Abstract
Multiple investigators have shown interdependence of lymphatic contractions on nitric oxide (NO) activity by pharmacological and traumatic suppression of endothelial NO synthase (eNOS). We demonstrated that lymphatic diastolic relaxation is particularly sensitive to NO from the lymphatic endothelium. The predicted mechanism is shear forces produced by the lymph flow during phasic pumping, activating eNOS in the lymphatic endothelium to produce NO. We measured [NO] during phasic contractions using microelectrodes on in situ mesenteric lymphatics in anesthetized rats under basal conditions and with an intravenous saline bolus (0.5 ml/100 g) or infusion (0.5 ml·100 g−1·h−1). Under basal conditions, [NO] measured on the tubular portions of the lymphatics was ∼200–250 nM, slightly higher than in the adjacent adipocyte microvasculature, whereas [NO] measured on the lymphatic bulb surface was ∼400 nM. Immunohistochemistry of eNOS in isolated lympathics indicated a much greater expression in the lymph valves and surrounding bulb area than in the tubular regions. During phasic lymphatic contractions, the valve and tubular [NO] increased with each contraction, and during intravenous saline infusion, [NO] increased in proportion to the contraction frequency and, presumably, lymph flow. The partial blockade of eNOS over ∼1 cm length with Nω-nitro-l-arginine methyl ester lowered the [NO]. These in vivo data document for the first time that both valvular and tubular lymphatic segments increase NO generation during each phasic contraction and that [NO] summated with increased contraction frequency. The combined data predict regional variations in eNOS and [NO] in the tubular and valve areas, plus the summated NO responses dependent on contraction frequency provide for a complex relaxation mechanism involving NO.
Keywords: lymph flow, frequency contraction
lymphatics that are not exposed to periodic compressions/expansion cycles by their host organ must contract in a phasic fashion to propel the lymph made in the organ to the downstream lymph vessels, through the nodes and on to the venous circulation. This intermittent contraction process has been likened to the cardiac cycle by Granger (14) and McHale and Meharg (19) with a systolic and diastolic phase with the frequency of contractions and stroke volume determining the flow of lymph. With the use of this analogy to the cardiac cycle, when the upstream organ produces more lymph, the lymphatic vessels should respond by an increased amplitude and frequency of contractions similar to the elevated stroke volume and heart rate to improve cardiac output. This process has been documented by Benoit et al. (4) for in vivo rat mesenteric lymphatics during hemodilution/volume expansion and with increased transmural pressure of artificial lymph in isolated lymphatics from numerous species and tissues (13, 15, 16, 20–22, 24, 25). In each study mentioned, a greater flow of lymph was associated with an increased frequency of contraction, a greater stroke volume equivalent, and/or a greater relaxation during lymphatic diastole. The latter is used to increase the diastolic diameter and enhance filling, analogous to enhanced cardiac lusitropy. An evaluation of the mechanisms that lead to the contraction and relaxation components of the lymphatic cycle has revealed a counteractive regulation of the two processes. Lymphatic contractions in the rat have been reported to have components of both an enhanced phasic contractile response to distension (4) and the release of constrictor prostaglandins from endothelial cells in response to shear flow forces (21, 22). The relaxation of lymphatics is known from pharmacological studies to be predominantly dependent on nitric oxide (NO) release, either in response to pharmacological activation of the lymphatic endothelium (10, 18, 34) or in response to shear forces as lymph flow is accelerated during the phasic contraction (12, 13, 22, 31). Gashev et al. (12), Tsunemoto et al. (31), and Gasheva et al. (13) reported that the suppression of NO synthase (NOS) with Nω-nitro-l-arginine methyl ester (l-NAME) increased the frequency of contraction under basal flow conditions and blocked the fall in contraction frequency associated with high-imposed flow in isolated rat lymphatics. A chronotropic inhibition of lymphatic contraction frequency by NO has also been demonstrated electrophysiologically by von der Weid et al. (32) in guinea pig mesenteric lymphatics. Using isolated lymphatic segments and intracellular electrical recordings, they found that both the perfusion rate and acetylcholine exposure slowed lymphatic contraction frequencies in an endothelial, NO-dependent manner. Additionally, acetylcholine exposure produced muscle cell membrane hyperpolarization and a decrease in the size of the underlying pacemaker activity, both of which were prevented by endothelial NOS (eNOS) inhibition by Nω-nitro-l-arginine and methylene blue. Furthermore, they documented an elevation of lymphatic endothelial cell calcium after acetylcholine exposure. This same group later demonstrated that sodium nitroprusside inhibited the lymphatic pacemaker frequency and amplitude and that this effect of sodium nitroprusside was abolished by the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one and was reduced by cGMP protein kinase inhibitors (33). However, the effects of eNOS suppression on the chronotropic effects on lymphatic contractions may be dependent on the type of lymph vessel studied or the conditions of the study. Koller et al. (16) found both l-NAME and the removal of endothelial cells of lumbar tract lymphatics prevented the increase in the frequency of contractions associated with an increased lymph flow. In vivo studies of the effects of pharmacological suppression of NO on the lymphatic contractile frequency indicate similar effects, l-NAME exposure to the mesenteric lymphatics increased contraction frequency, and this effect was reversed with l-arginine (28).
While numerous studies have demonstrated a role for NO in the pharmacological and physical modulation of lymphatic pumping, the direct measurement of NO generated by the lymphatic endothelial cells as a function of contractile activity has not been accomplished under any conditions. So while there is a substantial body of evidence that NO released by pharmacological agents most often inhibits phasic lymphatic contraction frequency and lymph pump flow, there is much less evidence relating lymph flow/shear-induced NO with the inhibition of the lymph pump. These gaps in knowledge need to be addressed to help resolve some of the controversies on the role of lymph flow and NO in the chronotropic and contractile processes of the phasic lymphatic pumping cycle, particularly under in vivo conditions.
Our first hypothesis is that the lymphatic wall [NO] must increase during the contraction event if it is to be part of the signaling to suppress a contraction, start diastole, and enhance lymphatic lusitropy. An underlying assumption about NO generation during the lymphatic pump cycle is that the rise in shear force during the increase in lymph flow induced by the phasic contraction (9) would cause a rapid increase in [NO] (13) that may be involved in the cessation of the active phasic contraction and the subsequent enhancement of the diastolic relaxation. If this is the case, the NO response would have to be quite rapid, since the entire contractile cycle can last <2 s (4). To further test this hypothesis, direct measurements of [NO] with a rapid responding NO-sensitive microelectrode (1, 2, 27) were correlated to the onset of the phasic contraction cycles of in vivo rat mesenteric lymphatics. During pilot studies to develop the required techniques, it became obvious that there were two components of the NO response to contractions: a transient component with each local contraction and a sustained component that was highly dependent on the frequency of contractions of the lymphatic network and potentially other NO sources. This led to the second hypothesis that NO generation during the phasic contractions increases in proportion to the lymphatic contraction frequencies that determine lymph flow and shear forces. In evaluating the first and second hypotheses, we found there were major differences in the [NO] between the lymphatic bulbs housing the valves and the tubular portions of the lymph vessel. In our third hypothesis, we proposed that regional variations in eNOS expression would favor higher NO generation in valves than tubular regions. This hypothesis was evaluated using an immunocytochemical detection of eNOS in the isolated mesenteric bulb and tubular regions, followed by an image analysis reconstruction of the vessel wall and valve leaflets.
