Abstract
Endothelium-denuded bovine pulmonary arteries (BPA) contract to hypoxia through a mechanism potentially involving removing a superoxide-derived hydrogen peroxide-mediated relaxation. BPA organ cultured for 24 h with 0.1 mM cobalt chloride (CoCl2) to increase the expression and activity of heme oxygenase-1 (HO-1) is accompanied by a decrease in 5 μM lucigenin-detectable superoxide and an increase in horseradish peroxidase-luminol detectable peroxide levels. Force development to KCl in BPA was not affected by increases in HO-1, but the hypoxic pulmonary vasoconstriction (HPV) response was decreased. Organ culture with a HO-1 inhibitor (10 μM chromium mesoporphyrin) reversed the effects of HO-1 on HPV and peroxide. Treatment of HO-1-induced BPA with extracellular catalase resulted in reversal of the attenuation of HPV without affecting the force development to KCl. Increasing intracellular peroxide scavenging with 0.1 mM ebselen increased force development to KCl and partially reversed the decrease in HPV seen on induction of HO-1. HO-1 induction increases extracellular (ec) superoxide dismutase (SOD) expression without changing Cu,Zn-SOD and Mn-SOD levels. HO-1-induced BPA rings treated with the copper chelator 10 mM diethyldithiocarbamate to inactivate ecSOD and Cu,Zn-SOD showed increased superoxide and decreased peroxide to levels equal to non-HO-1-induced rings, whereas the addition of SOD to freshly isolated BPA rings attenuated HPV similar to HO-1 induction with CoCl2. Therefore, HO-1 induction in BPA increases ecSOD expression associated with enhanced generation of peroxide in amounts that may not be adequately removed during hypoxia, leading to an attenuation of HPV.
Keywords: catalase, cobalt, hydrogen peroxide, superoxide, extracellular superoxide dismutase
in contrast to systemic arteries which dilate in the presence of hypoxia, pulmonary arteries constrict in response to hypoxia, and this response is known as “hypoxic pulmonary vasoconstriction” (HPV). Reactive oxygen species (ROS) have been shown by various groups (3, 12, 14, 21) to play a key role in the response to hypoxia in pulmonary arteries. Studies conducted in our laboratory (18) point toward a potential mechanism involving superoxide-derived hydrogen peroxide maintaining relaxation in the pulmonary arteries under aerobic conditions. Activation of soluble guanylate cyclase as a result of peroxide metabolism by catalase may be a factor in the expression of this relaxation (5–7). It was also observed that, under hypoxic conditions, there is a decrease in the production of superoxide and hence hydrogen peroxide, leading to a decrease in the activation of soluble guanylate cyclase associated with a loss of relaxation in the pulmonary vessels, leading to the contraction seen during hypoxia (6, 7). Because there is strong evidence supporting both increases and decreases in ROS in the mechanism of HPV, additional studies are needed to better define how these species are involved in the response of pulmonary arteries to hypoxia.
Interest in heme oxygenase (HO) expression influencing oxygen sensing has recently been stimulated by a report suggesting it regulates oxygen-sensitive potassium channels in the carotid body (22). Studies by Zhang et al. (24) have shown that HO activity functions to suppress an endothelium-dependent contractile response of rat pulmonary arteries seen when they are exposed to hypoxia for >10 min. Mice deficient in HO-1 showed a maladaptive response to hypoxia associated with right ventricular infarcts and emboli that resulted in pulmonary hypertension. HO has been observed to be upregulated in hepatopulmonary syndrome, a condition where blunting of HPV is observed (8). In addition, studies by Abraham and colleagues (13, 19, 20) have shown HO-1 induction leads to a decrease in vascular superoxide detection, and that HO activity appears to control extracellular (ec) superoxide dismutase (SOD) expression. Thus HO-1 induction may influence fundamental mechanisms involving ROS which contribute to the pulmonary vascular response to hypoxia. Because we observed in preliminary experiments that HO-1 induction by organ culturing endothelium-removed bovine pulmonary arteries (BPA) in the presence of CoCl2 depressed the contractile response of these arteries to hypoxia, the current study was developed to investigate if the activity of HO-1 and modulation of ecSOD activity had a role in the changes that were observed. Based on our previous work suggesting that HPV in BPA is mediated through removal of a basal relaxation maintained by peroxide under aerobic conditions, we focused on defining the role of superoxide conversion to peroxide in this response.
EXPERIMENTAL METHODS
Materials.
All solutions were made from Analyzed Reagent Grade salts from Baker Chemical while other material was obtained from Sigma Chemical (St. Louis, MO) unless mentioned. Chromium mesoporphyrin (CrMP) was obtained from Frontier Scientific. Inhibitor doses were selected based on standard reported doses in previous studies that had effectively inhibited the target without having influenced other systems controlling vascular function.
Organ culture.
