Abstract
Atherosclerosis is closely associated with disturbed flow characterized by low and oscillatory shear stress, but studies directly linking disturbed flow to atherogenesis is lacking. The major reason for this has been a lack of an animal model in which disturbed flow can be acutely induced and cause atherosclerosis. Here, we characterize partial carotid ligation as a model of disturbed flow with characteristics of low and oscillatory wall shear stress. We also describe a method of isolating intimal RNA in sufficient quantity from mouse carotid arteries. Using this model and method, we found that partial ligation causes upregulation of proatherogenic genes, downregulation of antiatherogenic genes, endothelial dysfunction, and rapid atherosclerosis in 2 wk in a p47phox-dependent manner and advanced lesions by 4 wk. We found that partial ligation results in endothelial dysfunction, rapid atherosclerosis, and advanced lesion development in a physiologically relevant model of disturbed flow. It also allows for easy and rapid intimal RNA isolation. This novel model and method could be used for genome-wide studies to determine molecular mechanisms underlying flow-dependent regulation of vascular biology and diseases.
Keywords: shear stress, endothelial dysfunction, inflammation, atherosclerosis, endothelium
atherosclerosis is a leading cause of morbidity and mortality in developed countries and is shown to be an inflammatory disease (22, 28). Although multiple systemic factors such as hypercholesterolemia, diabetes, hypertension, and smoking are well-known risk factors, atherosclerosis occurs preferentially at particular areas of disturbed flow characterized by low and oscillatory wall shear stress (WSS) in branched or curved arteries (19, 34). In contrast, straight arterial regions are exposed to high and stable shear stress and are well protected from atherosclerosis (34). Despite the close association between the two, evidence directly linking disturbed flow conditions to atherosclerosis has been lacking, and the mechanisms responsible for pro- and antiatherogenic effects of shear stress are still incompletely understood.
Shear stress is the tangential force imparted by viscous fluid flowing over endothelial cells (5, 16). Endothelial cells sense changes in shear stress and trigger mechanosensitive cell signaling events (2, 10). This in turn regulates endothelial function and structure, which affects vascular wall biology and pathophysiology (5). Endothelial cells in straight part of the arteries experience unidirectional, high time-averaged WSS (laminar shear). Laminar shear induces acute and chronic changes in endothelial cells leading to cell alignment, vasodilation, inhibition of inflammation, and coagulation, which are atheroprotective responses. In contrast, disturbed flow stimulates proatherogenic responses, including cell turnover, inflammation, thrombosis, and oxidative stress (2, 5, 10, 16).
The differential mechanisms by which disturbed and stable flow promotes and inhibits atherogenesis, respectively, have been a subject of intense study, mostly using cultured endothelial cells (2, 10, 16). To define molecular mechanisms responsible for these changes, investigators have carried out DNA microarray studies using endothelial cells and have identified shear sensitive genes such as kruppel-like factor 2 (klf-2), endothelial nitric oxide synthase (eNOS), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and bone morphogenic protein 4 (BMP-4) (3, 7, 9, 11, 12, 23, 29, 31). Although these in vitro studies have provided critical insights regarding shear sensitive mechanisms in cultured endothelial cells using modeled flow conditions, it cannot be assumed whether identical mechanisms are responsible for flow-dependent changes in vessels in vivo and vascular diseases such as atherosclerosis.
Several mouse models have been used to examine the role of shear sensitive genes and proteins in atherogenesis. These models include 1) naturally occurring atheroprone regions of arterial tree, 2) complete ligation of common carotid artery, and 3) perivascular shear modifier cuff placed around common carotid artery in mice deficient in apolipoprotein E (ApoE KO) or low-density lipoprotein receptor (LDLR KO). Naturally occurring atheroprone regions, such as lesser curvature of the aortic arch and root of innominate artery, are exposed to disturbed flow (33). These flow-disturbed areas in ApoE KO and LDLR KO mice develop measurable atherosclerotic lesions upon feeding atherogenic diets for at least 2–3 mo (25). Although these studies show that flow disturbance is associated with atherogenesis, they do not provide evidence directly linking disturbed flow to atherogenesis, since the flow disturbance is chronic from the early developmental stage and not due to an acute flow alteration. Other difficulties include small sample area of atherosclerosis, making reproducible endothelial RNA isolation from these areas in sufficient quantity and purity difficult (25). Complete ligation of common carotid artery has also been used to study atherosclerosis (17, 20). This model results in no flow through the left common carotid artery (LCA) and may be associated with endothelial denudation and thrombosis. It may not be a physiologically relevant model in the study of shear-mediated atherosclerosis. (36). The perivascular shear modifier cuff model was recently reported and applied to the study of shear-mediated vascular inflammation and atherogenesis. It was shown to create three distinct regions of shear stress via application of a perivascular cuff around LCA [lower shear (proximal to the cuff), high shear (inside the cuff), or oscillatory shear (distal to the cuff)] (6). It is important to note that low and oscillatory shear stress, two characteristics of disturbed flow, were predicted to be in two separate regions. The region of oscillatory shear stress (distal to cuff) had high average shear stress, and the region of low average shear stress (proximal to cuff) did not have an oscillatory shear stress component (6). This dissociation of oscillatory shear from low shear stress is a unique characteristic of the cuff model, and it is different from typical disturbed flow conditions displaying colocalized low and oscillatory shear stress.