METHODS
Animal and tissue setup.
The protocols used in these studies were approved by both the Texas A&M and Indiana University Medical School Institutional Animal Care and Use Committees. The in situ studies were done in Indianapolis at the Indiana University Medical School. The rats were Sprague-Dawley males (Harlan, Indianapolis, IN) in the weight range of 300–400 g. The animals were taken off food for about 18 h but given water ad libitum. The goal was to have a quiescent bowel with minimal lymph formation associated with food processing and thus to maintain the animals' lymph dynamic status in basal conditions, as has been done in previous studies by our group (3, 4, 9, 12, 37, 38). The animals were anesthetized with thiopental sodium (200 mg/kg; Abbott, Chicago, IL), diluted to 50 mg/ml of saline, and given subcutaneously in four separate subcutaneous locations over the lower back and thighs. This anesthetic protocol avoided any possibility of direct contact of anesthetic solution and mesenteric tissue. The animal was placed on a heating mat for the remainder of the experiment at 35–37°C, because rats anesthetized with thiopental sodium have a poor ability to thermoregulate and become hypothermic. Core temperature was measured by passing a flexible probe through the mouth to the stomach (Yellow Springs Instruments) and was kept ∼37.5°C. If a heating mat temperature of 37°C was inadequate to maintain the core temperature, the animal's body was covered by a plastic bubble sheet as insulation.
The trachea was cannulated and the animal mechanically ventilated at 70 breathes/min, a routine conscious ventilation frequency for the weight of rats used and at a tidal volume based on the Harvard Apparatus (Holliston, MA) nomogram for weight, ventilation frequency, and tidal volume interaction. Additional tidal volume was added to compensate for dead space in the ventilation tubing. After the animal was ventilated for 10–15 min, an ear oximeter (Nonin Pulse Oximeter Model 8600V, Plymouth, MN) was used to measure the percent saturation of hemoglobin with oxygen. Percent saturations of 93–97% were satisfactory: saturation above 97% on mechanical ventilation was avoided to prevent hypocapnia.
The right femoral artery was cannulated to measure the mean arterial pressure and to allow the infusion of saline through the arterial catheter as required in later portions of the protocol. The infusion caused a small offset of the arterial pressure, as the flow of fluid acted against the hydraulic resistance of the combined catheter and cannulated vascular segment. The amount of increased pressure was known from the immediate rise in apparent mean arterial pressure as soon as the infusion pump was activated. The offset was typically 2–4 mmHg and compensated by subtraction from the recorded arterial pressure. The fluid protocol was used after the animal had been allowed to equilibrate about 30 min, and one or more lymphatics had been selected and basal activity measured. The animal was then given 0.5 ml/100 g of saline as a bolus, followed by 0.5 ml·100 g−1·h−1 of saline by continuous infusion. The slow infusion is routinely used to maintain cardiovascular parameters since fluids are lost by ventilation, urine formation, and dehydration from surgical sites. The empty intestine appeared to generate basal lymph flow to the mesenteric lymphatic system before saline infusion. However, the initial fluid bolus and slow infusion generally increased lymphatic activity for several hours, as will be shown in detail.
The intestinal and mesenteric vasculatures were exposed through a 1.5–2-cm abdominal incision at the linea alba spanning the umbilicus. A loop of distal jejunum or early ileum was moved into the tissue support system. The mesentery consistently had a side with the mesenteric arteries and lymphatics in a relatively clear presentation to allow the micropipette tips to touch the mesentery monocellular layer over the lymph vessel wall. The entire mesentery was supported by a sloping portion of the tissue support, whereas the intestine itself floated free. The tissue support is composed of a hollow stainless steel shell similar to that used in prior studies by this laboratory (7, 35). To maintain the tissue in place, small 4-0 silk sutures were tied to the anti-mesenteric border of the intestine. The sutures were secured by a metal and plastic housing over the tissue that was also used to direct bicarbonate-buffered complete physiological solution over the intestine and mesentery (6). The buffer flow was set at 5 ml/min over the tissue and was immediately removed by suction. The buffer was equilibrated with 5% oxygen, 5% carbon dioxide, and balance nitrogen to duplicate the gas tensions in the peritoneal fluids. In addition, concentrated calcium chloride solution (10% in distilled water, 0.7 ml/l) was added after the carbon dioxide had reduced the pH to ∼7.4 to avoid crystallization. The buffer was preheated just before it entered the chamber, and the chamber system itself was heated by flowing distilled water (1.5–1.7 l/min) from a heated water pump. The tissue fluids were held at 37.5 ± 0.2°C both to preserve tissue integrity and to provide a thermally stable environment for NO microelectrodes that are very sensitive to temperature fluctuations.
The selection of lymph vessels followed specific guidelines. Only lymph vessels associated with the main trunk of mesenteric arteries en route to the intestine and before they arborized near the bowel wall were studied. Lymph vessels were chosen that were not near either the large mesenteric artery or vein and that were away from any type of microvessel larger in diameter than ∼25 μm. All arteries and many larger arterioles and even some venules have a NO concentration ([NO]) higher than that of most mesenteric lymph vessels and thus could donate NO to the lymph vessels in close proximity. It is very difficult to avoid the extensive microvascular capillary network in the adipose tissue that surrounds most of the lymph vessels, so the interaction of lymphatic and adipocyte microvessels possibly exchanging NO was evaluated.
NO measurements.
NO measurements for lymphatic vessels were based on earlier reports of NO-sensitive microelectrodes similar to those used by Buerk et al. (8) and Friedemann et al. (11) and modified by this laboratory to achieve the best characteristics of both designs (1, 35). Only the open-glass enclosed tip of the electrode surrounding the 7- to 8-μm carbon fiber was sensitive to NO, and the total outer width of the beveled tip was 11–14 μm. Nafion was electrically plated onto the surface of the carbon fiber at +0.7 V for 20–30 min to minimize the interference from proteins and negatively charged ions and organic compounds, including l-NAME used in this study (1, 27). As a frame of reference, most lymphatic tubular areas are ∼50–75 μm in diameter, and valve areas were typically 100–150 μm in diameter. All measurements were made with the microelectrode tip on the middle of the lymphatic vessel and slightly pressed onto the vessel surface to avoid the loss of contact between the microelectrode tip and the wall during lymphatic contractions. The monocellular layer of mesentery overlying the lymphatic vessel was neither removed nor penetrated. However, the penetration of the mesentery made very little difference in the [NO] measured. The microelectrodes were polarized at either 0.7 or 0.9 V depending on which voltage yielded the most stable “0” NO baseline and larger response to 600 and 1,200 nM NO calibrations. Microelectrode stability for long periods was essential because the microelectrode tip had to stay in contact with the lymph vessel for over an hour in some cases. Any electronic drift over time was compensated by determining a virtual baseline for “0” NO over time. The actual [NO] of the tissue was known from the difference of the baseline and tissue values and the calibration of the [NO]/current relationship generated by the microelectrode as measured by a Keithley model 6517A electrometer (Cleveland, OH). A PowerLab analog-to-digital chart recorder system (AD Instruments, Colorado Springs, CO) was used to synchronize the electrode data with measurements of vessel motion. During high-speed data recording, measurements were recorded 10 times/s; slow recording was at 1- or 5-s intervals depending on the needs of the protocol.