Organ cultures were performed for inducing HO-1 expression in BPA by exposing them to either 0.1 mM cobalt chloride (CoCl2) alone or with 10 μM CrMP, as previously described (15). Branches of BPA were isolated from fresh slaughterhouse-derived calf lungs transported in chilled buffered saline. Second- and third-order branches of the main lobar pulmonary arteries were then cleaned of their connective tissue and deendothelialized using a wooden stick. This was done because the pulmonary artery segments examined in this study show a prominent response to hypoxia in the absence of the influence of the endothelium, avoiding the complications of studying additional potential changes due to endothelial function. Rings measuring 2–3 mm in width made from the arteries were then incubated for 24 h at 37°C in DMEM containing 10% FBS, antibiotics (penicillin, streptomycin, fungizone) in the presence and absence of CoCl2 and CrMP, in an air-5% carbon dioxide atmosphere. After the incubation, these rings were used for experiments involving vascular reactivity studies, Western blotting for protein estimation, enzyme activity measurements, and chemiluminescence measurements for superoxide and hydrogen peroxide in the absence of CoCl2 or CrMP.
Vascular reactivity.
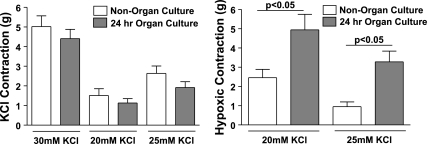
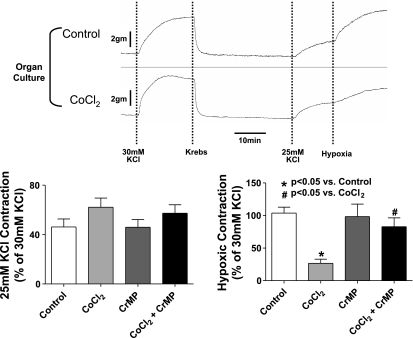
Noncultured and cultured BPA rings described above were mounted on wire hooks attached to either Grass (FT-03) force displacement transducers attached to a Grass Instruments polygraph recorder or to the Coulborne Instruments force transducers, using a Powerlab data acquisition system from ADInstruments for the measurement of isometric force development by the BPA rings, as previously described (5, 15). Arteries were then incubated for 1 h at a passive tension of 5 grams in Krebs-bicarbonate buffer solution (in mM: 118 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, and 5.6 glucose) gassed with 21% O2-5% CO2-74% N2. The buffer solution was maintained at 37°C by the individually thermostated baths. The arteries were then depolarized with Krebs-bicarbonate buffer containing 123 mM KCl in place of NaCl, after which they were reequilibrated with normal Krebs-bicarbonate buffer for another 30 min. The BPA rings were then contracted with 30 mM KCl containing Krebs-bicarbonate solution after which they were then again reequilibrated with normal Krebs-bicarbonate solution for 30 min. After 30 min, some of the BPA rings were exposed to probes [10 mM diethyldithiocarbamate (DETCA), 0.1 mM ebselen, 1 μM catalase from bovine liver, and 1 μM SOD from bovine erythrocytes] for another 30 min. The arteries were then precontracted with either 20 or 25 mM KCl to generate ∼20% or ∼40% of maximal force development (see Fig. 1). The conditions of 20 mM KCl were used to document the effects of interventions that potentially increase force under aerobic conditions, whereas 25 mM KCl was used to enhance the detection of conditions that might decrease force under aerobic conditions. Following this, they were exposed to 20 min of hypoxia by changing the gassing in the baths from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2. Finally, they were reoxygenated by changing the gas mixture to 21% O2-5% CO2-74% N2. Data are reported as a percent of the force generated by the initial contraction to 30 mM KCl, which generates ∼60% of maximal force. Organ culture with CoCl2 did not significantly alter the force generated by 30 mM KCl compared with pulmonary arteries not exposed to organ culture (see Fig. 1).
Fig. 1.
Effects of a 24 h organ culture on the contraction of isolated endothelium-rubbed bovine calf pulmonary arteries to the various doses of KCl examined in the protocols used in this study, and on the increase in force elicited by an acute exposure to hypoxia. The pooled data are a summary of the actual increases in force measured in freshly isolated (nonorgan cultured, n = 9–37) and organ cultured (n = 18–46) arteries in the absence of additional treatments for the initial contractions to 30 mM KCl (n = 37–46) and subsequent contractions to 20 (n = 9–18) or 25 (n = 24–33) mM KCl, which were exposed to hypoxia for the experiments reported in this study.
Superoxide and peroxide measurement using chemiluminescence.
Rings from BPA were placed in plastic scintillation minivials containing 5 μM lucigenin and other probes in 1 ml of Krebs solution buffered with 10 mM HEPES-NaOH (pH 7.4). The chemiluminescence from superoxide was measured by a liquid scintillation counter (LS6000IC; Beckman Instruments, San Diego, CA) with a single active photomultiplier tube in a dark room. Background chemiluminescence in the absence of tissue was subtracted from subsequent measurements made in the presence of BPA. The tissue was weighed at the end of the experiment, and the counts were divided by weight to give the readings in counts per minute per gram of tissue. For peroxide measurements, 10 μM luminol and 1 nM horseradish peroxidase (HRP) were substituted for lucigenin.