Partial carotid ligation has previously been described as a model of flow reduction and has been used in the study of vascular remodeling (18, 32). In this model, three of the four caudal branches of LCA were ligated, resulting in a substantial flow reduction in LCA (18). Korshunov and Berk (18) showed that partial ligation of LCA in C57Bl6 mice results in outward remodeling of LCA with intima-media-adventitial thickening. Mechanistically, the carotid remodeling by partial ligation involved macrophage infiltration, smooth muscle proliferation, and extracellular matrix reorganization (18). Sullivan and Hoying (32) used partial ligation in fibroblast growth factor 2 (FGF2) KO and wild-type mice to study the role of FGF2 in flow-dependent vascular remodeling. Although these studies have provided some insight into flow-mediated vascular remodeling, changes in shear stress levels or development of atherosclerosis in response to partial carotid ligation have not been described. We hypothesized that partial carotid ligation causes low and oscillatory shear stresses two major characteristics of disturbed flow that have been closely associated with atherogenesis (5). We further hypothesized that this disturbed flow would cause atherosclerosis in hyperlipidemic conditions. To test these hypotheses, we first measured flow velocity and direction as well as vessel dimensions by a high-resolution ultrasound system before and after partially ligating LCA. These measurements were then used for computational fluid dynamics (CFD) modeling to estimate shear stress magnitudes and directions. Next, ApoE KO mice were partially ligated and fed a high-fat diet to determine if this would result in atherosclerosis in LCA. In addition, we used a simple method of isolating intimal RNA in significant quantity and purity from carotid arteries for mechanistic studies. Here, we report that partial ligation causes disturbed flow, induces atherosclerosis rapidly within 2 wk upon feeding a high-fat diet, and that the additional simple RNA isolation method is extremely useful to determine gene expression changes occurring in carotid intima in response to changes in shear stress.
METHODS
Animal studies with partial ligation.
All animal studies were carried out by procedures approved by the Emory University Institutional Animal Care and Use Committee (IACUC). Male and female mice were ligated between 6 and 8 wk of age. C57Bl/6 and ApoE KO mice were obtained from Jackson Laboratories, and mice deficient in p47phox in ApoE KO background (p47phox_ApoE DKO) were generated as previously described (1). All mice were fed a chow diet and water ad libitum until partial ligation. Partial ligation of LCA was carried out as previously described (32) with minor modifications. Briefly, anesthesia was induced by intraperitoneal injection of xylazine (10 mg/kg) and ketamine (80 mg/kg) mixture. Epilated area was disinfected with betadine, and a ventral midline incision (4–5 mm) was made in the neck. LCA was exposed by blunt dissection. Three of four caudal branches of LCA (left external carotid, internal carotid, and occipital artery) were ligated with 6–0 silk suture (Fig. 1A) while the superior thyroid artery was left intact. The incision was then closed with Tissue-Mend (Veterinary Product Laboratories). Mice were monitored until recovery in a chamber on a heating pad following surgery. A single subcutaneous injection of buprenorphine (0.1 mg/kg) was given 12 h after partial ligation for additional pain relief. For atherosclerosis studies, ApoE KO and p47phox_ApoE DKO mice were fed the Paigen's high-fat diet (25) (Science Diets) immediately following partial ligation until killed, from 2 days up to 6 wk. C57Bl/6 mice were continued on chow diet postligation.
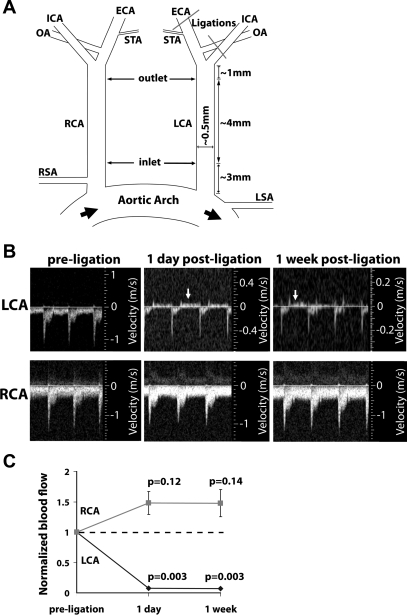
Fig. 1.