Lymphatic motion measurements.
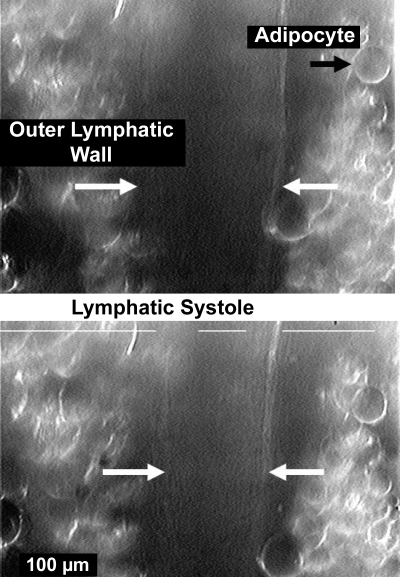
Lymphatic motion was followed with a Living System Vessel Dimension Analyzer (Living Systems, Burlington, VT). For the in vivo status of a young adult rat that weighed 300–400 g, the adipocytes on either side of the lymph vessels made the following of the exact diameter of the lymph vessel very difficult, as shown in Fig. 1. However, the key data needed were motion of the walls at the start of contraction, which could be tracked by one or both of the location sensors on either wall edge, as shown in Fig. 1. The lines on Fig. 1, left and right, mark the outer boundaries of the measurement system. The inner line marks the inner left and right boundary. The actual measurement marks are small “dots” (not shown) that follow a set light level on a structural feature along each side of the lymph vessel. Each side of the vessel had a different light level feature. The operator (H. G. Bohlen) marked the electronic chart record with a time mark to indicate the presence of a contraction. Whereas the human “timer” was not accurate enough to judge the precise start of the contraction, the operator marks did serve to help find the motion data for the beginning of the first sign of the lymphatic wall motion with each contraction. This was useful because both breathing and arterial pulse pressure motion of the mesenteric tissues added mechanical noise to the motion record. The frequency of contraction was determined by counting the number of contractions over a known time interval of >90 s and calculating contractions per minute.
Fig. 1.
A video image of an intact, in vivo lymphatic in diastole (top) and at full systole (bottom). The mesenteric lymphatic vessels associated with major mesenteric arteries are virtually surrounded by adipocytes, and the exact inner diameter locations are difficult to resolve. The white broken line along the top of each image was generated by the Living Systems Dimension Analyzer and is part of an automated system to track changes in dimensions, in this case, the edges of adipocytes adhered to the lymphatic. Normally, the 2 longer lines would terminate before crossing over the outer walls of the lymphatic. The limited contrast of the images, despite oblique lighting and modification of the image with the camera controls, made simultaneous measurements of both wall locations and thereby diameter difficult. However, 1 of the 2 measurement windows did consistently track motion to allow the start of systole to be accurately determined.
l-NAME application to single lymphatics.
To suppress the eNOS of the lymph vessel region being studied without changing intestinal vascular conditions, 1 mM l-NAME in mammalian Ringer solution (pH 7.3 to 7.4) was suffused onto the lymphatic wall with a 200–300 μm outer diameter micropipette at a flow of 50 μl/min. The 5 ml/min flow of buffer, a 100-fold greater flow, over the entire preparation would quickly dilute the l-NAME solution before it could significantly influence nearby vessels. The bathing media passes over the preparation and is immediately removed by aspiration to avoid building up the concentration of any ejected material. The rationale of the localized l-NAME application was to demonstrate that the NO signal from the lymph vessel wall declined when the eNOS of the lymphatic vessel was suppressed but when lymph flow from the intestine was unaltered. Although the microelectrodes are insensitive to l-NAME because of their Nafion coating (27), the l-NAME fluid release was stopped just before NO measurements resumed after eNOS suppression to avoid any unforeseen artifacts of fluid ejection. Upstream and downstream portions of the lymph vessel continued to function, and lymph flow through the blocked area was obvious from the motion of the lymphocytes moving inside the vessel. The [NO] of the blocked region began to decrease within 5 min after the start of the local l-NAME application. In trial and error studies, 20 min of application was deemed adequate to cause significant eNOS inhibition. Longer application times did cause much greater reductions in [NO] than will be reported. However, at these longer time intervals, the lymph vessel began a series of fast, small contractions that were hard to follow with the vessel wall motion measurement system available.
eNOS expression in isolated rat mesenteric lymphatics.
Because of the differences in NO production that we observed along the lymphatic, we isolated similar rat mesenteric lymphatics to measure the expression of eNOS using immunofluorescence histochemistry. After anesthesia and exteriorization of the small intestine and attached mesentery, an appropriate lymphatic was found (about 1.5 cm long) and isolated from the mesentery and surrounding adipocytes. The vessel is transferred to an isolated vessel chamber (Living Systems CH1) where it was cannulated and pressurized at 2 cmH2O. It was then fixed in 2% paraformaldehyde in PBS for 60 min and washed in PBS three times for 5 min each. The vessel was then permeabilized in −20°C methanol for 5 min and washed three times with PBS. The vessel was then divided into two pieces. One section was intralumenally incubated with primary antibody, mouse anti-eNOS (eNOS/NOS Type III, 5 μg/ml, BD Transduction, San Jose, CA) in blocking solution (1% BSA, 5% normal goat serum in PBS) and stored at 4°C overnight. The second section was treated for the same amount of time with the same concentration of mouse preimmune IgG. Both sections were then extra- and intralumenally rinsed three times for 5 min each, followed by an incubation with the secondary antibody, goat anti-mouse antibody conjugated to Alexa Fluor 488 (1: 200 dilution; Molecular Probes, Carlsbad, CA) for 1 h at room temperature and then rinsed for 5 min, three times each. The vessels were finally washed with PBS, three times for 5 min. The vessels were cannulated and tied onto two glass pipettes, pressurized at 2 cmH2O, and secured to the stage of a multiphoton/confocal microscope (Leica TCS SP2, Germany) for immediate observation with a Leica HC PL APO 20X (0.7 na) objective. The stained vessels were confocally scanned throughout the entire vessel diameter in 0.5-μm z-axis steps. An image reconstruction on the image stacks was performed using the Leica Confocal Software package. The negative controls for all experiments were produced and analyzed via similar procedures, except that the control samples were incubated with normal mouse IgG in a corresponding concentration to the primary antibody. The corresponding negative controls were scanned at the same instrument settings as the unknowns for a valid comparison of relative fluorescence intensities. The relative eNOS expression was evaluated from the whole vessel reconstructions by integrating the fluorescence intensity of the fluorescently tagged antibody labeling of eNOS along the axis of the vessel (binned in 10-μm segments). Since the absolute fluorescent intensity varies from vessel to vessel, to combine data from different vessels, within a vessel we normalized the fluorescence intensity axially to the value of the last upstream point before the upstream insertion of the valve leaflets. This integrates the eNOS signal across the diameter of each whole 10-μm-long segment of the lymphatic and includes the signal from the valve leaflets where present. However, to look at different sections of the lymphatic wall along the axis of the vessel and the valve leaflets separately, we used some of the vessels (n = 6 different vessels from different animals) that allowed a complete analysis of the valve leaflets to do a reconstruction of the middle quarter of the vessel which was radially oriented such that the sections through the lymphatic wall and valve were at 90° to the points of the leaflets' downstream insertions. We quantified eNOS expression in separate sections (25–30 μm long) of the vessels wall and valve leaflets (again normalized to the value at the valve leaflets' upstream insertion point).