Western blot analysis of HO-1 and SOD expression.
Western blot analysis was performed on rings frozen in liquid nitrogen immediately after exposure to the experimental conditions. The rings were pulverized in a mortar and pestle under liquid nitrogen and immediately placed in the lysis buffer [50 mM Tris·HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease cocktail (Sigma Chemical)]. The samples were then centrifuged, and the supernatant was collected for protein analysis by the modified Bradford (4) method. After determining the concentration of proteins in the supernatant on the plate reader, 10 μg of protein were added from each sample and run on a 10% SDS-PAGE. It was then transferred to a nitrocellulose membrane and subsequently exposed to the primary and secondary antibodies in 5% milk/TBS-Tween buffers. The bands were then detected by electrochemiluminescence by using the ECL kit (Amersham, La Jolla, CA) on standard X-ray film (Kodak X-Omat, Rochester, NY). Protein levels were measured by densitometric analysis using the Kodak 1D software (Kodak). Antibodies for human HO-1 (OSA-110D) were from Biotechnologies; for Cu,Zn-SOD (ab13498) and Mn-SOD (ab13533) from Abcam; and ecSOD (no. 07-704) from Upstate.
Measurement of HO activity.
HO activity was measured by spectrophotometric methods previously described by Abraham et al. (2). Briefly, pulmonary artery rings were homogenized in Tris-sucrose solution. Glucose 6-phosphate (1 mM), 0.2 U/ml glucose-6-phosphate dehydrogenase, 0.8 mM NADPH, and 0.025 mg/ml hemin were added to sucrose solution containing 2–3 mg/ml of pulmonary artery homogenate and incubated for 1 h at 37°C, under conditions protected from light exposure. Following this, chloroform extraction was used for removal of bilirubin, which was measured using the differences in absorbance at wavelengths from 460 to 530 nm with an absorption coefficient of 40 mM−1/cm with a dual beam spectrophotometer.
Statistical analysis.
Data are shown as means ± SE, and n is the number of blood vessels studied from different animals. Statistical significance between two groups was analyzed by using Student's t-tests within the same vessels. ANOVA were used for comparisons between multiple groups, and a Bonferroni correction was employed to determine significance between groups. A value of P < 0.05 was used to establish statistical significance.
RESULTS
Effects of organ culture on the response of BPA to hypoxia.
The data in Fig. 1 include a comparison of the effects of organ culture on the force generated by pulmonary arteries to various doses of KCl employed in the protocols used in this study, and on the increase in force elicited by acute exposure to hypoxia. While the data showed a trend toward organ culture for 24 h slightly decreasing the force generated by the various doses of KCl used in this study, none of these changes were statistically significant. However, the increase in force elicited by hypoxia was statistically significant when examined in arteries contracted with either 20 or 25 mM KCl.
Effects of organ culture on the role of peroxide in the response of BPA to hypoxia.
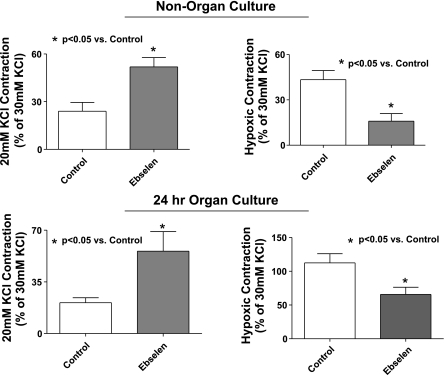
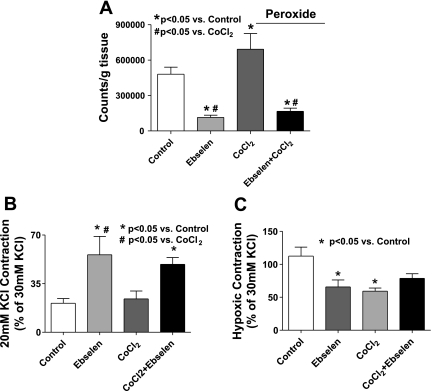
Ebselen was used to examine if organ culture alters the role of peroxide in the response to hypoxia due to its ability to enhance the consumption of intracellular peroxide in a manner that mimics the activities of enzymes other than catalase, such as glutathione peroxidase and peroxiredoxins, because this probe is thought to consume peroxide in a manner that is linked to the cooxidation of glutathione or thioredoxin (26). Because 100 μM ebselen has been previously observed to attenuate ∼50% of responses associated with exogenous and endogenous peroxide in bovine pulmonary and coronary arteries without having detectible nonspecific effects on the concentration-dependent generation of force by KCl in coronary arteries (16, 17), its effects on the response to hypoxia were examined at the low levels of force generated by contracting BPA with 20 mM KCl to better demonstrate its force-enhancing effects. The data in Fig. 2 show that force generation in response to 20 mM KCl is significantly increased by the presence of ebselen in noncultured and organ-cultured pulmonary arteries, suggesting organ culture did not detectibly alter the influence of endogenous peroxide on depressing force under aerobic conditions. The subsequent increase in force development elicited by acute exposure to hypoxia hypothesized (5–7) to be mediated by hypoxia decreasing endogenous peroxide was decreased by the presence of ebselen in both noncultured and organ-cultured pulmonary arteries. These data are consistent with organ culture not substantially changing the role of peroxide in the response of pulmonary arteries to hypoxia, with ebselen potentially functioning to partially remove a basal relaxation under aerobic conditions that appears to also be removed by hypoxia.