Partial ligation of left common carotid artery (LCA) causes low and oscillatory flow. A: three branches of the LCA [external carotid artery (ECA), internal carotid artery (ICA), and occipital artery (OA)] were ligated in the LCA, while leaving the superior thyroid artery (STA) open. B: ultrasound showing flow velocity profiles and revealing that partial ligation induces flow reversal (indicated by arrows) in LCA during diastole. Flow in the right common carotid artery (RCA) remains unchanged after ligation. Images shown were obtained from an ApoE KO mouse and are representative of at least 20 mice deficient in apolipoprotein E (ApoE KO). Partial ligation in C57BL/6 mice results in similar flow reversal profiles (data not shown). C: partial ligation reduces blood flow through the LCA, without significantly raising flow in RCA. The dotted line indicates the preligation flow level. Shown are means ± SE, n = 4 experiments.
High-resolution ultrasound measurements.
All ultrasound measurements were taken using a VEVO 770 high-resolution in vivo microimaging ultrasound system with a 30-MHz mouse probe (Visualsonics). Mice were anesthetized with inhaled isoflurane, and body temperature was maintained on a heated stage for the duration of studies. Levels of anesthesia, heart rate, temperature, and respirations were continuously monitored. Pulse wave Doppler mode was used at the inlet, midpoint, and outlet of the common carotid arteries (see Fig. 1A) for measuring flow velocity, M-mode for vessel dimensions, and B-mode for vessel length. All measurements were gated to an electrocardiogram and respiration.
CFD.
The CFD models incorporated the geometry of mouse carotid arteries as determined by the ultrasound system. LCA and RCA of mouse are similar in geometry to a straight tube with different diameters at two ends (inlet and outlet; Fig. 1A), which made a moving mesh CFD model simple. The geometric resolution of ultrasound images was 10 μm. After segmentation and measurement of the diameter waveforms, sequential diameters at three sections (inlet, outlet, and middle sections) over one cardiac cycle were obtained. A three-dimensional model was reconstructed based on the diameters at end diastole, and then a moving mesh was designed for the model according to individual diameter waveform (33). The moving model replicated the geometry and compliance characteristics of the mouse carotid artery. Computations were performed using the commercial CFD-ACE code based on a finite volume method for solving the Navier-Stokes equations as previously described (33). The velocity waveforms at three sections (inlet, outlet, and midpoint; Fig. 1A) were acquired from Doppler measurements. Two end-velocity waveforms were transformed to correspond with flow waveforms according to the diameter waveform. The flow waveforms were the inflow and outflow boundary conditions for CFD modeling. The midpoint velocity waveform was used later to compare with the CFD results at the same section. The blood was assumed to be a Newtonian and incompressible fluid, and the flowing state was assumed to be laminar flow.
Intimal RNA isolation from carotid arteries.
Mice were killed by CO2 inhalation according to Emory University's IACUC protocol and pressure perfused with saline containing heparin (10 U/ml) via the left ventricle after severing the inferior vena cava. LCA and the right common carotid artery (RCA) were then isolated and carefully cleaned of periadventitial fat. The carotid lumen was quickly flushed (few seconds) with 150 μl of QIAzol lysis reagent (QIAGEN) using a 29-gauge insulin syringe in a microfuge tube. The eluate was then used for intimal RNA isolation using an miRNeasy mini kit (QIAGEN) according to the manufacturer's instructions. The carotid artery left over after flushing with QIAzol was used to prepare RNA from media and adventitia. Media and adventitia was snap-frozen in liquid nitrogen, pulverized by mortar and pestle, and lysed in QIAzol lysis reagent (700 μl per carotid), and RNA was isolated using the miRNeasy mini kit as detailed in Supplemental Figs. 1–3 (Supplemental material for this article can be found on the American Journal of Physiology: Heart and Circulatory Physiology website).
Immunohistochemical staining studies.
Mice were killed and pressure perfused with saline containing heparin as described above. LCA and RCA were collected en bloc with the trachea and esophagus. For frozen sections, tissue was embedded in Tissue-Tek optimum cutting temperature medium, frozen on dry ice, and stored at −80°C until used. Frozen sections (7 μm) were fixed in acetone for 8 min, blocked with 10% goat serum for 1 h at room temperature, and incubated with primary antibodies overnight at 4°C (3). To visualize primary antibodies, rhodamine-conjugated secondary antibody was used for 1 h at room temperature. Nuclei were counterstained with Hoechst no. 33258. Oil red O staining was carried out using frozen sections as described (37). For pentachrome staining, fixed tissue was paraffin embedded, sectioned at 5 μm, and stained with the Russell-Movat pentachrome staining kit (American Master Tech Scientific). Micrographs were taken with a Zeiss (Jena, Germany) epifluorescence microscope. Images were analyzed with NIH Image J software to quantify lesion size in each animal as described (21).