Statistics.
Statistical differences were evaluated with one- and two-way analysis of variance for repeated measures followed by the Tukey least significant difference test to determine specific significant events. The analysis was performed with either Statistica software (Statsoft, Tulsa, OK) or Statview (SAS, Cary, NC).
RESULTS
Lymphatic valvular and tubular variations in [NO].
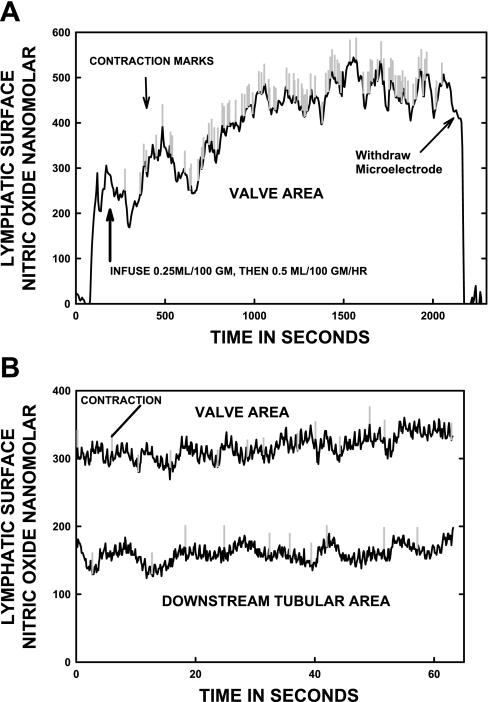
Figure 2A illustrates a long-term recording after a saline bolus was given intra-arterially followed by a sustained saline infusion. Figure 2B illustrates the rapid data collection from a paired lymphatic valve and downstream tubular area that were not measured simultaneously, but within 10 min of each other. These measurements were made after the bolus infusion had increased the activity of the lymph vessel to a new steady state. This faster recording of [NO] was very typical and is used to illustrate the correlation of changes in [NO] with the onset of individual lymphatic contractions. The marks on the record indicate the start of each lymphatic contraction. The motion of the lymphatic wall due to ventilation at 70 breathes/min did slightly influence the [NO] recorded. This is seen as the rapid, small variations in [NO] with a duration of ∼1 s shown in Fig. 2B, even though the microelectrode tip was pressed lightly against the lymphatic wall at all times.
Fig. 2.
A: a typical long-term recording of nitric oxide concentration [NO] at a slow speed (1 point/5 s) as a vessel responded to saline infusion. The recording shown is over more than 30 min. The vertical marks are individual contractions, and their decreasing spacing indicates increased frequency of contractions after the infusion of saline to augment lymph production by the small intestine. B: [NO] recorded at high speed (10 points/s) in the valvular and tubular area of the same lymph vessel measured after saline infusion. The smaller variations in [NO] in B are artifacts of the 70 breathes/min ventilation of the animal. In A and B, the larger variations in [NO] that start at or near vertical marks are excursions of [NO] with each lymphatic contraction.
In Fig. 2A, as the general frequency of contractions increased in response to greater intestinal production of lymph, the baseline [NO] also increased. These increases in contractile and NO activity began to occur generally within 5–10 min after saline infusion began and could take up to an hour to reach full activity. As shown in Fig. 2A, the new steady state for this particular vessel was reached at about 30 min post-bolus infusion, which was typical. Over longer periods of time in Fig. 2A, the [NO] responses of multiple contractions close in time can be seen to cause a progressive or summated increase in [NO]. When contractions spontaneously subsided, the [NO] began to decline until another group of contractions began to occur.
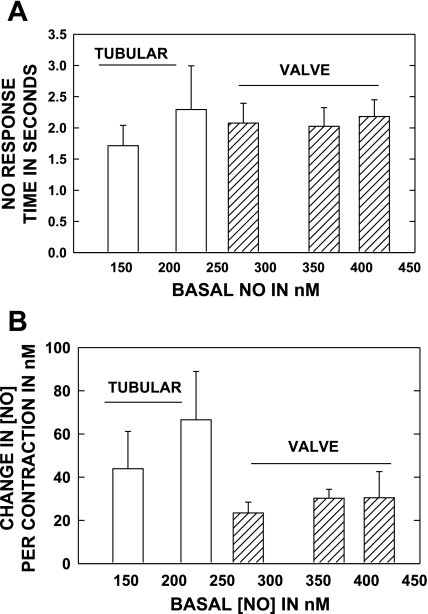
To better appreciate the rapidity and magnitude of the increase in [NO] with each contraction of lymph vessels, the time required to increase the [NO] from the nadir just before a contraction to the peak of contraction and the absolute increase in [NO] per contraction was measured on six tubular (6 rats) and seven valve areas (7 rats) from initial to activated states during systemic saline infusion. As shown in Fig. 3A, the time to peak response per contraction was of the order of ∼2 s for both tubular and valve regions and the response time was not appreciably altered by increasing NO generation during saline infusion. The increase in [NO] per contraction, shown in Fig. 3B, was greater in tubular than valvular regions, but as can be seen by the x-axis, the tubular regions operated at a lower basal range of [NO] than did the valve regions.
Fig. 3.
A: the response time of the [NO] from basal to peak during single lymphatic contraction cycle is shown for tubular and valve areas. Both regions have fast NO responses during contractions. The data were collected at basal conditions and as lymphatic activity increased during saline infusion. This allowed the comparison of timed and NO responses to the average [NO] in A and B. B: the increase in [NO] from basal to peak of contraction for the same vessels and conditions as in A. Tubular areas are able to generate as large an increase in NO per contraction as are valvular areas. Data set based on 6 tubular (6 rats) and 7 valve (7 rats) areas.
Regional variations in [NO] along lymphatic vessels.