Fig. 2.
Effects of increasing peroxide consumption by the presence of 100 μM ebselen on the contractile response of non-organ-cultured and 24-h organ-cultured bovine pulmonary arteries (BPA) to 20 mM KCl, and on the increase in force elicited by an acute exposure to hypoxia. Force generation in BPA to 20 mM KCl is increased while force development to hypoxia is decreased in the presence of 0.1 mM ebselen in both fresh and organ-cultured arteries (n = 9).
Influence of increased HO-1 expression on the response of pulmonary arteries to hypoxia.
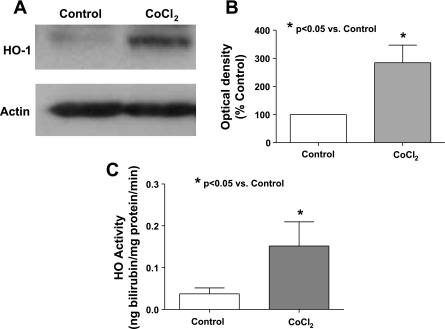
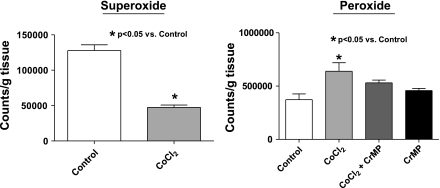
Figure 3 contains data from arteries examined in the present study confirming previous observations (15) that culturing BPA for 24 h in the presence of CoCl2 increases the expression of HO-1 (Fig. 3, A and B) and HO activity (Fig. 3C). Because HO-1 induction was previously reported to lower the detection of superoxide in pulmonary arteries (15), the effects of HO-1 induction were further studied to compare changes in superoxide detected by lucigenin chemiluminescence with measurements of peroxide release detected by the luminol chemiluminescence generated by HRP. The data in Fig. 4 show that treatment of BPA with CoCl2 leads to a significant decrease in superoxide and a significant increase in peroxide levels. Because this increase in peroxide is prevented in the presence during organ culture of an inhibitor of HO-1 CrMP, the increase in peroxide appears to be due to CoCl2 increasing the activity of HO. We then studied the force generation in BPA cultured in the presence of CoCl2, and, as shown in Fig. 5, the force generated by 25 mM KCl precontraction before hypoxia was not affected by the induction of HO-1 expression with CoCl2. However, the data in Fig. 5 also show that hypoxic contraction is decreased significantly in arteries organ cultured in the presence of CoCl2, and this decrease is prevented by organ culturing in the presence of CrMP. The apparent increase in peroxide generation under conditions where superoxide appeared to be decreased in HO-1-induced arteries was further investigated to determine if these changes potentially originated from an increase in the scavenging of superoxide. The effects of ebselen were examined to determine if the increased generation of peroxide associated with HO-1 induction was a contributing factor in decreasing the contraction to hypoxia. The data in Fig. 6A show that peroxide levels are reduced significantly in the presence of ebselen in both CoCl2-treated and untreated vessels. Force development to 20 mM KCl is increased in a similar manner by ebselen in both CoCl2-treated arteries and control organ-cultured vessels (Fig. 6B), suggesting that HO-1 induction did not change the role of endogenous peroxide in depressing force under aerobic conditions. Although ebselen decreased the contraction to hypoxia in non-CoCl2-treated arteries (Fig. 6C), ebselen appeared to partially reverse the attenuation of force developed to hypoxia seen with HO-1 induction, suggesting that the observed increase in peroxide generation elicited by organ culture with CoCl2 was functioning to attenuate the contraction to hypoxia.
Fig. 3.
Western blot (A) and summary data (n = 7) (B) documenting that heme oxygenase (HO)-1 expression is increased on exposure to 24 h of CoCl2 (0.1 mM) in organ culture. C: HO activity is also significantly (n = 12) increased by organ culture with CoCl2.
Fig. 4.
Lucigenin-detected superoxide levels are decreased (n = 12) while luminol-detected peroxide levels are increased (n = 4) by organ culture of BPA with CoCl2 (0.1 mM) for 24 h. When the HO inhibitor chromium mesoporphyrin (CrMP, 10 μM) is present during organ culture, it prevents detection of the increase in peroxide seen on induction of HO-1 with CoCl2.