Vascular relaxation study.
The vascular relaxation study was carried out using carotid rings obtained from LCA and RCA of partially ligated ApoE KO mice as previously described (24).
Dihydroethidium staining.
Thick frozen sections (30 μm) obtained from LCA and RCA of partially ligated mice were stained in 2 μM dihydroethidium (DHE) in PBS for 30 min at 37°C. Slides were mounted with DAKO mounting media and immediately imaged with a Zeiss LSM 510 META confocal microscope as described (30).
Statistical analysis.
Data are presented as means ± SE. Student's t-test for two groups and ANOVA with Games-Howell post hoc tests for comparing multiple groups were carried out using the SPSS program. P < 0.05 was considered statistically significant.
RESULTS
Partial ligation results in low and oscillatory shear stress.
To determine whether partial ligation caused changes in shear stress levels and direction, we ligated the external carotid, internal carotid, and occipital arteries of LCA, while leaving the superior thyroid artery intact in C57Bl/6 and ApoE KO mice (Fig. 1A). Next, flow velocity and direction and vessel dimensions of LCA and RCA were determined by high-resolution ultrasonography. This showed a reduction in flow velocity during systole in LCA at 1 and 7 days after ligation as expected (Fig. 1B). Moreover, flow direction was reversed during diastole in LCA at 1 and 7 days after ligation (Fig. 1B). Similar flow profiles were observed at both the predefined inlet and outlet. Flow profiles as shown in Fig. 1B were obtained from the inlet. Blood flow in LCA decreased by 90% at 1 and 7 days after ligation, but blood flow in RCA did not change significantly compared with that of preligation (Fig. 1C). Blood pressure and pulse did not change by partial ligation (Supplemental Fig. 1).
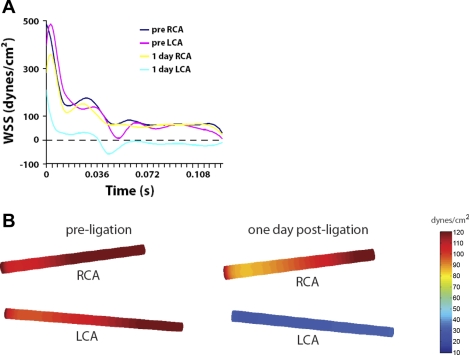
Using values of flow velocity, direction, and vessel dimensions, we performed CFD modeling of LCA and RCA pre- and 1 day postligation. This showed that the WSS value remained positive without any significant difference in RCA both before and after ligation of LCA during the entire cardiac cycle (Fig. 2, A and B). In contrast, the WSS level was reduced during systole compared with that of RCA and became negative (because of flow reversal) during diastole in ligated LCA (Fig. 2, A and B). Time-averaged WSS was reduced from ∼110 dyn/cm2 preligation to 30 dyn/cm2 postligation (Fig. 2B). These results show that partial ligation causes low and oscillatory shear stress in LCA, characteristic of disturbed flow.
Fig. 2.
Computational fluid dynamics (CFD) study. Partial ligation results in low and oscillatory shear stress. A and B: CFD was carried out using the values shown in Fig. 1 (ligated ApoE KO mice). A: wall shear stress (WSS) over a cardiac cycle. B: pseudocolor images of time-averaged WSS levels over the cardiac cycle in carotid arteries before and 1 day after partial ligation.
Method of isolating intimal RNA from carotid arteries.
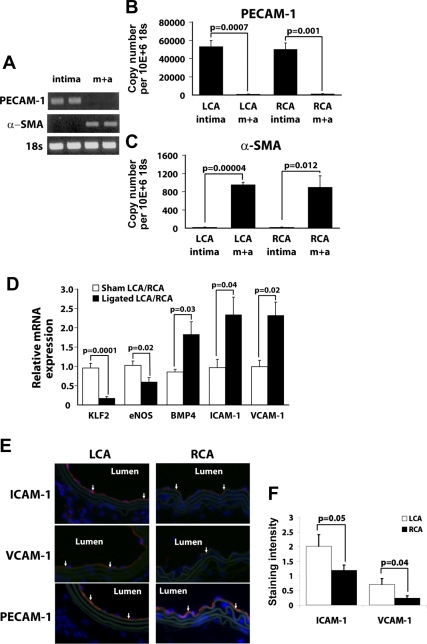
Lumens of LCA and RCA from sham-ligated C57 mice were flushed one time with Qiazol (Qiagen). The leftover LCA and RCA after collecting intimal RNA were also saved for analysis. RNA was then isolated with the miRNeasy kit (Qiagen), resulting in 10–20 ng total RNA from each eluate (intima), while each leftover vessel (media + adventitia) resulted in ∼800 ng to 1 μg of total RNA. To test for endothelial purity of intimal RNA, we performed RT-PCR for platelet endothelial cell adhesion molecule-1 (PECAM-1) and α-smooth muscle actin (α-SMA) (Fig. 3A). Intimal eluate showed endothelial marker PECAM-1 without any evidence of smooth muscle specific α-SMA. Conversely, media + adventitial RNA contained α-SMA without any evidence of PECAM-1. Quantitative real-time PCR (qPCR) and en face protein staining further confirmed the results (Fig. 3, B and C, and Supplemental Fig. 3). This shows that intimal RNA can be obtained from carotid arteries by our simple and reproducible method in sufficient quantity and purity without significant smooth muscle RNA contamination. In addition, media + adventitial RNA can also be isolated from the arteries without significant endothelial contamination.