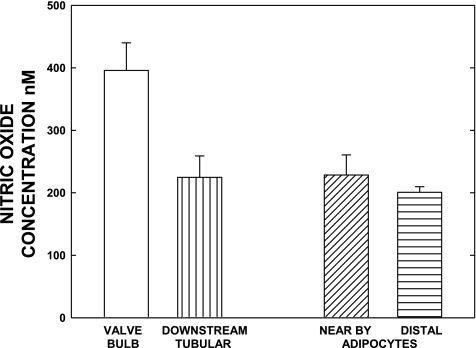
The vessel wall [NO] of fully activated lymph vessels varied by location and was influenced by the surrounding tissue area. For the data in Fig. 4, the mean data for this comparison were obtained after stable increases in lymphatic activity had occurred following saline infusion in 12 lymphatics of 10 rats. The concentrations used are those between successive contractions, which are lower than during the height of the contraction. The data in Fig. 4 also demonstrate that perilymphatic [NO] from the microvessels of adipose tissue or the adipocytes themselves was similar to that of the tubular areas of lymph vessels even when lymphatic pumping was initially activated by a bolus of saline infusion. The [NO] generated by the adipocytes/microvessels adjacent to a lymph vessel was not unique, because a displacement of the microelectrode about 500 μm away from the lymphatic wall but near the adipose tissue yielded virtually the same [NO]. As most lymph vessels of the mesentery were usually surrounded on three sides by adipose tissue with the fourth side as the mesentery exposed face, the microvasculature of the adipose tissue serves as both a source of oxygen and could affect the gradient of NO gained or lost from the lymphatic. During the low-level activity of lymph tubular vessels before saline infusion, the adipocyte tissue [NO] was consistently higher than that in the lymphatic tubular areas. However, the [NO] was always higher at the surface of the lymph bulb than the nearby adipose tissue in the valve areas during the basal activity period (before saline infusion).
Fig. 4.
Paired valvular and tubular area [NO] of lymphatics during stable activity shortly after bolus saline infusion are shown along with measurements of adipocyte [NO] one cell distance (20–30 um) from the lymph wall and 500 μm from the wall. The lymphatic wall concentrations used are those between successive contractions, which are lower than during the height of the contraction. The high [NO] of the adipocyte tissue, presumably generated by their microvascular network, was equivalent to the [NO] found basally in the tubular lymphatic regions. The lymphatic valvular areas did consistently have a much higher [NO] than either the [NO] found in adipose tissue or tubular regions of the lymphatic. The data set is based on measurements of 12 lymphatics and associated adipose tissues in 10 rats.
Imaging of eNOS expression in lymph vessels.
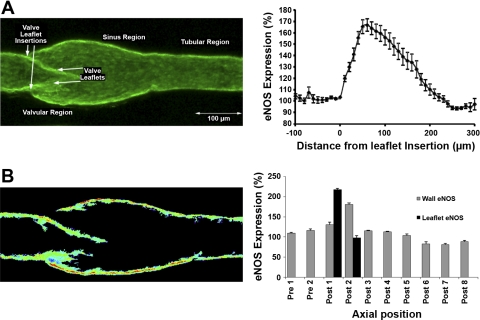
Based on the microelectrode results in Figs. 3 and 4 that demonstrated that the valvular areas had a much higher [NO] than did the tubular areas of the lymphatics, isolated mesenteric lymph vessels were evaluated for eNOS distribution by immunofluorescence histochemistry. The goal was to determine whether valvular areas, particularly the valve leaflets, had a greater eNOS expression than did the tubular areas. The various results are shown in Fig. 5. The three-dimensional reconstruction of the vessel wall stained for eNOS demonstrates in Fig. 5A the unique structure of the lymphatic wall and leaflets along the axis of the vessel. Like many small lymphatics, the vessel displays the typical postvalvular sinus region that is significantly larger than either the tubular section upstream of the valve leaflets or the tubular section downstream of the valve and sinus region (38). This creates a large sinus volume between the leaflets and the vessel sinus wall; Fig. 5A, left, depicts a representative vessel image of eNOS expression, and Fig. 5A, right, presents the mean intensity data for eNOS fluorescence of 18 vessels evaluated from 15 animals. In Fig. 5A, right, the origin of the valve leaflet was used as a “0” position intensity reference for upstream and downstream distance measurements, and this locations intensity was set as a relative value of 100% for estimating the relative intensity for all other locations along the lymphatic vessel. The eNOS is highly expressed in the rat mesenteric lymphatic endothelium all along the lymphatic. Note the high signal (integrated eNOS expression across a 10-μm transverse section) from the valve leaflets area as seen in Fig. 5A, right. The elevation in the eNOS signal starts at the upstream valve leaflet insertion point and peaks as a 60–70% increase in the section of the lymphatic that contains the valve leaflet. In part, this is because of the greater endothelial cellular volume on both sides of the valve leaflet structures and the increased volume and surface area of the lymphatic sinus regions that are included in the reconstructed image. However, to evaluate the eNOS expression in more detail on the valve leaflets, and the different sections of the lymphatic wall in the pre valve, the sinus region of the lymphatic bulb, and the downstream tubular section of the lymphatic, we performed reconstructions of the three-dimensional eNOS image stack through the middle quarter of the vessel (Fig. 5B, right; spectrally color coded to reflect relative eNOS fluorescence intensity). We radially oriented these reconstructions such that these sections were at 90° to the valve leaflet downstream insertion points. While we did not expressly quantify the absolute eNOS concentration, the relative concentration of eNOS is highest in the sinus wall endothelium (the red-orange color in the example in Fig. 5B, left). The fluorescent intensity signal was normalized in each vessel to the value measured at the point immediately before the upstream insertion of the valve leaflets. The signals were then integrated axially along a 25–30-μm section of the vessel and plotted (Fig. 5B, right). These data demonstrate that both the upper half of the valve leaflets and most of the sinus region wall have higher levels of eNOS expression than do the downstream or upstream tubular areas (Fig. 5B). The expression of eNOS along the tubular sections of the lymphatic appears relatively constant axially, beyond ∼200 μm downstream of any valve down to ∼60 μm upstream of the next set of valve leaflets.
Fig. 5.
A: the expression of endothelial NO synthase (eNOS) was measured by immunofluorescence histochemistry using isolated rat mesenteric lymphatic vessels and confocal microscopy. A, left: a typical complete reconstruction of the three-dimensional eNOS expression depicting the labeling in the lymphatic endothelium of the valvular, sinus, valve leaflets, and tubular regions. A, right: the integrated fluorescence intensity of eNOS rose in the valvular region of the lymphatic starting about the upstream point of insertion of the lymphatic valves (n = 18 vessels from different animals, bars = means ± SE). The relative eNOS expression then declined in the downstream axial direction of the lymphatic sinus beyond the valve leaflets. B, left: a typical reconstruction of the middle quarter of the scanned vessel cutting through the lymphatic wall and valve at 90° to the points of leaflets downstream insertion. The image was background subtracted and color coded to represent relative eNOS expression spectrally with higher expression being hotter colors. The normalized eNOS expression was then integrated over axial sections of the vessel (binned in 25–30 μm long-axial sections) and plotted in the bar graph (n = 6 vessels from different animals, bars = means ± SE) (B, right).