Fig. 5.
Effects of HO-1 induction by CoCl2 on the contraction of BPA to 25 mM KCl and the contraction elicited by hypoxia. The effects of 24 h organ culture in the absence and presence of CoCl2 (0.1 mM) on responses of BPA to 30 mM KCl, 25 mM KCl, and subsequent exposure of arteries contracted with 25 mM KCl to hypoxia are shown. Summary data for the increase in force elicited by 25 mM KCl and the additional increase in force elicited by hypoxia are reported for BPA organ cultured in the absence and presence of combinations of CoCl2 and the inhibitor of HO activity CrMP. The data indicate that force development to 25 mM KCl is not changed on treatment with CoCl2 and CrMP (n = 10); however, force development to hypoxia is decreased on treatment with CoCl2, and CrMP prevents this attenuation in force.
Fig. 6.
A: peroxide levels are increased in BPA organ cultured with CoCl2 (0.1 mM) and decreased when measured in the presence of ebselen (n = 6). B: 20 mM KCl contraction is increased (n = 7–9) (B) and attenuation of hypoxic force seen on induction of HO-1 through CoCl2 (0.1 mM) is removed in the presence of ebselen (n = 7–9) (C).
Influence of increased ecSOD on the response of pulmonary arteries to hypoxia.
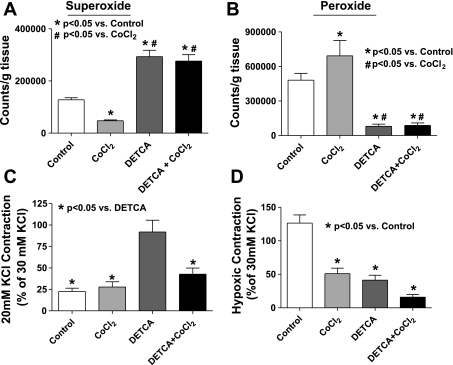
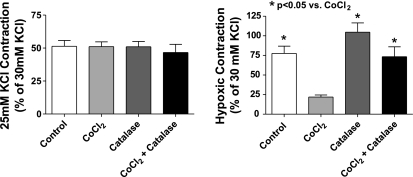
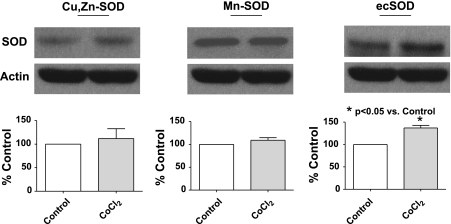
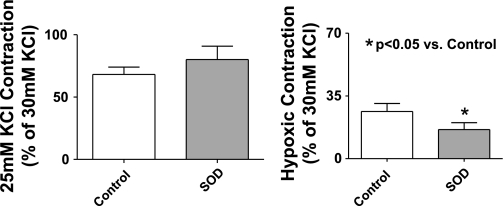
Studies were conducted to evaluate if an increase in superoxide scavenging and its potential role in enhancing peroxide generation could be contributing to modulation of the hypoxic response by HO-1 induction. The data in Fig. 7A show that inhibiting Cu-dependent SOD activity by pretreatment of organ-cultured pulmonary arteries with 10 mM of the Cu-binding agent DETCA for 30 min followed by washout of the DETCA (9) causes a significant increase in superoxide levels compared with untreated control and HO-1-induced vessels. However, there is no difference in superoxide levels between HO-1 induced and control vessels treated with DETCA. These data suggest that the generation of superoxide is the same in control organ-cultured vessels and HO-1-induced vessels and that the decrease in superoxide levels seen on induction of HO-1 is likely due to increased scavenging by a copper-containing form of SOD. Peroxide levels detected by HRP-luminol shown in Fig. 7B are decreased in both HO-1-induced and noninduced vessels to similar levels following treatment with DETCA, consistent with the metabolism of superoxide to peroxide being decreased by inhibition of SOD. Treatment of organ-cultured arteries with DETCA increased force generation to 20 mM KCl (see Fig. 7C). However, although DETCA treatment also appeared to slightly increase force in BPA treated with CoCl2, this change did not reach statistical significance. Hypoxic contraction is also significantly decreased following DETCA treatment in both HO-1-induced and noninduced organ-cultured pulmonary arteries (Fig. 7D) under the conditions where the DETCA treatment decreased the detection of peroxide shown in Fig. 7B. The data in Fig. 8 show that the addition of catalase in the tissue bath to pulmonary arteries organ cultured with CoCl2 caused a reversal of the attenuation of hypoxic force development caused by the HO-1-associated induction of ecSOD, without having an effect on the hypoxic response of control organ-cultured arteries or the initial force generated by 25 mM KCl under aerobic conditions. These data appear to be detecting a role for increased extracellular peroxide generation in the changes elicited by organ culture with CoCl2 that were observed. Figure 9 shows that HO-1 induction with CoCl2 leads to an increase in the expression of ecSOD compared with untreated organ-cultured BPA, while intracellular Cu,Zn-SOD and mitochondrial Mn-SOD levels remained unchanged. The effects of adding SOD to the tissue bath on the response of pulmonary arteries not exposed to organ culture were examined to detect if there was a source of extracellular superoxide generated by freshly isolated tissue that could influence the response to hypoxia by its conversion to peroxide. The data in Fig. 10 show that force development to 25 mM KCl was not affected by the added SOD, but hypoxic force development was decreased significantly.
