suggested that nNOS, but not iNOS nor eNOS, is associated with sympathoexcitatory activity in the rVLM. Our data imply that nNOS likely serves as the principal source of NO in the rVLM in excitatory responses to cardiac stimulation. As such, the present study amplifies past studies, suggesting that NO in the rVLM, especially that derived from nNOS, participates in processing cardiac sympathoexcitatory responses (7, 12).
Epicardial stimulation with BK activates barosensitive, but not baro-insensitive, neurons in the rVLM (22). Thus, during ischemia, BK likely stimulates cardiac sympathetic (spinal) afferents to increase rVLM neuronal activity in a population of cells that are inhibited by baroreceptor input and hence are sympathoexcitatory (31, 33). In the present study, we specifically examined a population of rVLM neurons, which responded to epicardial cardiac stimulation with BK that were barosensitive and showed strong correlation with cardiac sympathetic efferent activity, as determined by time domain analysis (Fig. 5). Moreover, their activities were tightly related to BP, as identified by both time and frequency domain analyses using pulse-triggered averaging and coherence between neuronal activity and the cardiac cycle (Fig. 5). Thus these neurons could be classified as cardiovascular-related sympathoexcitatory cells. Iontophoresis of the nNOS inhibitor into the region where we recorded neuronal firing attenuated the increased discharge of sympathoexcitiatory neurons associated with cardiac stimulation. As such, NO formed by the action of nNOS facilitates discharge of rVLM sympathoexcitatory cardiovascular neurons responsive to cardiac stimulation.
Our previous data using triple labeling showed that rVLM neurons activated by stimulation of cardiac sympathetic pathways contain glutamate, as well as nNOS (13). Through a cGMP/protein kinase G mechanism, NO facilitates the action of glutamate in the rVLM (4, 17). Thus, in combination, our findings suggest that NO may regulates cardiac reflexes through glutamate in rVLM. Further studies will be required to explore this possibility.
In summary, in the present study, we extended our laboratory's previous anatomic and physiological findings (12, 34) to investigate the role of the NO and isoforms of NOS in rVLM in processing cardiac sympathoexcitatory reflexes. We found that nonselective inhibition of NOS in the rVLM attenuates sympathoexcitatory cardiac-cardiovascular reflexes. In particular, we noted that these reflexes are attenuated by specific inhibition of rVLM nNOS, but not by iNOS or eNOS inhibition, suggesting that endogenous NO, especially that derived from nNOS, contributes to the processing of cardiac sympathoexcitatory reflexes in the rVLM. This information helps elucidate the role of NO in the rVLM in the regulation of excitatory visceral cardiovascular reflexes, including sympathoexcitatory responses induced by cardiac stimulation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-66217, the Larry K. Dodge and Susan-Samueli Endowed Chairs (J. C. Longhurst), and American Heart Association Western Affiliate Grants 0160077Y and 0365064Y (Z.-L. Guo).
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Vu Thai Nguyen.
REFERENCES
- 1.Barman SM, Gebber GL. Subgroups of rostral ventrolateral medullary and caudal medullary raphe neurons based on patterns of relationship to sympathetic nerve discharge and axonal projections. J Neurophysiol 77: 65–75, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Berman AL. The Brainstem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates Madison, WI: University of Wisconsin, 1968 [Google Scholar]
- 3.Chan JY, Wang LL, Wu KL, Chan SH. Reduced functional expression and molecular synthesis of inducible nitric oxide synthase in rostral ventrolateral medulla of spontaneously hypertensive rats. Circulation 104: 1676–1681, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chan SH, Wang LL, Chan JY. Differential engagement of glutamate and GABA receptors in cardiovascular actions of endogenous nNOS or iNOS at rostral ventrolateral medulla of rats. Br J Pharmacol 138: 584–593, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan SH, Wang LL, Wang SH, Chan JY. Differential cardiovascular responses to blockade of nNOS or iNOS in rostral ventrolateral medulla of the rat. Br J Pharmacol 133: 606–614, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang AY, Chan JY, Chan SH. Differential distribution of nitric oxide synthase isoforms in the rostral ventrolateral medulla of the rat. J Biomed Sci 10: 285–291, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chen SY, Mao SP, Chai CY. Role of nitric oxide on pressor mechanisms within the dorsomedial and rostral ventrolateral medulla in anaesthetized cats. Clin Exp Pharmacol Physiol 28: 155–163, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Dempesy CW, Richardson DE, Fontana CJ. Cardiovascular sympathoinhibitory neurons form an extended longitudinal column in cat lateral medulla. Brain Res 603: 328–332, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Forstermann U, Kleinert H, Gath I, Schwarz P, Closs E, Dun N. Expression and expressional control of nitric oxide synthases in various cell types. Adv Pharmacol 34: 171–186, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Fu LW, Phan A, Longhurst JC. Myocardial ischemia-mediated excitatory reflexes: a new function for thromboxane A2? Am J Physiol Heart Circ Physiol 295: H2530–H2540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebber GL, Barman SM. Lateral tegmental field neurons of cat medulla: a potential source of basal sympathetic nerve discharge. J Neurophysiol 54: 1498–1512, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Guo ZL, Longhurst J. Activation of nitric oxide-producing neurons in the brain stem during cardiac sympathoexcitatory reflexes in the cat. Neuroscience 116: 167–178, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Guo ZL, Longhurst J. Responses of neurons containing VGLUT3/nNOS-cGMP in the rVLM to cardiac stimulation. Neuroreport 17: 255–259, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Guo Z, Lai H, Longhurst J. Medullary pathways involved in cardiac sympathoexcitatory reflexes in the cat. Brain Res 925: 55–66, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Guyenet PG, Haselton JR, Sun MK. Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog Brain Res 81: 105–116, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Hirooka Y, Polson JW, Dampney RAL. Pressor and sympathoexcitatory effects of nitric oxide in the rostral ventrolateral medulla. J Hypertens 14: 1317–1324, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Huang CC, Chan SH, Hsu KS. cGMP/protien kinase G-dependent potentiation of glutamatergic transmission induced by nitric oxide in immature rat rostral ventrolateral medilla neurons in vitro. Mol Pharmacol 64: 521–532, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Huang HS, Stahl G, Longhurst J. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. Am J Physiol Heart Circ Physiol 268: H2114–H2124, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Kagiyama S, Tsuchihashi T, Abe I, Fujishima M. Cardiovascular effects of nitric oxide in the rostral ventrolateral medulla of rats. Brain Res 757: 155–158, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension 38: 896–901, 2001 [PubMed] [Google Scholar]
- 21.Kocsis B, Gebber GL, Barman SM, Kenney MJ. Relationships between activity of sympathetic nerve pairs: phase and coherence. Am J Physiol Regul Integr Comp Physiol 259: R549–R560, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Li DP, Pan HL. Responses of neurons in rostral ventrolateral medulla to activation of cardiac receptors in rats. Am J Physiol Heart Circ Physiol 279: H2549–H2557, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Martins-Pinge M, Baraldi-Passy I, Lopes O. Excitatory effects of nitric oxide within the rostral ventrolateral medulla of freely moving rats. Hypertension 30: 704–707, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Morimoto S, Sasaki S, Miki S, Kawa T, Itoh H, Nakata T, Takeda K, Nakagawa M. Pressor response to pulsatile compression of the rostral ventrolateral medulla mediated by nitric oxide and c-fos expression. Br J Pharmacol 129: 859–864, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morimoto S, Sasaki S, Miki S, Kawa T, Nakamura K, Itoh H, Nakata T, Takeda K, Nakagawa M, Fushiki S. Nitric oxide is an excitatory modulator in the rostral ventrolateral medulla in rats. Am J Hypertens 13: 1125–1134, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Nerdrum T, Baker D, Coleridge H, Coleridge J. Interaction of bradykinin and prostaglandin E1 on cardiac pressor reflex and sympathetic afferents. Am J Physiol Regul Integr Comp Physiol 250: R815–R822, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol 531: 445–458, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purves RD. Ionophoresis. In: Microelectrode Methods for Intracellular Recording and Ionophoresis New York: Academic, 1981, p. 92–102 [Google Scholar]
- 29.Rees D, Palmer R, Schulz R, Hodson H, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 101: 746–752, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjen-ALooi SC, Li P, Longhurst JC. Role of medullary GABA, opioids, and nociceptin in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am J Physiol Heart Circ Physiol 293: H3627–H3635, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Tjen-ALooi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton Neurosci 106: 119–131, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Tjen-ALooi S, Bonham A, Longhurst J. Interactions between sympathetic and vagal cardiac afferents in nucleus tractus solitarii. Am J Physiol Heart Circ Physiol 272: H2843–H2851, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Tjen-ALooi S, Pan HL, Longhurst JC. Endogenous bradykinin activates ischaemically sensitive cardiac visceral afferents through kinin B2 receptors in cats. J Physiol 510: 633–641, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjen-ALooi SC, Phan NT, Longhurst JC. Nitric oxide modulates sympathoexcitatory cardiac-cardiovascular reflexes elicited by bradykinin. Am J Physiol Heart Circ Physiol 281: H2010–H2017, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Tseng CJ, Liu HY, Lin HC, Ger LP, Tung CS, Yen MH. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension 27: 36–42, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Wei J, Markis J, Malagold M, Braunwald E. Cardiovascular reflexes stimulated by perfusion of ischemic myocardium in acute myocardial infarction. Circulation 67: 796–801, 1983 [DOI] [PubMed] [Google Scholar]
- 37.Wu WC, Wang Y, Su CK, Chai CY. The nNOS/cGMP signal transducing system is involved in the cardiovascular responses induced by activation of NMDA receptors in the rostral ventrolateral medulla of cats. Neurosci Lett 310: 121–124, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Zanzinger J, Czachurski J, Seller H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am J Physiol Regul Integr Comp Physiol 268: R958–R962, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Zanzinger J, Seller H. Species differences in the distribution of nitric oxide synthase in brain stem regions that regulate sympathetic activity. Brain Res 764: 265–268, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Fu LW, Guo Z, Longhurst J. Role of glutamate in rostral ventrolateral medulla in acupuncture-related modulation of visceral reflex sympathoexcitation. Am J Physiol Heart Circ Physiol 292: H1868–H1875, 2007 [DOI] [PubMed] [Google Scholar]