Interaction of contraction frequency and NO generation.
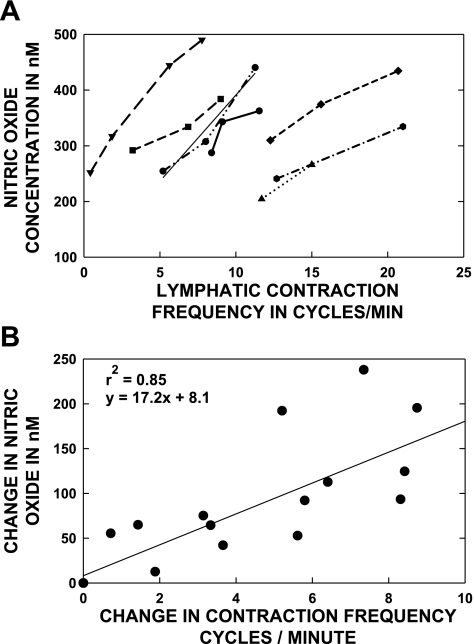
In in situ preparations, the recorded [NO] increased following intravascular saline infusion, and both the frequency of lymphatic contractions and basal [NO] increased together. Long-term recordings of lymph vessels from basal states to activated status after saline infusion, such as in the Fig. 2A, were used to correlate the local [NO] to the frequency of lymphatic contraction. The pattern of behaviors shown in the Fig. 2A were so prevalent in individual experiments as to be accepted as what should be a normal response to elevated lymph flow. The assumption is that the increased frequency of contractions after saline infusion reflected the elevated lymph flow that would exert greater average shear forces on their endothelial cells. Examples of the frequency of contraction versus [NO] for individual valvular lymphatic areas in seven different rats are shown in Fig. 6A. There is a good deal of variation in contraction frequency between lymph vessels in the same animal. We intentionally did not follow only the faster contracting lymph vessels but randomly selected vessels to show the range of activity that we encountered. Note that some valve areas of the lymphatic vessels had a simple step increase in activity after saline infusion was started, whereas others had a progressive increase in the frequencies of contraction. Variability of individual lymphatic contraction frequencies is commonly observed (12). When we evaluated the data, we noticed that the slope of the contraction frequency versus the [NO] relationship was similar between different vessels and animals. Therefore, the data were evaluated as the change in frequency versus the change in [NO] as shown in Fig. 6B. The data set for all vessels fit a straight line to relate the [NO] responses to the frequency of the lymphatic contractions in the valvular regions. The regression correlation was 0.85, which was significant at P ≤ 0.05, and the equation of the line was as follows: change in [NO] = (17.2 × change in frequency) + 8.1.
Fig. 6.
The contraction frequency and [NO] was measured during basal lymphatic activity at the beginning of the experiment and at various times after infusion of saline. Each line in A is for a different animal (N = 7). Note that considerable variability of contraction frequency existed for the basal activity at the beginning of each experiment, but the [NO] range is not as variable. Saline infusion was used to elevate lymphatic activity and also increased both [NO] and contraction frequency in every case. The slope of the contraction frequency vs. [NO] was similar between animals, and the data are plotted as change in contraction frequency vs. change in [NO] in B. The data set fit a straight line with a high correlation coefficient and may indicate a relatively common [NO] vs. contraction frequency, i.e., flow mechanism, between lymph vessels.
Effect of eNOS suppression on [NO] and contraction frequency.
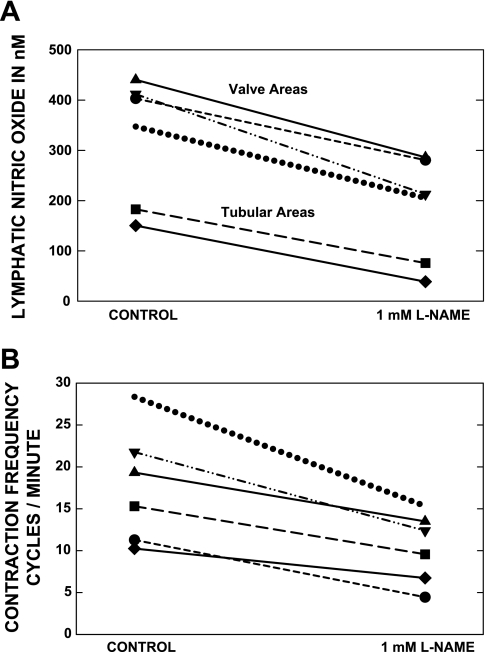
At the end of six in vivo experiments, l-NAME was applied to a small section of an activated lymph vessel to determine how the frequency of contractions and [NO] would be altered. In addition, these studies illustrated that the presumptive signal measured by the NO-sensitive microelectrode was lowered in the in vivo environment by a known blocker of NOS enzymes. The assumption of the study was that [NO] would decrease and confirm that most of the current measured by the electrode was from the oxidation of NO. Figure 7A presents the data that perilymphatic [NO] for both bulbular and tubular areas did decline after l-NAME exposure. The average [NO] decreased significantly (P < 0.05) to 50.6 ± 7.6% of control after partial eNOS suppression. As mentioned earlier, l-NAME was used to suppress eNOS, but a full suppression was intentionally avoided. The vessels exposed to the higher or prolonged doses of l-NAME simply contracted to a small diameter with frequent small contractions. With the partial blockade protocol used, as shown in Fig. 7B, the frequency of contractions for the same six vessels in six rats appeared to decrease because, functionally, the vessels would contract and hold the phasic contraction before eventually relaxing. The frequency of contractions decreased significantly (P < 0.01) to 61.9 ± 3.6% of control after exposure to l-NAME.
Fig. 7.
A and B: (l-name) was used to partially suppress eNOS in a small section of an activated lymphatic to demonstrate that [NO] declined as would be expected and to evaluate the change in contraction frequency. In B, a decrease in contraction frequency with partial eNOS suppression is consistently shown. After application of l-NAME, the [NO] had decreased significantly (P < 0.01) to 50.6 ± 7.6% of control and the frequency of contractions had decreased significantly (P < 0.04) to 61.9+3.6% of control.
DISCUSSION
Our first hypothesis is that the lymphatic wall [NO] must increase during the contraction event if it is to be part of the signaling to suppress a contraction and start diastole and enhance lymphatic lusitropy. This can be seen most readily in Fig. 2, particularly Fig. 2B, by the small rise in [NO] that occurs over 3–6 s following each phasic contraction (the slow waves in NO). Figure 3 provides details on the time required for the [NO] to increase with each lymphatic contraction (in Fig. 3A) and the magnitude of the increase (in Fig. 3B) for both tubular and valve areas. These measurements were made for the basal state and as the activity increased following saline infusion. Note that the time to peak [NO] from the nadir of [NO] just before contraction is about 2 s for all regions and not appreciably influenced by the level of activity, i.e., as judged by increasing basal [NO]. The data in Fig. 2B illustrate that the tubular regions are as or more capable of transient increases in [NO] with each contraction as are the valve areas. The rapid, transient increases in [NO] presumably reflect the local increase in NO production that accompanies the local rise in shear forces that occur with each phasic contraction (9). Similar shear-dependent mechanisms to increase NO generation have been reported in the blood vascular endothelium (26, 29, 30) and, given the documented changes in shear (9), appear likely during phasic lymphatic contractions.