Fig. 7.
Superoxide levels are increased (n = 12) (A) while peroxide levels are decreased (B) to similar levels in pulmonary arteries organ cultured either in the absence or presence of CoCl2, that were subsequently treated with the Cu-binding agent 10 mM diethyldithiocarbamate (DETCA, n = 6). C: 20 mM KCl force is significantly increased in the presence of DETCA in organ-cultured vessels (n = 9–13). D: hypoxic force development is decreased in the organ-cultured arteries subsequently treated with DETCA (n = 9–13).
Fig. 8.
Force development to 25 mM KCl is not affected by a 30-min treatment of pulmonary arteries previously organ cultured in the absence or presence of CoCl2 by catalase (1 μM) (22–23), but the attenuation in hypoxic force development by HO-1 induction with CoCl2 is reversed in the presence of catalase (n = 23–29).
Fig. 9.
HO-1 induction with CoCl2 causes an increase in the expression of extracellular (ec) superoxide dismutase (SOD) (n = 6) while the expression of Cu,Zn-SOD (n = 14) and Mn-SOD, (n = 6) is not affected.
Fig. 10.
Freshly isolated BPA rings treated for 30 min with SOD (1 μM) do not show a change in force development to 25 mM KCl, but hypoxic force development is attenuated significantly in these rings (n = 24).
DISCUSSION
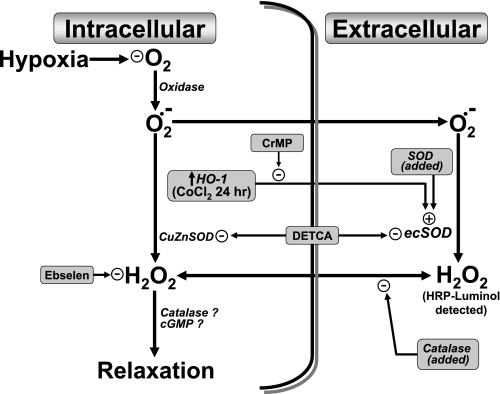
Data in the present study support previous studies from our laboratory (5, 6, 18) providing evidence that the metabolism of superoxide-derived peroxide by catalase participates in causing relaxation of BPA under aerobic conditions, and this relaxation appears to be removed during hypoxia leading to HPV. Because ebselen is a glutathione peroxidase and peroxiredoxin mimetic, it should scavenge peroxide in a manner that would prevent its metabolism by catalase and the actions peroxide has on other systems. The observed increases in force development to KCl caused by ebselen under aerobic conditions and decreased force development to hypoxia in the pulmonary arteries studied are consistent with ebselen decreasing the amount of peroxide available for metabolism by catalase and maintaining the relaxation that is removed by hypoxia. While organ culture enhanced the contraction to hypoxia, it did not appear to change the role of peroxide in the response. Our results also show that the removal of Cu by pretreatment of pulmonary arteries with DETCA leads to an increase in superoxide, presumably by inactivating the Cu-containing forms of SOD, and this is associated with a decrease in the detection of peroxide by the HRP-luminol chemiluminescence method used. This decrease in peroxide formation was accompanied with an increase in force development to KCl under aerobic conditions and a decrease in the contraction elicited by hypoxia. Because the addition of catalase does not directly alter force generation under aerobic conditions or the subsequent contraction to hypoxia (in the absence of changes in SOD activity), the intracellular generation of peroxide that is derived from superoxide metabolism through Cu,Zn-SOD appears to be the main source of peroxide involved in mediating the basal aerobic relaxation. While these observations support our previously published hypotheses suggesting that a decrease in superoxide-derived peroxide metabolized by endogenous catalase is the main ROS involved in the contractile response of pulmonary arteries to hypoxia (5, 6, 18), data in the present study examining the effects of HO-1 induction by organ culture with CoCl2 have detected additional ROS-mediated processes shown in Fig. 11 regulating the response to hypoxia that appear to originate from increased ecSOD expression enhancing peroxide generation to levels that function to impair the removal of its relaxing effects by hypoxia.
Fig. 11.
Model showing how an increase in ecSOD expression as a result of HO-1 induction by organ culture with CoCl2 potentially inhibits the contraction of BPA to hypoxia by enhancing the conversion of extracellular superoxide normally present in BPA to peroxide. The model shows how the interventions used in this study modulate a hypoxia-elicited contractile mechanism hypothesized to be mediated by removal of the oxygen-dependent relaxing effects of peroxide that are normally derived from intracellular superoxide.