Fig. 3.
A–C: method of intimal RNA preparation. Intimal RNA and medial + adventitial (m + a) RNA were obtained from sham-operated RCA and LCA in C57BL/6 mice. RNAs were analyzed by semiquantitative RT-PCR (A) and quantitative real-time PCR (qPCR) (B and C) for platelet endothelial cell adhesion molecule-1 (PECAM-1) and α-smooth muscle actin (α-SMA) using 18s as an internal control. Bar graphs are means ± SE, n = 3. D: partial ligation results in decreases in kruppel-like factor 2 (KLF2) and endothelial nitric oxide synthase (eNOS) while increasing bone morphogenic protein 4 (BMP4), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1). Intimal RNA from sham and partially ligated C57BL/6 mice were collected from LCA and RCA, respectively, 2 days after surgery and analyzed by qPCR using 18S as an internal control. Data are shown as a ratio of mRNA expressed in LCA over RCA of sham and partially ligated mice. Means ± SE are shown, n = 3 sham 5 ligated. E and F: partial ligation increases ICAM-1 and VCAM-1 protein expression in LCA. C57BL/6 mice underwent partial ligation, and LCA and RCA were collected 2 days postligation. Frozen sections were stained for ICAM-1, VCAM-1, and PECAM-1 (red). Nuclei were counterstained with Hoechst (blue). Images are representative of n = 4 (E). Average staining intensity per stained area for each was quantified and shown as the mean ± SE (F).
Partial ligation downregulates klf-2 and eNOS while upregulating ICAM-1, VCAM-1, and BMP4.
We tested whether intimal RNA obtained from LCA and RCA by our method could be used to study regulation of mechanosensitive genes in endothelial cells. Intimal RNA from sham and partially ligated C57BL/6 mice were collected 2 days after surgery and analyzed by qPCR. We chose this 2-day time point because we did not observe measurable accumulation of CD11b-positive leukocytes in the intima in either LCA or RCA in these mice (Supplemental Fig. 2), whereas this seems to be a sufficient duration to ensure robust gene expression changes following the partial ligation. Klf-2 and eNOS were significantly downregulated in LCA by 80 and 50%, respectively, whereas BMP4, ICAM-1, and VCAM-1 were significantly upregulated in LCA by approximately twofold compared with RCA (Fig. 3D). In sham-operated mice, mRNA levels of these genes did not differ between LCA and RCA. Protein levels for ICAM-1 and VCAM-1 were verified by immunohistochemical staining with PECAM-1 as an endothelial marker (Fig. 3, E and F). These results are consistent with previous observations in vitro and in vivo that KLF-2 and eNOS are downregulated in areas of disturbed flow while BMP4, ICAM-1, and VCAM-1 are upregulated in areas of disturbed flow (3, 4, 7, 11, 12, 29, 31, 33, 38).
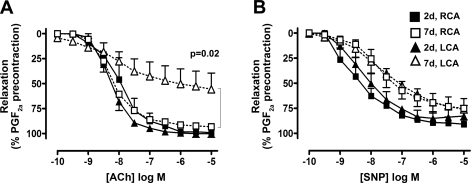
Partial ligation in ApoE KO mice impairs endothelium-dependent vasorelaxation.
To determine if disturbed flow causes endothelial dysfunction, we examined the vasorelaxation response to acetylcholine. Carotid artery rings were obtained from LCA and RCA of partially ligated ApoE KO mice fed a high-fat diet for 2 or 7 days. At 2 days postligation, LCA and RCA showed a normal relaxation response to acetylcholine, exceeding 90% of precontracted tone (Fig. 4A). At 7 days, however, relaxation of LCA by acetylcholine was significantly inhibited, reaching only 50% of precontracted tone, whereas the RCA vasorelaxtion response remained unchanged (Fig. 4A). To determine whether the impaired response was due to endothelial defect, response to the endothelium-independent vasodilator sodium nitroprusside (SNP) was studied. At 2 and 7 days postligation, both LCA and RCA relaxed to similar degrees in response to SNP (Fig. 4B), suggesting that disturbed flow induces endothelial dysfunction in ApoE KO mice in 7 days.