While evaluating hypothesis 1, the measurements revealed that the [NO] along the in vivo mesenteric lymphatics varied by location and was influenced by local blood microvessels. The adipose tissue with its dense capillary network has a relatively high concentration of NO (∼200 nM) compared with tissue measurements in both the small intestine and skeletal muscle of rats, which are seldom higher than 150 nM (17, 23, 35, 36). Thus adipose tissue may be a significant source of NO to the tubular portions of the adjacent lymph vessels. However, the high perilymphatic [NO] generated by the adipocytes and microvasculature cells by comparison was only about half of the [NO] in the valvular portion of the lymph vessel. These data are shown for typical examples in Fig. 2 and averaged for the data group in Figs. 3 and 4. The valvular regions exhibited [NO] levels that were at least comparable with the periarteriolar [NO] levels measured previously in both the small intestine and skeletal muscle (17, 23, 35, 36) under basal conditions.
When saline infusion occurred, lymphatic contraction frequency rose as we have previously shown (4). Under these edemagenic conditions, the forces responsible for lymph formation and flow increased, thus stimulating the lymph pump. Accompanying the expected rise in lymph flow and pumping, the valvular and tubular regions [NO] increased substantially, as shown in Fig. 6. Presumably, this increase in [NO] is related to the increase in lymph flow and thus shear forces, acting on the lymphatic endothelium. How much of the elevation of [NO] under these edemagenic conditions is due to the local versus distant generation of NO by the lymphatic endothelium remains to be determined. The probable cause of the high valvular [NO] is the larger content of eNOS of the sinus wall and the endothelial cell-coated valve leaflets, as clearly shown in Fig. 5. However, it is also likely that the high-shear force of lymph flowing through the open valve leaflets was a contributing factor to the elevated NO near the valve. Whereas the valvular portions of the lymph vessels did have much higher [NO] than the tubular portions, the tubular areas did exhibit both eNOS expression (Fig. 5) and an increase in [NO] with contractions (Figs. 2A, 3, and 6A), indicating locally competent NO production. In Fig. 3, the tubular regions raised [NO] more and as fast, as judged by time to peak [NO] with each contraction, as the valve areas. However, tubular regions operated at lower basal [NO] than did the valve areas at all levels of activity (see also Fig. 4). The regional variations in eNOS expression with the higher relative values in the valve areas may in part explain the differences in basal [NO] concentrations, plus there are likely differences in lymph shear forces.
Our second hypothesis was that NO generation during pumping increases in proportion to the phasic lymphatic contraction frequencies that help determine lymph flow and shear forces. One of the more unique observations in regard to [NO] and contraction frequency was that behaviors by vessels in different animals all followed a pattern of increased [NO] as the frequency of phasic contractions increased. These data are shown in Fig. 6. As mentioned in the preceding paragraphs, with each contraction there was a rapid, transient increase in [NO]. However, the basal [NO] between contractions increased as the frequency of the phasic activity was elevated during saline infusion. As shown in Fig. 6A, the initial [NO] and contraction frequency of individual lymph vessels were quite variable between animals. For individual vessels, such as the example shown in Fig. 2A and for the averaged data in Fig. 6, it was very clear that the [NO] increased in approximate proportion to the frequency of lymphatic contractions. However, once the lymph vessels were activated by saline infusion, the slopes of the increase in [NO] versus the increase in contraction frequency were quite similar, as shown in Fig. 6A and replotted in Fig. 6B as changes in frequency versus changes in [NO]. While correlations are not necessarily predictive of the mechanisms, it would appear that mesenteric lymph vessels have a common facility to increase NO generation in response to elevated lymph flow. Elevated contraction frequency is consistent with elevated flow, which also increases the phasically generated shear stress. The lymph flow being pumped is generated by the intestinal tissue upstream from the mesenteric lymphatics, and the lymphatic pumping behavior was in response to the increased fluid load and lymph pressure imposed on them. The basis of this was already established by the work of Benoit et al. (4) that quantified the lymph pressure and lymph flow to a frequency and pumping stroke volume of in vivo mesenteric lymphatics during saline loading. As the lymph flow increased because of saline infusion, lymph vessels responded with increased chronotropic and inotropic behavior. Thus the enhanced lymph formation, pressure, and flow that result from the saline infusion stimulated lymphatic contraction frequency and increased lymph pump flow/shear, which in turn enhanced the lymphatic production of NO. Depending on how long the NO survives in the lymph as it flows downstream, this may result in the elevation of the [NO] both locally and at sites downstream along the lymphatic tree, essentially increasing the lymph NO load.
To evaluate that the third hypothesis of proposed regional variations in eNOS expression would favor higher NO generation in valves than tubular regions, immunocytochemical methods were employed. As shown in Figs. 3 and 4, the regional variations in measured averaged [NO] with higher valvular than tubular [NO] were supported by the regional relative differences in eNOS total and membrane content as shown in Fig. 5, A and B. While these data are given in relative terms because image analysis histochemistry was necessary, the results support the potential for greater NO generation in valve than tubular regions. Exactly how this dichotomy of eNOS expression and NO generation between valvular and tubular regions may be influential in the overall lymphatic regulation remains to be determined. However, as mentioned for Fig. 3, the tubular regions were as or more competent to generate rapid transient increases in NO with each contraction as were the valvular areas, but from a lower basal [NO] between contractions. Therefore, contraction-cycle increases in NO generation to presumably promote the relaxation phase appear to be a common physiological process of the valvular and tubular sections of microscopic lymphatic vessels.