Induction of HO-1 modulates the metabolism of ROS by pulmonary arteries through increasing the expression of ecSOD.
The induction of HO-1 expression by organ-culturing endothelium-denuded BPA with CoCl2 appeared to modulate the detection of ROS in a manner that seemed to be dependent on the activity of HO during the organ culture conditions because the increased detection of peroxide was attenuated by culturing arteries in the presence of an inhibitor of HO activity. Based on the detection of decreased superoxide by lucigenin chemiluminescence and increased peroxide release by HRP-elicited luminol chemiluminescence, there appeared to be increased conversion of superoxide to peroxide. Examination of the expression of the different forms of SOD present in vascular tissue resulted in the detection of increased ecSOD, without changes in Cu,Zn-SOD or Mn-SOD, which are observations consistent with previous work suggesting HO activity increases the expression of ecSOD (13, 19, 20). Because inactivation of Cu,Zn-SOD and ecSOD by binding the copper present in these enzymes with DETCA increased the detection of superoxide by lucigenin to similar levels in control and HO-1 induced arteries, the activities of oxidases generating superoxide do not appear to be influenced by the CoCl2 organ culture approaches employed to induce HO-1 and ecSOD. Thus the induction of HO-1 appears to be associated with increased generation of peroxide from sources of superoxide that are metabolized by ecSOD.
Induction of HO-1 reduces the contraction of pulmonary arteries to hypoxia through increased generation of peroxide by ecSOD.
The results reported in this study provide evidence for a potentially novel interaction involving increased ecSOD activity being able to enhance the metabolism of superoxide to peroxide, generating peroxide levels that seem to suppress the contraction to hypoxia. Because HO-1 induction inhibited the contraction to hypoxia in a manner that was completely reversed by the addition of catalase, the increased expression of ecSOD resulted in the generation of levels of extracellular peroxide that promoted relaxation under the hypoxic conditions examined. Although increasing intracellular peroxide consumption with ebselen decreased the contraction to hypoxia in control and organ-cultured endothelium-denuded BPA, it increased the depressed contraction to hypoxia seen in the arteries exposed to organ culture with CoCl2 for induction of HO-1 expression. Ebselen also increased force generation by KCl under aerobic conditions in organ-cultured control and HO-1-induced arteries, consistent with detection of the presence of tonic peroxide-mediated relaxation which could be removed by hypoxia. However, it appears that the presence of ebselen decreased the inhibitory effect of increased generation of peroxide on hypoxic force generation by elevated ecSOD, enabling the response to hypoxia to appear to be somewhat enhanced by ebselen in HO-1-induced pulmonary arteries. Previous studies examining the effects of glutathione depletion with diethylmaleate had detected evidence that this treatment inhibited the contraction of BPA to hypoxia (23). Glutathione depletion was also observed to increase the metabolism of endogenous peroxide by catalase to a degree that it did not decrease under hypoxia below the levels seen under aerobic conditions in control arteries. Thus the decreased contraction to hypoxia associated in the present study with HO-1 induction and increased ecSOD expression appears to originate from an incomplete removal by hypoxia of the relaxing effect of the observed increased generation of peroxide.
Inhibition by pretreatment with DETCA of the Cu-dependent superoxide-scavenging activities of Cu,Zn-SOD and ecSOD essentially eliminated the contraction to hypoxia in organ-cultured control and HO-1-induced pulmonary arteries. This suggests that removal of SOD-dependent sources of peroxide are essential for the contraction to hypoxia. Because inhibition of SOD by Cu depletion decreases peroxide generation and increases force under aerobic conditions, the data further support the dominant role of removing a tonic relaxation to peroxide in the contraction to hypoxia seen in the BPA studied. The observation that increasing ecSOD activity by adding Cu,Zn-SOD to the tissue bath caused a depression of the contraction to hypoxia seen in freshly isolated pulmonary arteries suggests that pulmonary arteries normally release superoxide in amounts that could depress the contraction to hypoxia if it could be converted to peroxide in a more efficient manner. Recent studies have detected evidence that vascular smooth muscle cells from bovine coronary arteries release superoxide in amounts that have been suggested to have regulatory effects (25). Data in the present study are consistent with the level of ecSOD activity present in the vessel wall having a major role in enabling this potential source of superoxide to influence vascular function through its ability to increase the generation of peroxide.