Fig. 4.
Partial ligation induces endothelial dysfunction. Arterial rings were obtained from LCA and RCA that were partially ligated as in Fig. 1 and fed high-fat diet for 2 and 7 days in ApoE KO mice. Rings preconstricted with PGF2α were dilated with increasing concentrations of acetylcholine (A) or sodium nitroprusside (SNP; B) for endothelial-independent relaxation. Shown are means ± SE, n = 2 for 2 days, and 6 for 7 days.
Partial ligation in ApoE KO mice causes accelerated atherosclerosis in a NADPH oxidase-dependent manner.
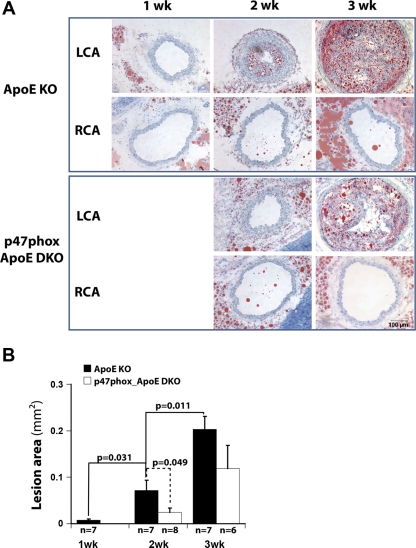
To examine whether partial ligation causes accelerated atherosclerosis, ApoE KO mice were partially ligated and fed the high-fat diet for 1, 2, or 3 wk. At 1 wk, LCA did not show any evidence of atherosclerotic lesion as determined by Oil red O staining (Fig. 5A). By 2 wk, LCA developed robust lipid lesion that increased dramatically by 3 wk after ligation (Fig. 5, A and B). However, RCA did not show any lesions for as long as 6 wk postligation (Fig. 6). Next, we examined the hypothesis that NADPH oxidases play a key role in atherosclerosis development in response to disturbed flow. To test this, we used p47phox_ApoE DKO mice. p47phox_ApoE DKO showed significantly less atherosclerosis at 2 wk postligation (Fig. 5B). By 3 wk, p47phox_ApoE DKO showed a trend toward less atherosclerotic lesion compared with ApoE KO mice, although it did not reach statistical significance (P = 0.08). This suggests that p47phox deficiency delays but does not fully prevent development of atherosclerosis induced by disturbed flow.
Fig. 5.
Partial ligation and high-fat diet rapidly induce atherosclerosis in LCA of ApoE KO mice, and this atherogenesis is delayed in mice deficient in p47phox in ApoE KO background (p47phox_ApoE DKO). ApoE KO and p47phox_ ApoE DKO mice were partially ligated and fed the high-fat diet for 1–3 wk. Shown are representative images of at least n = 6 (A). Frozen sections from LCA were stained with Oil red O. Intimal lesion was quantified for each and shown as the mean ± SE (B).
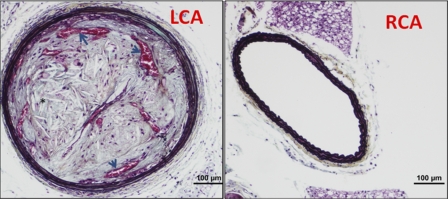
Fig. 6.
Partial ligation and high-fat diet induce features of advanced atherosclerosis in LCA. ApoE KO mice were partially ligated and fed high-fat diet for 4 wk. Paraffin sections obtained from LCA and RCA were stained with pentachrome. Note needle-shaped cholesterol clefts (*) and intraplaque neovessels (arrows) containing red blood cells.
Partial ligation in ApoE KO mice develops complex lesion.
Next we examined whether partial ligation can induce advanced atherosclerotic lesion. Pentachrome staining of LCA of ApoE KO mice 4–6 wk postpartial ligation and high-fat diet showed robust cholesterol clefts and several remarkable intraplaque neovessels (Fig. 6). These results show that at least some features of advanced lesions develop quickly within 4–6 wk following partial ligation, providing additional unique advantage of our model in the study of atherosclerosis.
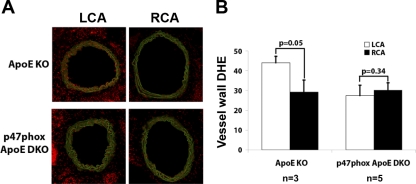
Partial ligation stimulates superoxide production in LCA by a p47phox NADPH oxidase-dependent manner.