As a check of the technical reliability of the NO measurement methodology in this study and to evaluate the in vivo consequences of the partial suppression of NO generation, l-NAME was used to verify that the signal measured by the NO-sensitive microelectrodes was in fact due to NO and that the suppression of eNOS had functional consequences on lymphatic activity. We have previously shown that the microelectrodes are unable to directly respond to ∼1 mM l-NAME when their Nafion membrane is intact (27). Furthermore, when the electrodes do respond to l-NAME at 0.1–1 mM concentrations, the current measured is increased and not decreased as shown in Fig. 7A. The decrease in signal, presented as a decline in [NO] in Fig. 7, provided evidence consistent with the suppression of NOS. This local inhibition of NO produced an unexpected effect of decreasing the local phasic contraction frequency, as seen in Fig. 7B. Here it appeared that the vessels contracted but only slowly relaxed as the [NO] declined because of NOS inhibition. In fact, we had to be very careful not to overly suppress with l-NAME because the phasic contractions of the vessel appeared to lengthen and subsequently had only very small transient relaxations. The decrease in frequency of contraction occurred because of the greater time spent in the systolic contraction phase with increased tone and decreased lusitropy. The sustained contraction and lengthening of relaxation time after NOS blockade is similar to what we previously observed in the rat thoracic duct (13). But the general effect of NOS blockade on phasic contraction frequency observed with local blockade is somewhat different than what we have previously seen with global NOS inhibition. For example, using isolated mesenteric and thoracic duct lymphatics, Gasheva et al. (13) found that l-NAME increased the frequency of contractions during increased imposed flow conditions, and Gasheva et al. (13) found l-NAME increased the frequency of contractions even without an imposed flow. Mizuno et al. (21), using isolated rat lumbar lymphatics, found that the removal of endothelial cells, which in principle is similar to the suppression of NO formation by chemical methods, resulted in a constriction and an increased frequency of contractions. However, these studies were also done without an imposed flow. The current study demonstrated a decrease in contraction frequency in response to l-NAME in vivo. The precise cause for this apparent discrepancy between in vivo and isolated vessel studies is not known and needs to be further evaluated. Although great care was taken not to disturb lymph flow generated by the small intestine by using a very localized application of l-NAME to a small section of the lymph vessel, the complexity of the in vivo situations with intact neurohumoral responses necessitates further study.
In summary, in vivo measurements of the mesenteric lymphatic wall [NO] have shown that the valvular areas generated a large NO signal in proportion to the elevated lymphatic contractile inotropic and chronotropic activity associated with tissue hydration and lymph formation. The tubular areas of the mesenteric lymphatic vessels responded similarly, but at a lower [NO]. While lymph vessels were capable of rapid increases in [NO] with each lymphatic systole during the periods of basal flow, a greatly increased average [NO] occurred as the pumping cycle frequency was elevated during the periods of enhanced flow. This elevation of [NO] presumably relaxes lymphangions during lymphatic diastole and reduces outflow resistance as well as enhances lusitropy to make the lymph pump more efficient. The suppression of NOS, while lymph vessels are activated, disrupted the contraction/relaxation cycle in favor of excessive contraction. In effect, NO generation by activated lymph vessels is essential to diastolic filling and, thereby, the maintenance of elevated lymph pump flow.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-70308.
ACKNOWLEDGMENTS
We thank Randal Bills for technical assistance during these studies and Dr. James Moore for critical review of the manuscript.
REFERENCES
- 1.Bauser-Heaton HD, Bohlen HG. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS. Am J Physiol Heart Circ Physiol 293: H2193–H2201, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bauser-Heaton HD, Song J, Bohlen HG. Cerebral microvascular nNOS responds to lowered oxygen tension through a bumetanide-sensitive cotransporter and sodium-calcium exchanger. Am J Physiol Heart Circ Physiol 294: H2166–H2173, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Benoit JN, Zawieja DC. Effects of f-Met-Leu-Phe-induced inflammation on intestinal lymph flow and lymphatic pump behavior. Am J Physiol Gastrointest Liver Physiol 262: G199–G202, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Bohlen HG. Intestinal mucosal oxygenation influences absorptive hyperemia. Am J Physiol Heart Circ Physiol 239: H489–H493, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Bohlen HG. Integration of intestinal structure, function, and microvascular regulation. Microcirculation 5: 27–37, 1998 [PubMed] [Google Scholar]
- 8.Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvasc Res 52: 13–26, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ferguson MK, DeFilippi VJ. Nitric oxide and endothelium-dependent relaxation in tracheobronchial lymph vessels. Microvasc Res 47: 308–317, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Friedemann MN, Robinson SW, Gerhardt GA. o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal Chem 68: 2621–2628, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granger HJ. Role of the interstitial matrix and lymphatic pump in regulation of transcapillary fluid balance. Microvasc Res 18: 209–216, 1979 [DOI] [PubMed] [Google Scholar]
- 15.Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol Heart Circ Physiol 233: H57–H65, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol Regul Integr Comp Physiol 277: R1683–R1689, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Lash JM, Nase GP, Bohlen HG. Acute hyperglycemia depresses arteriolar NO formation in skeletal muscle. Am J Physiol Heart Circ Physiol 277: H1513–H1520, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Leak LV, Cadet JL, Griffin CP, Richardson K. Nitric oxide production by lymphatic endothelial cells in vitro. Biochem Biophys Res Commun 217: 96–105, 1995 [DOI] [PubMed] [Google Scholar]
- 19.McHale NG, Meharg MK. Co-ordination of pumping in isolated bovine lymphatic vessels. J Physiol 450: 503–512, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno R, Dornyei G, Koller A, Kaley G. Myogenic responses of isolated lymphatics: modulation by endothelium. Microcirculation 4: 413–420, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol 274: R790–R796, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Nase GP, Tuttle J, Bohlen HG. Reduced perivascular Po2 increases nitric oxide release from endothelial cells. Am J Physiol Heart Circ Physiol 285: H507–H515, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 239: H88–H95, 1980 [DOI] [PubMed] [Google Scholar]
- 25.Orlov RS, Lobacheva A. Intravascular pressure and spontaneous contraction of the lymphatics. Bull Exp Biol Med 83: 448–450, 1977 [PubMed] [Google Scholar]
- 26.Peng X, Haldar S, Deshpande S, Irani K, Kass DA. Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension 41: 378–381, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Pezzuto L, Bohlen HG. Extracellular arginine rapidly dilates in vivo intestinal arteries and arterioles through a nitric oxide mechanism. Microcirculation 15: 123–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirasawa Y, Ikomi F, Ohhashi T. Physiological roles of endogenous nitric oxide in lymphatic pump activity of rat mesentery in vivo. Am J Physiol Gastrointest Liver Physiol 278: G551–G556, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol 101: 1751–1759, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Takeda H, Komori K, Nishikimi N, Nimura Y, Sokabe M, Naruse K. Bi-phasic activation of eNOS in response to uni-axial cyclic stretch is mediated by differential mechanisms in BAECs. Life Sci 79: 233–239, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Tsunemoto H, Ikomi F, Ohhashi T. Flow-mediated release of nitric oxide from lymphatic endothelial cells of pressurized canine thoracic duct. Jpn J Physiol 53: 157–163, 2003 [DOI] [PubMed] [Google Scholar]
- 32.von der Weid PY, Crowe MJ, van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol 493: 563–575, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von der Weid PY, Zhao J, van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol 280: H2707–H2716, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 264: H1460–H1464, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Zani BG, Bohlen HG. Sodium channels are required during in vivo sodium chloride hyperosmolarity to stimulate increase in intestinal endothelial nitric oxide production. Am J Physiol Heart Circ Physiol 288: H89–H95, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Zani BG, Bohlen HG. Transport of extracellular l-arginine via the cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol 289: H1381–H1390, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Zawieja DC, Barber BJ. Lymph protein concentration in initial and collecting lymphatics of the rat. Am J Physiol Gastrointest Liver Physiol 252: G602–G606, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol Heart Circ Physiol 264: H1283–H1291, 1993 [DOI] [PubMed] [Google Scholar]