The data in this study suggest that conditions inducing HO-1 appear to activate the expression of ecSOD, which functions to metabolize an extracellular source of superoxide normally present in the BPA studied to promote increased amounts of peroxide that are not adequately removed by hypoxia, causing an apparent attenuation of the HPV response. Because HO-1 expression appears to increase under pathophysiological conditions (1) such as pulmonary hypertension, and function in a manner that appears to prevent the development of this disease and lung injury (10, 11), the mechanism of increased ecSOD detected in the present study may function to help protect the pulmonary circulation through reducing oxidative stress and diminishing the expression of pulmonary hypertension. In addition, conditions associated with increased ecSOD may have important vascular regulatory roles through enhanced peroxide formation in the diverse pathological conditions (1) associated with increased HO-1 expression. For example, although increased nitric oxide and HO-1 products could be a contributing factor in the depressed pulmonary response to hypoxia seen in hepatopulmonary syndrome (8), the role of changes in ecSOD expression and the potential influence of increased peroxide could also be a factor that remains to be determined. Thus HO-1 induction could also alter how the endothelium influences the HPV response, and additional processes may be a factor in the changes seen in vivo under conditions where altered HO-1 expression is observed.
GRANTS
This study was support by National Institutes of Health Grants HL-31069, HL-43023, and HL-66331 (to M. S. Wolin) and HL-056601, DK-068134, and HL-34300 (N. G. Abraham).
ACKNOWLEDGMENTS
Portions of this study were presented at the Experimental Biology 2005 Meeting in San Diego, CA (Faseb J 19: A1310, 2005) and at the 2007 American Heart Association Scientific Sessions in Orlando, FL (Circulation 116: II–35, 2007).
Present address for N. G. Abraham: Dept. of Physiology and Pharmacology, Univ. of Toledo, Toledo, OH.
REFERENCES
- 1.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Abraham NG, Lin JH, Dunn MW, Schwartzman ML. Presence of heme oxygenase and NADPH cytochrome P-450 (c) reductase in human corneal epithelium. Invest Ophthalmol Vis Sci 28: 1464–1472, 1987 [PubMed] [Google Scholar]
- 3.Archer SL, Will JA, Weir EK. Redox status in the control of pulmonary vascular tone. Herz 11: 127–141, 1986 [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol 252: H721–H732, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Burke-Wolin TM, Wolin MS. H2O2 and cGMP may function as an O2 sensor in the pulmonary artery. J Appl Physiol 66: 167–170, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Burke-Wolin TM, Wolin MS. Inhibition of cGMP-associated pulmonary arterial relaxation to H2O2 and O2 by ethanol. Am J Physiol Heart Circ Physiol 258: H1267–H1273, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Carter EP, Hartsfield CL, Miyazono M, Jakkula M, Morris KG, Jr, McMurtry IF. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol 283: L346–L353, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cherry PD, Omar HA, Farrell KA, Stuart JS, Wolin MS. Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol Heart Circ Physiol 259: H1056–H1062, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res 86: 1224–1229, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol 36: 158–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 288: H13–H21, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 111: 3126–3134, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 90: 1307–1315, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Mingone CJ, Ahmad M, Gupte SA, Chow JL, Wolin MS. Heme oxygenase-1 induction depletes heme and attenuates pulmonary artery relaxation and guanylate cyclase activation by nitric oxide. Am J Physiol Heart Circ Physiol 294: H1244–H1250, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohazzab-HKM, Agarwal R, Wolin MS. Influence of glutathione peroxidase on coronary artery responses to alterations in Po2 and H2O2. Am J Physiol Heart Circ Physiol 276: H235–H241, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Mohazzab-HKM, Wolin MS. Oxidant signalling and vascular oxygen sensing. Role of H2O2 in responses of the bovine pulmonary artery to changes in Po2. Adv Exp Med Biol 475: 249–258, 2000 [PubMed] [Google Scholar]
- 18.Mohazzab-HKM, Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery Po2 sensor. Am J Physiol Lung Cell Mol Physiol 267: L823–L831, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Turkseven S, Drummond G, Rezzani R, Rodella L, Quan S, Ikehara S, Abraham NG. Impact of silencing HO-2 on EC-SOD and the mitochondrial signaling pathway. J Cell Biochem 100: 815–823, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol 289: H701–H707, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 91: 719–726, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306: 2093–2097, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Wolin MS, Burke-Wolin TM, Kaminski PM, Mohazzab-HKM Reactive oxygen species and vascular oxygen sensors. In: Nitric Oxide and Radicals in the Pulmonary Vasculature, edited by Weir EK, Archer SL, Reeves JT. Armonk, NY: Futura, 1996, p. 245–263 [Google Scholar]
- 24.Zhang F, Kaide JI, Yang L, Jiang H, Quan S, Kemp R, Gong W, Balazy M, Abraham NG, Nasjletti A. CO modulates pulmonary vascular response to acute hypoxia: relation to endothelin. Am J Physiol Heart Circ Physiol 286: H137–H144, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Zhang G, Zhang F, Muh R, Yi F, Chalupsky K, Cai H, Li PL. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. Am J Physiol Heart Circ Physiol 292: H483–H495, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Zhao R, Holmgren A. A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem 277: 39456–39462, 2002 [DOI] [PubMed] [Google Scholar]