To determine the underlying mechanism by which p47phox deficiency delayed atherosclerosis as shown above (Fig. 5), we tested whether disturbed flow increased superoxide production in a p47phox-dependent manner. DHE staining intensity in the vessel walls of LCA obtained from ApoE KO mice was significantly higher than that of RCA (Fig. 7). This increase was significantly blunted in LCA of p47phox_ApoE DKO mice. This suggests that reactive oxygen species (ROS) was produced in response to disturbed flow in a p47phox NADPH oxidase-dependent manner. This is consistent with previous reports in vitro and in vivo (1, 15, 33a).
Fig. 7.
Partial ligation in ApoE KO mice increases superoxide production in LCA in a p47phox NADPH oxidase-dependent manner. ApoE KO and p47phox_ApoE DKO mice underwent partial ligation and were fed high-fat diet for 2 days. Frozen sections were stained with dihydroethidium (DHE; red), and autofluorescence of elastic laminas is shown in green (A). Shown in A are representative images of n = 3–5 mice. DHE staining intensity was quantified and shown as the mean ± SE (B).
DISCUSSION
Here, we characterized partial carotid ligation as a model of disturbed flow with low and oscillatory shear stress that causes endothelial dysfunction and accelerated atherosclerosis. We also describe a simple method of isolating intimal RNA from mouse carotid arteries. In this mouse model, we found that partial ligation of LCA causes 1) low and oscillatory shear, 2) upregulation of proatherogenic genes, 3) downregulation of antiatherogenic genes, 4) ROS production in a p47phox-dependent manner, 5) endothelial dysfunction in ApoE KO mice in 1 wk, 6) rapid atherosclerosis within 2 wk in a p47phox-dependent manner in ApoE KO mice, and 7) advanced lesions by 4 wk.
Partial ligation causes not only low shear stress but also oscillatory shear stress, two characteristics of pathophysiologically relevant disturbed flow associated with atherogenesis (3). Previously, Cheng et al. (6) used the perivascular cuff model to create three distinct regions of shear stress: lower shear (100 dyn/cm2), higher shear (250 dyn/cm2), or oscillatory shear (140 dyn/cm2). Using 15- to 20-wk-old ApoE KO mice fed an atherogenic diet for minimum of 8 wk, they demonstrated that lower shear was more atherogenic than oscillatory shear, although flow oscillation was not directly demonstrated in their mouse model (6). It should be noted, however, that typical atheroprone regions in carotid bifurcations and aortic arches are exposed to disturbed flow characterized by colocalization of both low and oscillatory shear stress (5, 33, 34). Perhaps one reason that our partial ligation induced atherosclerosis much faster than the perivascular cuff model is that LCA is exposed to both low and oscillatory shear in our partial ligated artery while the perivascular cuff exposes LCA to either low or oscillatory shear, but not both. Complete ligation of the carotid artery (20) has also been used to induce rapid atherosclerosis (17). However, its pathophysiological relevance is debated, since it is a model of no flow and likely does not mimic disturbed flow with low and oscillatory shear (36). We propose that the partial ligation model provides a physiologically relevant model well-suited for the study of atherosclerosis induced by disturbed flow. It has been reported that harmonic frequencies above the heart rate may be a risk factor for atherosclerosis development (14). We have not yet examined whether flow frequencies change significantly in LCA by partial ligation. If that is the case, further studies are warranted to determine whether changes in flow frequencies are an important atherogenic component of the disturbed flow created by partial ligation.
Intraplaque neovessel formation and cholesterol clefts are important features of advanced atherosclerotic lesions (13). In our model, pentachrome staining revealed numerous large vascular structures containing red blood cells within the plaque. It is interesting to note that many of these intraplaque neovessels were found near internal elastic lamina, consistent with a previous report showing that intraplaque hemorrhage in innominate artery of 60-wk-old ApoE KO mice occurred in a similar region (27). In addition, we observed abundant needle-like cholesterol clefts within the plaque, providing further evidence of advanced lesions. This could serve as a very useful model to study the mechanisms underlying intraplaque neovascularization and its importance in atherosclerosis.
Here we describe a simple method of isolating intimal RNA from carotid arteries in sufficient quantity with virtually no appreciable contamination from underlying media and adventitia. Intimal RNA obtained by this method is nearly free of medial smooth muscle RNA contamination. Previous efforts to isolate intimal RNA from atheroprone and atheroprotected areas of vasculature have been reported using a scrapping method (35). However, we found our method of simply flushing the carotid artery with lysis reagent easier and highly reproducible. Having our partial ligation model, which exposes the entire length of the common carotid artery to disturbed flow, enabled us to apply this easy method of endothelial RNA isolation. This method could easily be applied to obtain sufficient quantities of RNA for high-throughput genomewide studies, including DNA microarray and microRNA array. Although genomewide cDNA array analysis using endothelial RNAs obtained from the region of the porcine aortic arch that were chronically exposed to disturbed flow from birth was reported (26), our acute mouse model offers unique advantages over the porcine model: in the mouse model, nearly the entire length of the carotid was acutely exposed to relatively uniform disturbed flow conditions, resulting in rapid atherosclerosis development within 2 wk postligation. This accelerated atherogenesis makes it possible to reveal underlying mechanisms directly linking disturbed flow to atherogenesis. The availability of the large portion of the carotid artery allows easy and reproducible intimal RNA isolation from them, enabling RNA studies such as qPCR and genomewide analysis of cDNA and microRNA to identify mechanosensitive transcripts and pathways.
We applied this method to study mechanisms by which disturbed flow induces endothelial dysfunction and atherosclerosis. By comparing endothelial RNA from LCA and RCA, we found that partial ligation downregulated expression of antiatherogenic genes such as eNOS and Klf-2 while upregulating proatherogenic genes such as ICAM-1, VCAM-1, and BMP4. These gene expression changes are consistent with known endothelial responses to disturbed flow in vitro and in vivo (3, 7, 11, 12, 29, 31). Furthermore, using this model in p47phox_ApoE DKO mice, we showed that p47phox-dependent NADPH oxidases play an important role in atherogenesis induced by disturbed flow.
We believe that this is the first report demonstrating that acute induction of disturbed flow in vivo causes endothelial dysfunction. It was reported previously that endothelial dysfunction could be induced in carotid arteries of ApoE KO mice by feeding a Western diet for >26 wk (8). By comparison, our model impairs the vasorelaxation response within 1 wk, providing a rapid and useful model to study flow-dependent endothelial dysfunction.
In conclusion, we characterized partial carotid ligation as a model for acutely induced disturbed flow, which results in accelerated endothelial dysfunction and atherosclerosis and allows for a simple method of intimal RNA isolation. These could be used in ApoE, LDL receptor KO, or other transgenic mice to further study molecular mechanisms underlying flow-dependent regulation of vascular biology and disease, such as neovascularization and atherosclerosis. Moreover, this in vivo model could be used to test various therapeutic interventions targeting endothelial dysfunction and atherosclerosis in considerably reduced study duration.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-87012 (HJ), HL-75209 (H. Jo), and UO1HL-80711 (D. Harrison, D. Giddens, and H. Jo) and by a World Class University Project (H. Jo) from the Ministry of Science, Technology, and Education of South Korea.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Marschall Runge for providing p47phox_ApoE DKO mice and Deborah Martinson for assistance with immunohistochemical staining studies.
REFERENCES
- 1.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest 108: 1513–1522, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation 117: 1082–1089, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 116: 1258–1266, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res 82: 532–539, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49: 2379–2393, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113: 2744–2753, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Cheng C, van Haperen R, de Waard M, van Damme LC, Tempel D, Hanemaaijer L, van Capellen GW, Bos J, Slager CJ, Duncker DJ, van der Steen AF, de Crom R, Krams R. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood 2005 [DOI] [PubMed] [Google Scholar]
- 8.d'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, Katusic ZS. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 21: 1017–1022, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101: 14871–14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res 89: 1073–1080, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol 49: 2073–2080, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol 293: H645–H653, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Jo H, Song H, Mowbray A. Role of NADPH Oxidases in Disturbed Flow- and BMP4- Induced Inflammation and Atherosclerosis. Antiox Redox Signal 8: 1609–1619, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109: 520–525, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol 23: 2185–2191, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5: 293–302, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 17: 2238–2244, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol 160: 2145–2155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby P. Inflammation in atherosclerosis. Nature 420: 868–874, 2002 [DOI] [PubMed] [Google Scholar]
- 23.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA 98: 8955–8960, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation 113: 2818–2825, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68: 231–240, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA 101: 2482–2487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol 20: 2587–2592, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 29.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 199: 1305–1315, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 278: 31128–31135, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arterioscler Thromb Vasc Biol 22: 1100–1105, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol 27: 346–351, 2007 [DOI] [PubMed] [Google Scholar]
- 33a.Tang PC, Qin L, Zielonka J, Zhou J, Matte-Martone C, Bergaya S, van Rooijen N, Shlomchik WD, Min W, Sessa WC, Pober JS, Tellides G. MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med 205: 3159–3171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 24: 12–22, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Patholy 171: 1691–1704, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q. Mouse models of arteriosclerosis: from arterial injuries to vascular grafts. Am J Pathol 165: 1–10, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 111: 1814–1821, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686–692, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.